In Silico Prediction for Intestinal Absorption and Brain Penetration of Chemical Pesticides in Humans
Abstract
:1. Introduction
2. Materials and Methods
2.1. Pesticide Selection
2.2. In Silico Evaluation of Intestine and Brain Permeation
2.3. Association of Pesticide Physicochemical Parameters and Predicted Permeation across Intestinal or Blood-Brain Barriers
2.4. Confrontation of Predicted and Measured Human Intestinal Absorption Values for Some Pesticides
3. Results
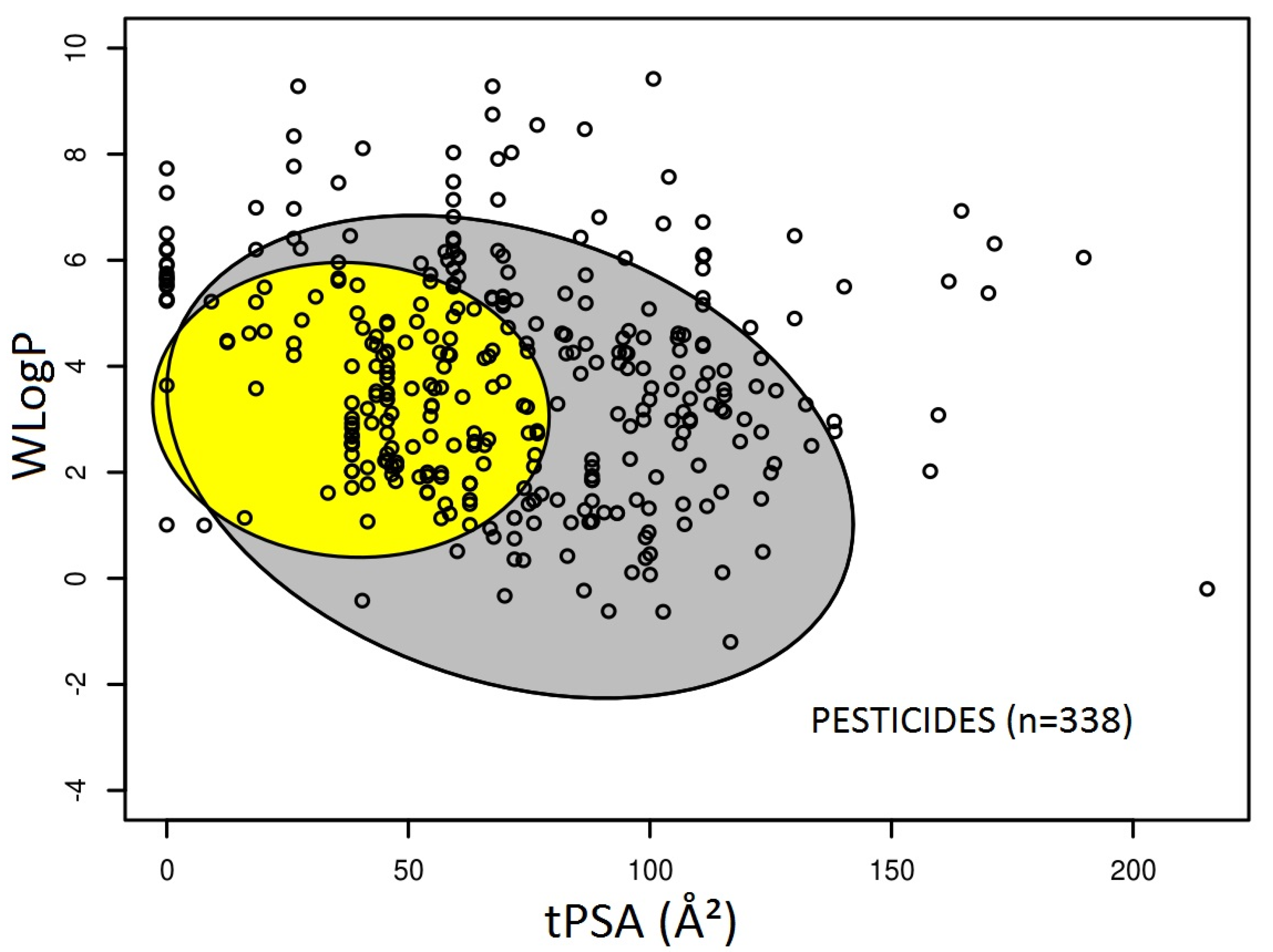
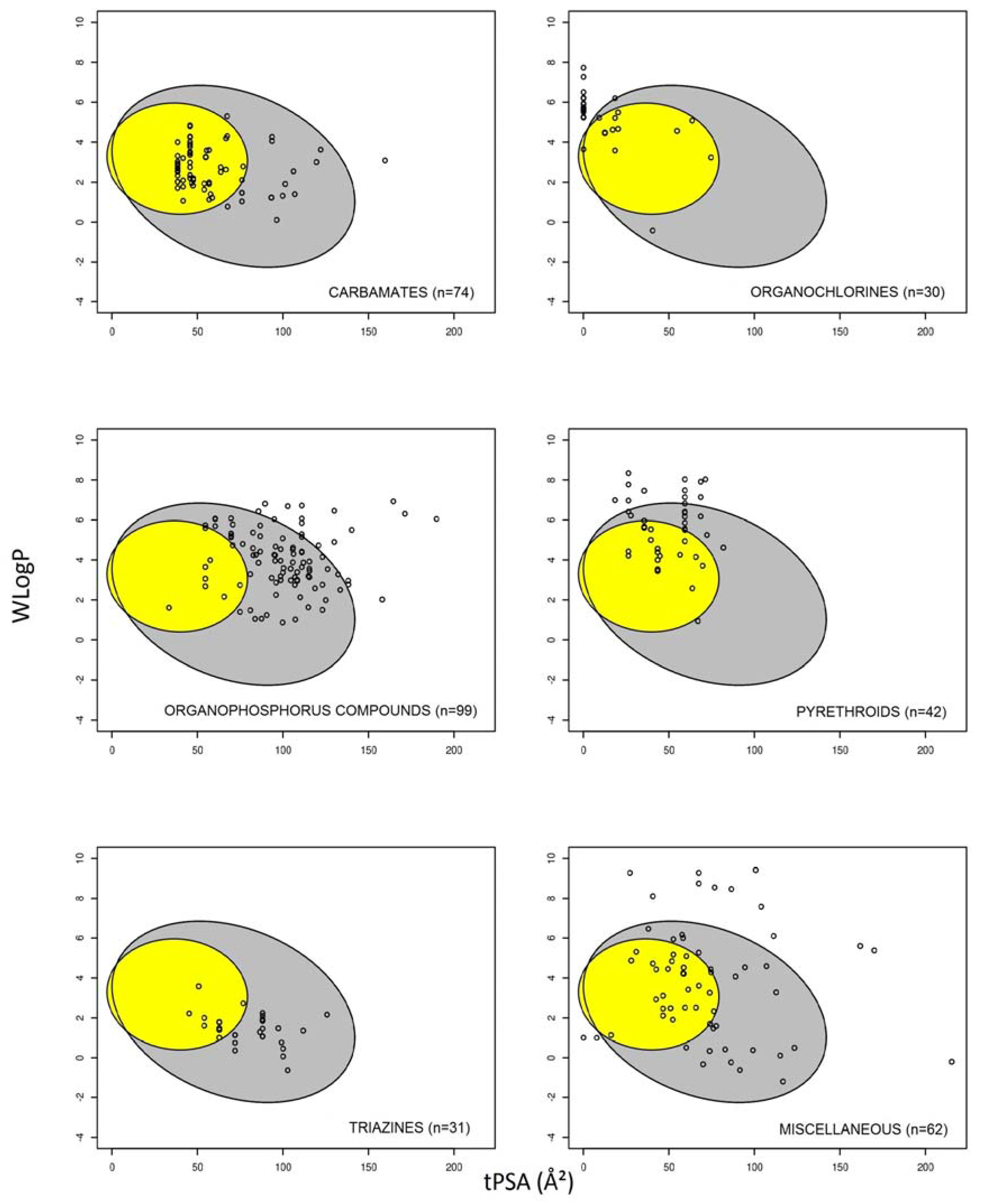
3.1. Prediction of Intestinal and Brain Permeation of Pesticides
3.2. Pesticide Physicochemical Parameters Associated with the Prediction of Intestinal or Brain Permeation
3.3. Confrontation of Predicted and Measured Human Intestinal Absorption for Some Pesticides
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Conflicts of Interest
Appendix A
References
- Damalas, C.A.; Eleftherohorinos, I.G. Pesticide exposure, safety issues, and risk assessment indicators. Int. J. Environ. Res. Public Health 2011, 8, 1402–1419. [Google Scholar] [CrossRef] [PubMed]
- Winter, C.K. Dietary pesticide risk assessment. Rev. Environ. Contam. Toxicol. 1992, 127, 23–67. [Google Scholar] [PubMed]
- Schafer, K.S.; Kegley, S.E. Persistent toxic chemicals in the US food supply. J. Epidemiol. Community Health 2002, 56, 813–817. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, D.; Ambrus, A.; Dieterle, R.; Felsot, A.; Harris, C.; Petersen, B.; Racke, K.; Wong, S.S.; Gonzalez, R.; Tanaka, K.; et al. Pesticide residues in food—Acute dietary exposure. Pest. Manag. Sci. 2004, 60, 311–339. [Google Scholar] [CrossRef] [PubMed]
- Mostafalou, S.; Abdollahi, M. Pesticides: An update of human exposure and toxicity. Arch. Toxicol. 2017, 91, 549–599. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Kabir, E.; Jahan, S.A. Exposure to pesticides and the associated human health effects. Sci. Total Environ. 2017, 575, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Mostafalou, S.; Abdollahi, M. Pesticides and human chronic diseases: Evidences, mechanisms, and perspectives. Toxicol. Appl. Pharmacol. 2013, 268, 157–177. [Google Scholar] [CrossRef] [PubMed]
- Soderlund, D.M. Molecular mechanisms of pyrethroid insecticide neurotoxicity: Recent advances. Arch. Toxicol. 2012, 86, 165–181. [Google Scholar] [CrossRef] [PubMed]
- Pope, C.N. Organophosphorus pesticides: Do they all have the same mechanism of toxicity? J. Toxicol Environ. Health B Crit. Rev. 1999, 2, 161–181. [Google Scholar] [CrossRef] [PubMed]
- Coecke, S.; Pelkonen, O.; Leite, S.B.; Bernauer, U.; Bessems, J.G.; Bois, F.Y.; Gundert-Remy, U.; Loizou, G.; Testai, E.; Zaldivar, J.M. Toxicokinetics as a key to the integrated toxicity risk assessment based primarily on non-animal approaches. Toxicol. In Vitro 2013, 27, 1570–1577. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.R.; Hatley, O.J.; Ungell, A.L.; Hilgendorf, C.; Peters, S.A.; Rostami-Hodjegan, A. Gut Wall Metabolism. Application of Pre-Clinical Models for the Prediction of Human Drug Absorption and First-Pass Elimination. AAPS J. 2016, 18, 589–604. [Google Scholar] [CrossRef] [PubMed]
- Abbott, N.J. Prediction of blood-brain barrier permeation in drug discovery from in vivo, in vitro and in silico models. Drug Discov. Today Technol. 2004, 1, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Sjogren, E.; Abrahamsson, B.; Augustijns, P.; Becker, D.; Bolger, M.B.; Brewster, M.; Brouwers, J.; Flanagan, T.; Harwood, M.; Heinen, C.; et al. In vivo methods for drug absorption—Comparative physiologies, model selection, correlations with in vitro methods (IVIVC), and applications for formulation/API/excipient characterization including food effects. Eur. J. Pharm. Sci. 2014, 57, 99–151. [Google Scholar] [CrossRef] [PubMed]
- Pellegatti, M. Preclinical in vivo ADME studies in drug development: A critical review. Expert. Opin. Drug Metab. Toxicol. 2012, 8, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, K.; Singh, S.K.; Mishra, D.N. Drug targeting to brain: A systematic approach to study the factors, parameters and approaches for prediction of permeability of drugs across BBB. Expert. Opin. Drug Deliv. 2013, 10, 927–955. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Li, W.; Liu, G.; Tang, Y. In silico ADMET prediction: Recent advances, current challenges and future trends. Curr. Top. Med. Chem. 2013, 13, 1273–1289. [Google Scholar] [CrossRef] [PubMed]
- Butina, D.; Segall, M.D.; Frankcombe, K. Predicting ADME properties in silico: Methods and models. Drug Discov. Today 2002, 7, S83–S88. [Google Scholar] [CrossRef]
- Toropov, A.A.; Toropova, A.P.; Beeg, M.; Gobbi, M.; Salmona, M. QSAR model for blood-brain barrier permeation. J. Pharmacol. Toxicol. Methods 2017, 88, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Toropova, M.A.; Raska, I.; Toropov, A.A.; Raskova, M. The Utilization of the Monte Carlo Technique for Rational Drug Discovery. Comb. Chem. High. Throughput Screen. 2016, 19, 676–687. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Zoete, V. A BOILED-Egg to predict gastrointestinal absorption and brain penetration of small molecules. ChemMedChem 2016, 11, 1117–1121. [Google Scholar] [CrossRef] [PubMed]
- Wilkes, M.F.; Woollen, B.H.; Marsh, J.R.; Batten, P.L.; Chester, G. Biological monitoring for pesticide exposure-the role of human volunteer studies. Int. Arch. Occup. Environ. Health 1993, 65, S189–S192. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.B.; Anand, S.S.; Kim, H.J.; White, C.A.; Bruckner, J.V. Toxicokinetics and tissue distribution of deltamethrin in adult Sprague-Dawley rats. Toxicol. Sci. 2008, 101, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Musther, H.; Olivares-Morales, A.; Hatley, O.J.; Liu, B.; Rostami Hodjegan, A. Animal versus human oral drug bioavailability: Do they correlate? Eur. J. Pharm. Sci. 2014, 57, 280–291. [Google Scholar] [CrossRef] [PubMed]
- Resnik, D.B.; Portier, C. Pesticide testing on human subjects: Weighing benefits and risks. Environ. Health Perspect 2005, 113, 813–817. [Google Scholar] [CrossRef] [PubMed]
- London, L.; Coggon, D.; Moretto, A.; Westerholm, P.; Wilks, M.F.; Colosio, C. The ethics of human volunteer studies involving experimental exposure to pesticides: Unanswered dilemmas. Environ. Health 2010, 9, 50. [Google Scholar] [CrossRef] [PubMed]
- Hazardous Substances Data Bank (HSDB). Available online: https://toxnet.nlm.nih.gov/newtoxnet/hsdb.htm (accessed on 26 March 2017).
- National Pesticide Information Center. Available online: http://npic.orst.edu/ (accessed on 28 March 2017).
- Holt, E.; Weber, R.; Stevenson, G.; Gaus, C. Formation of dioxins during exposure of pesticide formulations to sunlight. Chemosphere 2012, 88, 364–370. [Google Scholar] [CrossRef] [PubMed]
- PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 30 March 2017).
- The R Project for Statistical Computing. Available online: http://www.R-project.org/ (accessed on 18 April 2017).
- Cheng, T.; Zhao, Y.; Li, X.; Lin, F.; Xu, Y.; Zhang, X.; Li, Y.; Wang, R.; Lai, L. Computation of octanol-water partition coefficients by guiding an additive model with knowledge. J. Chem. Inf. Model. 2007, 47, 2140–2148. [Google Scholar] [CrossRef] [PubMed]
- Chedik, L.; Bruyere, A.; Le Vee, M.; Stieger, B.; Denizot, C.; Parmentier, Y.; Potin, S.; Fardel, O. Inhibition of Human Drug Transporter Activities by the Pyrethroid Pesticides Allethrin and Tetramethrin. PLoS ONE 2017, 12, e0169480. [Google Scholar] [CrossRef] [PubMed]
- PubMed. Available online: https://www.ncbi.nlm.nih.gov/pubmed/ (accessed on 28 March 2017).
- Fukuto, T.R. Mechanism of action of organophosphorus and carbamate insecticides. Environ. Health Perspect. 1990, 87, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Ellison, C.A.; Tian, Y.; Knaak, J.B.; Kostyniak, P.J.; Olson, J.R. Human hepatic cytochrome P450-specific metabolism of the organophosphorus pesticides methyl parathion and diazinon. Drug Metab. Dispos. 2012, 40, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Flaskos, J. The developmental neurotoxicity of organophosphorus insecticides: A direct role for the oxon metabolites. Toxicol. Lett. 2012, 209, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Norinder, U.; Haeberlein, M. Computational approaches to the prediction of the blood-brain distribution. Adv. Drug Deliv. Rev. 2002, 54, 291–313. [Google Scholar] [CrossRef]
- Saeedi Saravi, S.S.; Dehpour, A.R. Potential role of organochlorine pesticides in the pathogenesis of neurodevelopmental, neurodegenerative, and neurobehavioral disorders: A review. Life Sci. 2016, 145, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Eadsforth, C.V.; Bragt, P.C.; van Sittert, N.J. Human dose-excretion studies with pyrethroid insecticides cypermethrin and alphacypermethrin: Relevance for biological monitoring. Xenobiotica 1988, 18, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Woollen, B.H.; Marsh, J.R.; Laird, W.J.; Lesser, J.E. The metabolism of cypermethrin in man: Differences in urinary metabolite profiles following oral and dermal administration. Xenobiotica 1992, 22, 983–991. [Google Scholar] [CrossRef] [PubMed]
- Papalexiou, P.; Bitar, N.; Stockis, A. Absorption of Radiocarbon Labelled Deltamethrin Given Orally in Healthy Volunteers, Unpublished Proprietary Report 50/22, submitted to WHO by Roussel Uclaf. 1984.
- Sams, C.; Jones, K. Biological monitoring for exposure to deltamethrin: A human oral dosing study and background levels in the UK general population. Toxicol. Lett. 2012, 213, 35–38. [Google Scholar] [CrossRef] [PubMed]
- Cridland, J.S.; Weatherley, B.C. Urinary Excretion in Man of 3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropane Carboxylic acid (“CVA”) after Oral Ingestion of Permethrin (NRDC 143)—A First Report, Wellcome Research Laboratories (Report No. BDPE-77–1), Unpublished data, submitted to WHO. 1977.
- Adcock, J.W.; Challis, I.R. The Pharmacokinetics and Metabolism of Bendiocarb in Man, Report from Fisons Ltd. Unpublished Report, submitted to WHO by FBC Limited. 1976.
- Sams, C.; Patel, K.; Jones, K. Biological monitoring for exposure to pirimicarb: Method development and a human oral dosing study. Toxicol. Lett. 2010, 192, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Vandekar, M.; Plestina, R.; Wilhelm, K. Toxicity of carbamates for mammals. Bull. World Health Organ. 1971, 44, 241–249. [Google Scholar] [PubMed]
- Dawson, J.A.; Heath, D.F.; Rose, J.A.; Thain, E.M.; Ward, J.B. The excretion by humans of the phenol derived in vivo from 2-isopropoxyphenyl N-methylcarbamate. Bull. World Health Organ. 1964, 30, 127–134. [Google Scholar] [PubMed]
- Hayes, W.J., Jr.; Dale, W.E.; Pirkle, C.I. Evidence of safety of long-term, high, oral doses of DDT for man. Arch. Environ. Health 1971, 22, 119–135. [Google Scholar] [CrossRef] [PubMed]
- Braun, W.H.; Blau, G.E.; Chenoweth, M.B. The metabolism/pharmacokinetics of pentachlorophenol in man, and a comparison with the rat and monkey. Dev. Toxicol. Environ. Sci. 1979, 4, 289–296. [Google Scholar]
- Poiger, H.; Schlatter, C. Pharmacokinetics of 2,3,7,8-TCDD in man. Chemosphere 1986, 15, 1489–1494. [Google Scholar] [CrossRef]
- Nolan, R.J.; Rick, D.L.; Freshour, N.L.; Saunders, J.H. Chlorpyrifos: Pharmacokinetics in human volunteers. Toxicol. Appl. Pharmacol. 1984, 73, 8–15. [Google Scholar] [CrossRef]
- Griffin, P.; Mason, H.; Heywood, K.; Cocker, J. Oral and dermal absorption of chlorpyrifos: A human volunteer study. Occup. Environ. Med. 1999, 56, 10–13. [Google Scholar] [CrossRef] [PubMed]
- Garfitt, S.J.; Jones, K.; Mason, H.J.; Cocker, J. Exposure to the organophosphate diazinon: Data from a human volunteer study with oral and dermal doses. Toxicol. Lett. 2002, 134, 105–113. [Google Scholar] [CrossRef]
- Hutson, D.H.; Hoadley, E.C. The comparative metabolism of (14 C-vinyl)dichlorvos in animals and man. Arch. Toxikol. 1972, 30, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Sanderson, D.M.; Edson, E.F. Toxicologic properties of the organophosphorus insecticide dimethoate. Br. J. Ind. Med. 1964, 21, 52–64. [Google Scholar] [PubMed]
- Krieger, R.I.; Thongsinthusak, T. Metabolism and excretion of dimethoate following ingestion of overtolerance peas and a bolus dose. Food Chem. Toxicol. 1993, 31, 177–182. [Google Scholar] [CrossRef]
- Meaklim, J.; Yang, J.; Drummer, O.H.; Killalea, S.; Staikos, V.; Horomidis, S.; Rutherford, D.; Ioannides-Demos, L.L.; Lim, S.; McLean, A.J.; et al. Fenitrothion: Toxicokinetics and toxicologic evaluation in human volunteers. Environ. Health Perspect. 2003, 111, 305–308. [Google Scholar] [CrossRef] [PubMed]
- Morgan, D.P.; Hetzler, H.L.; Slach, E.F.; Lin, L.I. Urinary excretion of paranitrophenol and alkyl phosphates following ingestion of methyl or ethyl parathion by human subjects. Arch. Environ. Contam. Toxicol. 1977, 6, 159–173. [Google Scholar] [CrossRef] [PubMed]
- Garfitt, S.J.; Jones, K.; Mason, H.J.; Cocker, J. Oral and dermal exposure to propetamphos: A human volunteer study. Toxicol. Lett. 2002, 134, 115–118. [Google Scholar] [CrossRef]
- Gehring, P.J.; Kramer, C.G.; Schwetz, B.A.; Rose, J.Q.; Rowe, V.K. The fate of 2,4,5-trichlorophenoxyacetic acid (2,4,5-T) following oral administration to man. Toxicol. Appl. Pharmacol. 1973, 26, 352–361. [Google Scholar] [CrossRef]
- Sauerhoff, M.W.; Braun, W.H.; Blau, G.E.; Gehring, P.J. The fate of 2,4-dichlorophenoxyacetic acid (2,4-D) following oral administration to man. Toxicology 1977, 8, 3–11. [Google Scholar] [CrossRef]
- Kohli, J.D.; Khanna, R.N.; Gupta, B.N.; Dhar, M.M.; Tandon, J.S.; Sircar, K.P. Absorption and excretion of 2,4-dichlorophenoxyacetic acid in man. Xenobiotica 1974, 4, 97–100. [Google Scholar] [CrossRef] [PubMed]
- Woollen, B.H.; Hart, T.B.; Batten, P.L.; Laird, W.J.; Davies, D.S.; Dollery, C.T. Oral pharmacokinetics of fluazifop-butyl in human volunteers. Hum. Exp. Toxicol. 1991, 10, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Feldmann, R.J.; Wannag, A. Human urinary excretion of the herbicide 2-methyl-4-chloro-phenoxy-acetic acid. Scand. J. Work Environ. Health 1977, 3, 100–103. [Google Scholar]
- Conning, D.A.; Fletcher, K.; Swan, A.A.B. Paraquat and related bipyridyls. Br. Med. Bull. 1969, 25, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Nolan, R.J.; Freshour, N.L.; Kastl, P.E.; Saunders, J.H. Pharmacokinetics of picloram in male volunteers. Toxicol. Appl. Pharmacol. 1984, 76, 264–269. [Google Scholar] [CrossRef]
- Carmichael, N.G.; Nolan, R.J.; Perkins, J.M.; Davies, R.; Warrington, S.J. Oral and dermal pharmacokinetics of triclopyr in human volunteers. Hum. Toxicol. 1989, 8, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Breckenridge, A.; Orme, M. Kinetics of warfarin absorption in man. Clin. Pharmacol. Ther. 1973, 14, 955–961. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Lombardo, F.; Gifford, E.; Shalaeva, M.Y. In silico ADME prediction: Data, models, facts and myths. Mini Rev. Med. Chem. 2003, 3, 861–875. [Google Scholar] [CrossRef] [PubMed]
- Veber, D.F.; Johnson, S.R.; Cheng, H.Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef] [PubMed]
- Mukunthan, K.S.; Bhattacharya, A.; Patel, T.N.C. Regression Analysis: Identifying Molecular Descriptors for HIA, MDCK and Caco-2. Int. J. Pharm. Sci. Rev. Res. 2016, 37, 205–209. [Google Scholar]
- Lipinski, C.A. Lead- and drug-like compounds: The rule-of-five revolution. Drug Discov. Today Technol. 2004, 1, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Brunet, J.L.; Maresca, M.; Fantini, J.; Belzunces, L.P. Intestinal absorption of the acetamiprid neonicotinoid by Caco-2 cells: Transepithelial transport, cellular uptake and efflux. J. Environ. Sci. Health B 2008, 43, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Brunet, J.L.; Maresca, M.; Fantini, J.; Belzunces, L.P. Human intestinal absorption of imidacloprid with Caco-2 cells as enterocyte model. Toxicol. Appl. Pharmacol. 2004, 194, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kelder, J.; Grootenhuis, P.D.; Bayada, D.M.; Delbressine, L.P.; Ploemen, J.P. Polar molecular surface as a dominating determinant for oral absorption and brain penetration of drugs. Pharm. Res. 1999, 16, 1514–1519. [Google Scholar] [CrossRef] [PubMed]
- Amaraneni, M.; Sharma, A.; Pang, J.; Muralidhara, S.; Cummings, B.S.; White, C.A.; Bruckner, J.V.; Zastre, J. Plasma protein binding limits the blood brain barrier permeation of the pyrethroid insecticide, deltamethrin. Toxicol. Lett. 2016, 250–251, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, R.M.; Holden, J.E.; Nickles, R.J.; Murali, D.; Barbee, D.L.; Barnhart, T.E.; Christian, B.T.; DeJesus, O.T. Paraquat is excluded by the blood brain barrier in rhesus macaque: An in vivo pet study. Brain Res. 2009, 1259, 74–79. [Google Scholar] [CrossRef] [PubMed]
- La Merrill, M.; Emond, C.; Kim, M.J.; Antignac, J.P.; Le Bizec, B.; Clement, K.; Birnbaum, L.S.; Barouki, R. Toxicological function of adipose tissue: Focus on persistent organic pollutants. Environ. Health Perspect. 2013, 121, 162–169. [Google Scholar] [PubMed]
- Lee, D.H.; Steffes, M.W.; Sjodin, A.; Jones, R.S.; Needham, L.L.; Jacobs, D.R., Jr. Low dose organochlorine pesticides and polychlorinated biphenyls predict obesity, dyslipidemia, and insulin resistance among people free of diabetes. PLoS ONE 2011, 6, e15977. [Google Scholar] [CrossRef] [PubMed]
- Ruzzin, J.; Petersen, R.; Meugnier, E.; Madsen, L.; Lock, E.J.; Lillefosse, H.; Ma, T.; Pesenti, S.; Sonne, S.B.; Marstrand, T.T.; et al. Persistent organic pollutant exposure leads to insulin resistance syndrome. Environ. Health Perspect. 2010, 118, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Surak, J.G.; Bradley, R.L., Jr. Transport of organochlorine chemicals across cell membranes. Environ. Res. 1976, 11, 343–352. [Google Scholar] [CrossRef]
- Moser, G.A.; McLachlan, M.S. Modeling digestive tract absorption and desorption of lipophilic organic contaminants in humans. Environ. Sci. Technol. 2002, 36, 3318–3325. [Google Scholar] [CrossRef] [PubMed]
- Jamal, G.A. Neurological syndromes of organophosphorus compounds. Adverse Drug React. Toxicol. Rev. 1997, 16, 133–170. [Google Scholar] [PubMed]
- Voorhees, J.R.; Rohlman, D.S.; Lein, P.J.; Pieper, A.A. Neurotoxicity in Preclinical Models of Occupational Exposure to Organophosphorus Compounds. Front. Neurosci. 2016, 10, 590. [Google Scholar] [CrossRef] [PubMed]
- Abraham, M.H.; Takacs-Novak, K.; Mitchell, R.C. On the partition of ampholytes: Application to blood-brain distribution. J. Pharm. Sci. 1997, 86, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.E. Rapid calculation of polar molecular surface area and its application to the prediction of transport phenomena. 2. Prediction of blood-brain barrier penetration. J. Pharm. Sci. 1999, 88, 815–821. [Google Scholar] [CrossRef] [PubMed]
- Vilar, S.; Chakrabarti, M.; Costanzi, S. Prediction of passive blood-brain partitioning: Straightforward and effective classification models based on in silico derived physicochemical descriptors. J. Mol. Graph. Model. 2010, 28, 899–903. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, P.; Friis, C.; Gyrd-Hansen, N.; Kraul, I. Disposition of parathion in neonatal and young pigs. Pharmacol. Toxicol. 1991, 69, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Mutkus, L.A.; Sumner, D.; Stevens, J.T.; Eldridge, J.C.; Strandhoy, J.W.; Aschner, M. Transendothelial permeability of chlorpyrifos in RBE4 monolayers is modulated by astrocyte-conditioned medium. Brain Res. Mol. Brain Res. 2001, 97, 43–50. [Google Scholar] [CrossRef]
- Li, W.; Ehrich, M. Transient alterations of the blood-brain barrier tight junction and receptor potential channel gene expression by chlorpyrifos. J. Appl. Toxicol. 2013, 33, 1187–1191. [Google Scholar] [CrossRef] [PubMed]
- Balbuena, P.; Li, W.; Rzigalinski, B.A.; Ehrich, M. Malathion/oxon and lead acetate increase gene expression and protein levels of transient receptor potential canonical channel subunits TRPC1 and TRPC4 in rat endothelial cells of the blood-brain barrier. Int. J. Toxicol. 2012, 31, 238–249. [Google Scholar] [CrossRef] [PubMed]
- Fardel, O.; Kolasa, E.; Le Vee, M. Environmental chemicals as substrates, inhibitors or inducers of drug transporters: Implication for toxicokinetics, toxicity and pharmacokinetics. Expert. Opin. Drug Metab. Toxicol. 2012, 8, 29–46. [Google Scholar] [CrossRef] [PubMed]
- Lacher, S.E.; Skagen, K.; Veit, J.; Dalton, R.; Woodahl, E.L. P-Glycoprotein Transport of Neurotoxic Pesticides. J. Pharmacol. Exp. The.r 2015, 355, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Bucher, S.; Le Vee, M.; Jouan, E.; Fardel, O. Regulation of hepatic drug transporter activity and expression by organochlorine pesticides. J. Biochem. Mol. Toxicol. 2014, 28, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Bain, L.J.; LeBlanc, G.A. Interaction of structurally diverse pesticides with the human MDR1 gene product P-glycoprotein. Toxicol. Appl. Pharmacol. 1996, 141, 288–298. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, S.; Sorani, M.; Giacomini, K.M. Transport of paraquat by human organic cation transporters and multidrug and toxic compound extrusion family. J. Pharmacol. Exp. Ther. 2007, 322, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Chan, L.M.; Lowes, S.; Hirst, B.H. The ABCs of drug transport in intestine and liver: Efflux proteins limiting drug absorption and bioavailability. Eur. J. Pharm. Sci. 2004, 21, 25–51. [Google Scholar] [CrossRef] [PubMed]
- Zastre, J.; Dowd, C.; Bruckner, J.; Popovici, A. Lack of P-glycoprotein-mediated efflux and the potential involvement of an influx transport process contributing to the intestinal uptake of deltamethrin, cis-permethrin, and trans-permethrin. Toxicol. Sci. 2013, 136, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Kramer, R.E.; Wellman, S.E.; Rockhold, R.W.; Baker, R.C. Pharmacokinetics of methyl parathion: A comparison following single intravenous, oral or dermal administration. J. Biomed. Sci. 2002, 9, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Gammon, D.; Liu, Z.; Chandrasekaran, A.; ElNaggar, S. The pharmacokinetic properties of bifenthrin in the rat following multiple routes of exposure. Pest. Manag. Sci. 2015, 71, 835–841. [Google Scholar] [CrossRef] [PubMed]
- Genuis, S.J.; Lane, K.; Birkholz, D. Human Elimination of Organochlorine Pesticides: Blood, Urine, and Sweat Study. Biomed. Res. Int. 2016, 2016, 1624643. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Yu, L.S.; Zeng, S. Stereoselectivity of chiral drug transport: A focus on enantiomer-transporter interaction. Drug Metab. Rev. 2014, 46, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Houston, J.B.; Upshall, D.G.; Bridges, J.W. Pharmacokinetics and metabolism of two carbamate insecticides, carbaryl and landrin, in the rat. Xenobiotica 1975, 5, 637–648. [Google Scholar] [CrossRef] [PubMed]
- Eaton, D.L.; Daroff, R.B.; Autrup, H.; Bridges, J.; Buffler, P.; Costa, L.G.; Coyle, J.; McKhann, G.; Mobley, W.C.; Nadel, L.; et al. Review of the toxicology of chlorpyrifos with an emphasis on human exposure and neurodevelopment. Crit. Rev. Toxicol. 2008, 38 (Suppl. 2), 1–125. [Google Scholar] [CrossRef] [PubMed]
- Boobis, A.R.; Ossendorp, B.C.; Banasiak, U.; Hamey, P.Y.; Sebestyen, I.; Moretto, A. Cumulative risk assessment of pesticide residues in food. Toxicol. Lett. 2008, 180, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Konig, J.; Muller, F.; Fromm, M.F. Transporters and drug-drug interactions: Important determinants of drug disposition and effects. Pharmacol. Rev. 2013, 65, 944–966. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.Z.; Jang, G.R.; Tsunoda, S. Dietary effects on drug metabolism and transport. Clin. Pharmacokinet. 2003, 42, 1071–1088. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, P.; Sack, A.; Wampole, M.; Bobst, S.; Vracko, M. Integration of in silico methods and computational systems biology to explore endocrine-disrupting chemical binding with nuclear hormone receptors. Chemosphere 2017, 178, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Novic, M.; Vracko, M. QSAR models for reproductive toxicity and endocrine disruption activity. Molecules 2010, 15, 1987–1999. [Google Scholar] [CrossRef] [PubMed]
- Greget, R.; Dadak, S.; Barbier, L.; Lauga, F.; Linossier-Pierre, S.; Pernot, F.; Legendre, A.; Ambert, N.; Bouteiller, J.M.; Dorandeu, F.; et al. Modeling and simulation of organophosphate-induced neurotoxicity: Prediction and validation by experimental studies. Neurotoxicology 2016, 54, 140–152. [Google Scholar] [CrossRef] [PubMed]
- Knaak, J.B.; Dary, C.C.; Zhang, X.; Gerlach, R.W.; Tornero-Velez, R.; Chang, D.T.; Goldsmith, R.; Blancato, J.N. Parameters for pyrethroid insecticide QSAR and PBPK/PD models for human risk assessment. Rev. Environ. Contam. Toxicol 2012, 219, 1–114. [Google Scholar] [PubMed]
- Punt, A.; Peijnenburg, A.A.; Hoogenboom, R.L.; Bouwmeester, H. Non-animal approaches for toxicokinetics in risk evaluations of food chemicals. ALTEX 2017, in press. [Google Scholar] [CrossRef] [PubMed]

| Organophosphorus Pesticide | Brain Permeation Prediction | |
|---|---|---|
| Parent Molecule | Oxon Metabolite | |
| Bensulide | No | No |
| Chlorpyrifos | No | Yes |
| Chlorpyrifos-methyl | No | Yes |
| Coumaphos | No | No |
| Diazinon | No | No |
| Ethion | No | No |
| Fenthion | No | No |
| Fonofos | No | Yes |
| Malathion | No | No |
| Methyl-parathion | No | No |
| Parathion | No | No |
| Phorate | No | No |
| Phosmet | No | No |
| Sulprofos | No | No |
| Terbufos | No | No |
| Physicochemical Parameter | Parameter Value (Mean ± SD) | ||
|---|---|---|---|
| Low Intestinal Absorption (n = 63 Pesticides) | High Intestinal Absorption (n = 275 Pesticides) | Significance 2 | |
| Molecular weight (g/mol) | 420.4 ± 145.4 | 284.0 ± 79.3 | S (p < 0.0001) |
| Mean atomic van der Waals volume | 0.7 ± 0.1 | 0.6 ± 0.1 | S (p < 0.0001) |
| Mean atomic polarizability | 0.8 ± 0.5 | 0.7 ± 0.1 | S (p < 0.0001) |
| Number of heavy atoms | 24.2 ± 11.4 | 18.0 ± 5.3 | S (p < 0.0001) |
| Number of aromatic heavy carbons | 6.6 ± 6.3 | 5.5 ± 4.4 | NS (p = 0.1050) |
| Fraction Csp3 | 0.5 ± 0.3 | 0.5 ±0.3 | NS (p = 0.7335) |
| Number of rotatable bonds | 6.2 ± 4.2 | 5.4 ± 2.2 | S (p = 0.0252) |
| Number of H-bond acceptors | 4.5 ± 4.3 | 3.5 ± 1.6 | S (p = 0.0031) |
| Number of H-bond donors | 0.4 ± 0.9 | 0.6 ± 0.8 | NS (p = 0.0550) |
| LogS (Silicos-IT) | −6.0 ± 2.5 | −3.8 ± 1.9 | S (p < 0.0001) |
| XLogP3 | 5.2 ± 2.1 | 3.1 ± 1.8 | S (p < 0.0001) |
| Molar refractivity | 97.4 ± 37.6 | 73.2 ± 19.9 | S (p < 0.0001) |
| tPSA (Å2) | 68.1 ± 61.3 | 70.3 ± 27.7 | NS (p = 0.6675) |
| Physicochemical Parameter | Parameter Value (Mean ± SD) | ||
|---|---|---|---|
| No-Brain Permeation (n = 208 Pesticides) | Brain Permeation (n = 130 Pesticides) | Significance 2 | |
| Molecular weight (g/mol) | 332.2 ± 120.2 | 273.1 ± 74.6 | S (p < 0.0001) |
| Mean atomic van der Waals volume | 0.7 ± 0.1 | 0.6 ± 0.1 | S (p = 0.0225) |
| Mean atomic polarizability | 0.7 ± 0.3 | 0.7 ± 0.1 | NS (p = 0.0510) |
| Number of heavy atoms | 20.0 ± 8.3 | 17.8 ± 4.8 | S (p = 0.0061) |
| Number of aromatic heavy carbons | 5.9 ± 5.2 | 5.4 ± 4.2 | NS (p = 0.3634) |
| Fraction Csp3 | 0.5 ± 0.3 | 0.5 ± 0.3 | NS (p = 0.4746) |
| Number of rotatable bonds | 6.0 ± 2.9 | 4.8 ± 2.2 | S (p < 0.0001) |
| Number of H-bond acceptors | 4.3 ± 2.7 | 2.8 ± 1.3 | S (p < 0.0001) |
| Number of H-bond donors | 0.6 ± 0.9 | 0.6 ± 0.7 | NS (p = 0.6422) |
| LogS (Silicos-IT) | −4.2 ± 2.5 | −4.1 ± 1.6 | NS (p = 0.6153) |
| XLogP3 | 3.6 ± 2.4 | 3.4 ± 1.4 | NS (p = 0.4395) |
| Molar refractivity | 81.1 ± 29.1 | 72.2 ± 18.4 | S (p = 0.0021) |
| tPSA (Å2) | 83.3 ± 39.2 | 48.5 ± 15.2 | S (p < 0.0001) |
| Number of N and O atoms | 4.4 ± 2.5 | 3.4 ± 1.2 | S (p < 0.0001) |
| Pesticide | Class | Intestinal Absorption 1 | |
|---|---|---|---|
| Determined from Pharmacokinetics Studies | Predicted by SwissADME Webtool | ||
| Cypermethrin | Pyrethroid | High (Fa = 0.40 [40,41]) | High |
| Deltamethrin | Pyrethroid | High (Fa > 0.48 [42,43]) | High |
| Permethrin | Pyrethroid | High (Fa ≥ 0.32 [44]) | High |
| Bendiocarb | Carbamate | High (Fa ≥ 0.99 [45]) | High |
| Pirimicarb | Carbamate | High (Fa = 0.74 [46]) | High |
| Molinate | Carbamate | High (Fa > 0.40 [22]) | High |
| Propoxur | Carbamate | High (Fa > 0.37 [47,48]) | High |
| DDT 2 | Organochlorine | Low (Fa = 0.15 [49]) | Low |
| Pentachlorophenol | Organochlorine | High (Fa > 0.86 [50]) | High |
| TCDD 2 | Organochlorine | High (Fa > 0.87 [51]) | Low |
| Chlorpyrifos | Organophosphorus compound | High (Fa = 0.82 [52,53]) | High |
| Diazinon | Organosphosphorus compund | High (Fa > 0.66 [54]) | High |
| Dichlorvos | Organophosphorus compound | High (Fa > 0.36 [55]) | High |
| Dimethoate | Organosphosphorus compound | High (Fa = 0.86 [56,57]) | High |
| Fenitrothion | Organophosphorus compound | High (Fa = 0.81 [58]) | High |
| Parathion | Organophosphorus compound | High (Fa > 0.46 [59]) | High |
| Propetamphos | Organophosphorus compound | High (Fa > 0.41 [60]) | High |
| 2,4,5-T 2 | Miscellaneous | High (Fa > 0.89 [61]) | High |
| 2,4-D 2 | Miscellaneous | High (Fa = 0.85 [62,63]) | High |
| Fluazifop-butyl | Miscellaneous | High (Fa = 0.88 [64]) | High |
| MCPA 2 | Miscellaneous | High (Fa > 0.55 [65]) | High |
| Paraquat | Miscellaneous | Low (Fa ≤ 0.05 [66]) | Low |
| Picloram | Miscellaneous | High (Fa = 0.91 [67]) | High |
| Triclopyr | Miscellaneous | High (Fa > 0.82 [68]) | High |
| Warfarine | Miscellaneous | High (Fa > 0.93 [69]) | High |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chedik, L.; Mias-Lucquin, D.; Bruyere, A.; Fardel, O. In Silico Prediction for Intestinal Absorption and Brain Penetration of Chemical Pesticides in Humans. Int. J. Environ. Res. Public Health 2017, 14, 708. https://doi.org/10.3390/ijerph14070708
Chedik L, Mias-Lucquin D, Bruyere A, Fardel O. In Silico Prediction for Intestinal Absorption and Brain Penetration of Chemical Pesticides in Humans. International Journal of Environmental Research and Public Health. 2017; 14(7):708. https://doi.org/10.3390/ijerph14070708
Chicago/Turabian StyleChedik, Lisa, Dominique Mias-Lucquin, Arnaud Bruyere, and Olivier Fardel. 2017. "In Silico Prediction for Intestinal Absorption and Brain Penetration of Chemical Pesticides in Humans" International Journal of Environmental Research and Public Health 14, no. 7: 708. https://doi.org/10.3390/ijerph14070708





