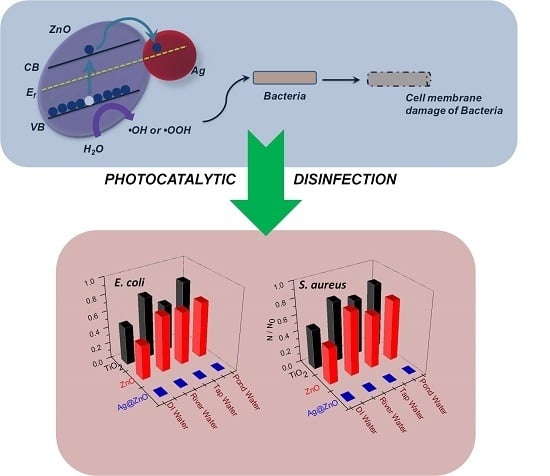
Disinfection of the Water Borne Pathogens Escherichia coli and Staphylococcus aureus by Solar Photocatalysis Using Sonochemically Synthesized Reusable Ag@ZnO Core-Shell Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
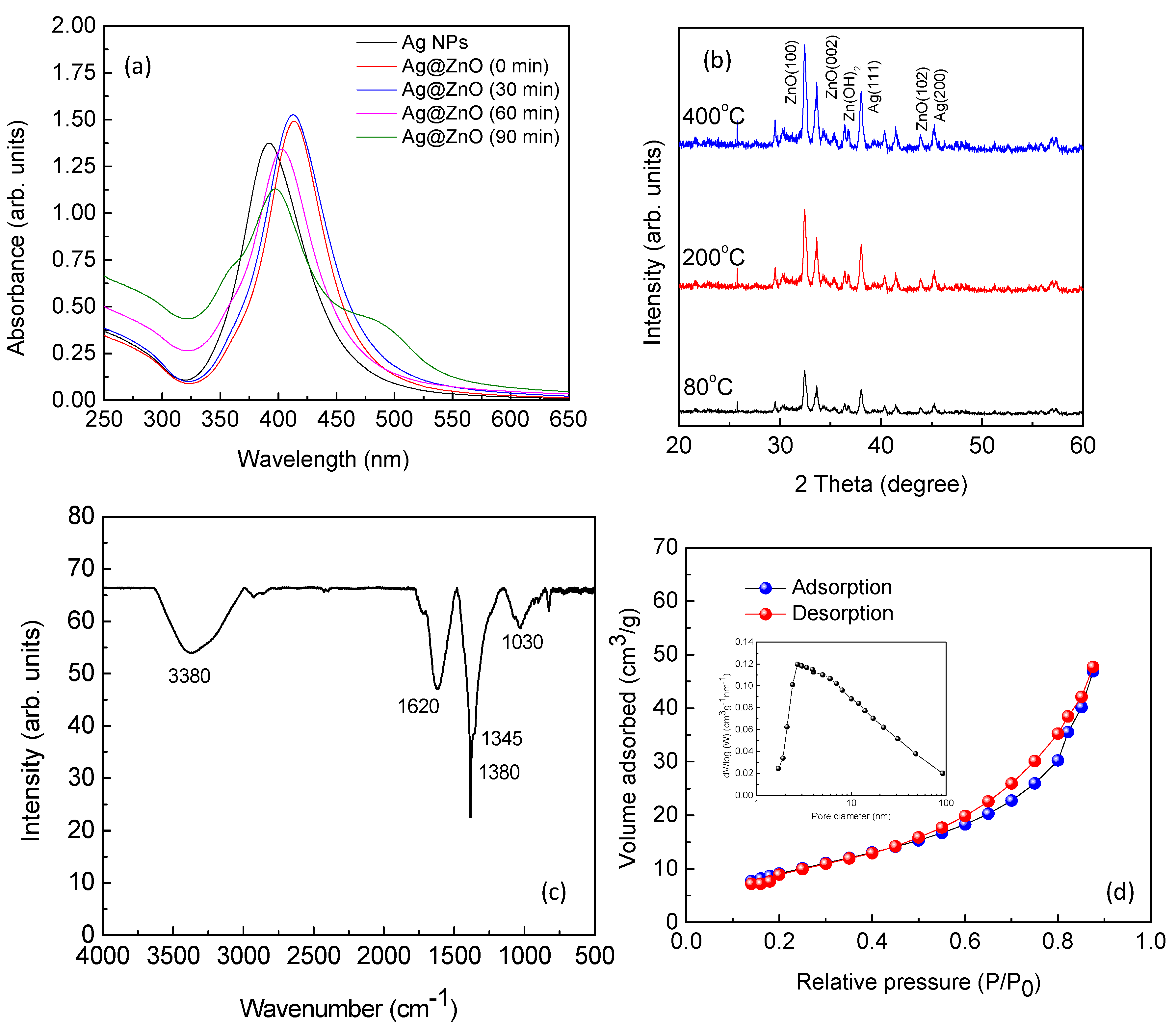
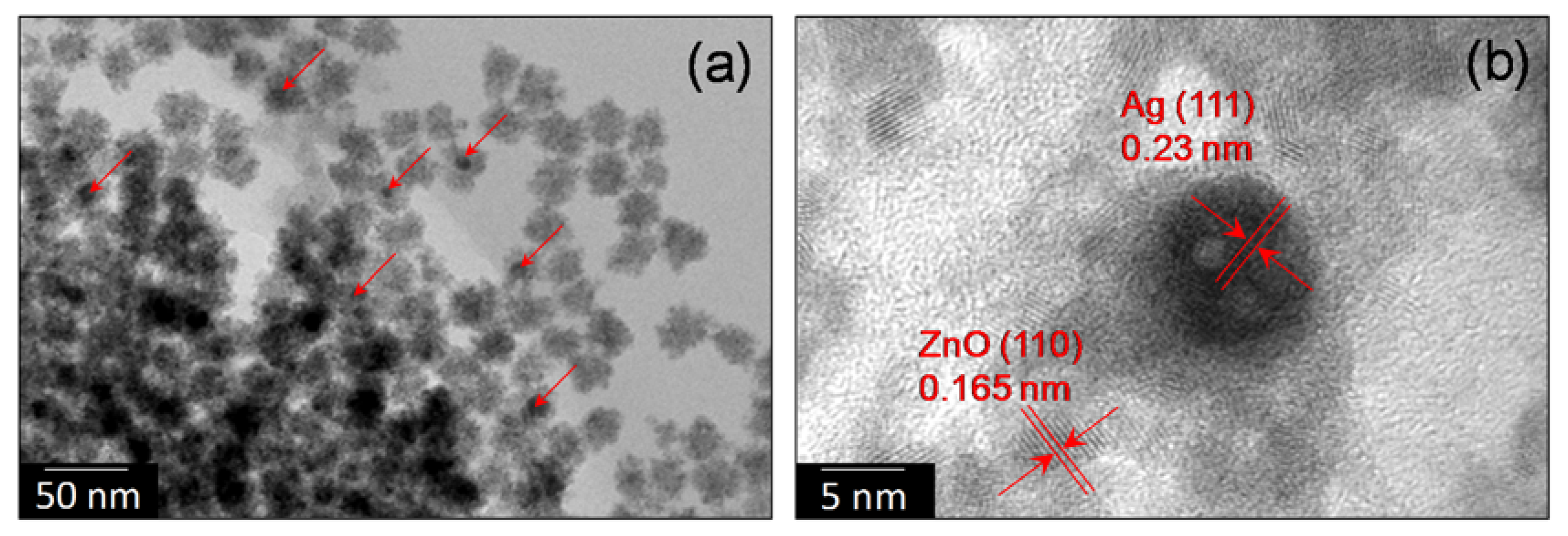
2.2. Synthesis and Characterization of Ag@ZnO Core-Shell Nanoparticles
2.3. Preparation of Bacterial Cultures
2.4. Photocatalytic Disinfection Experiments
2.5. Determination of Lipid Peroxidation
2.6. Potassium Ion (K+) Leakage Studies
2.7. Stability and Reusability of the Photocatalyst
3. Results and Discussion
3.1. Characterization of Nano-Photocatalyst
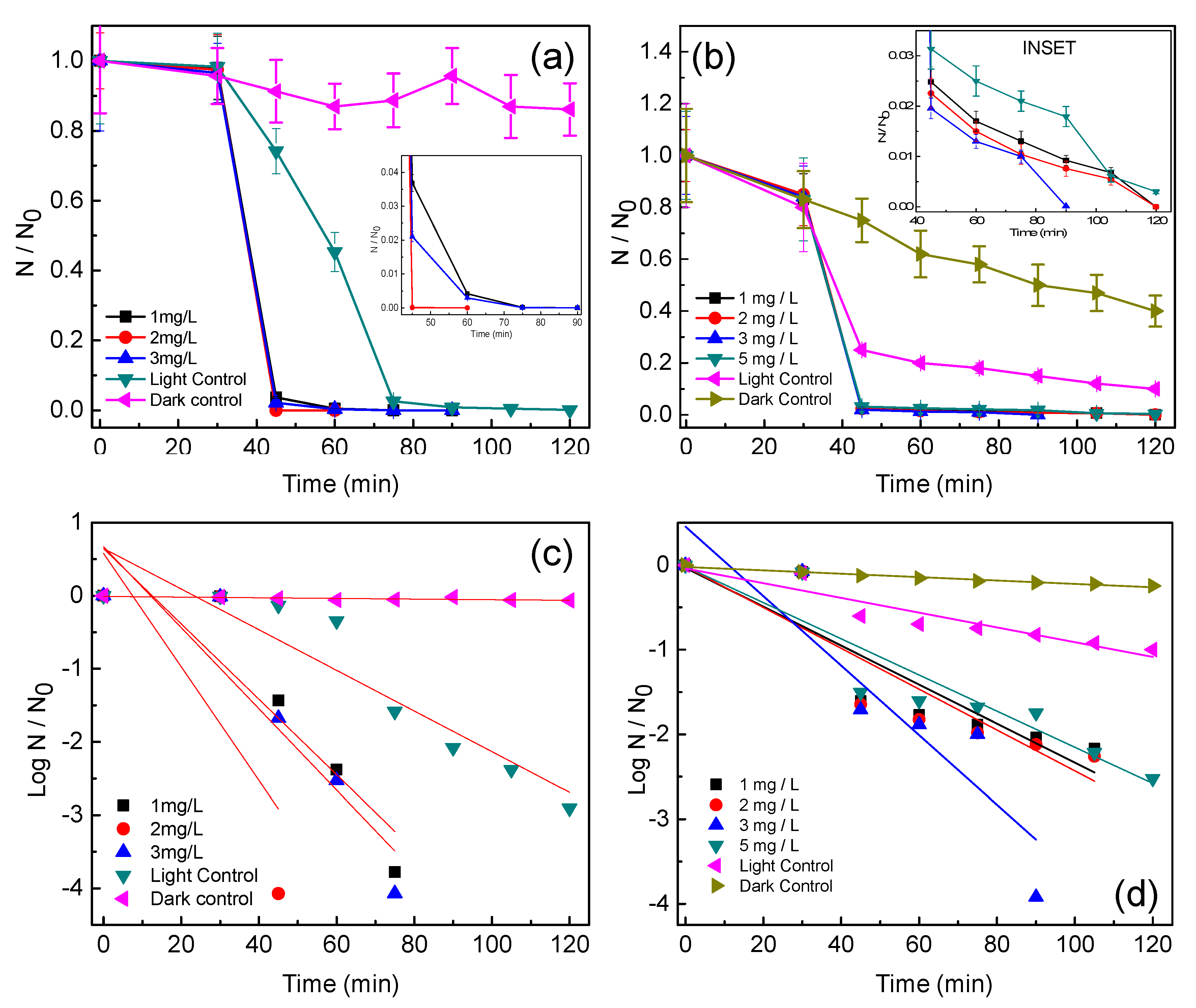
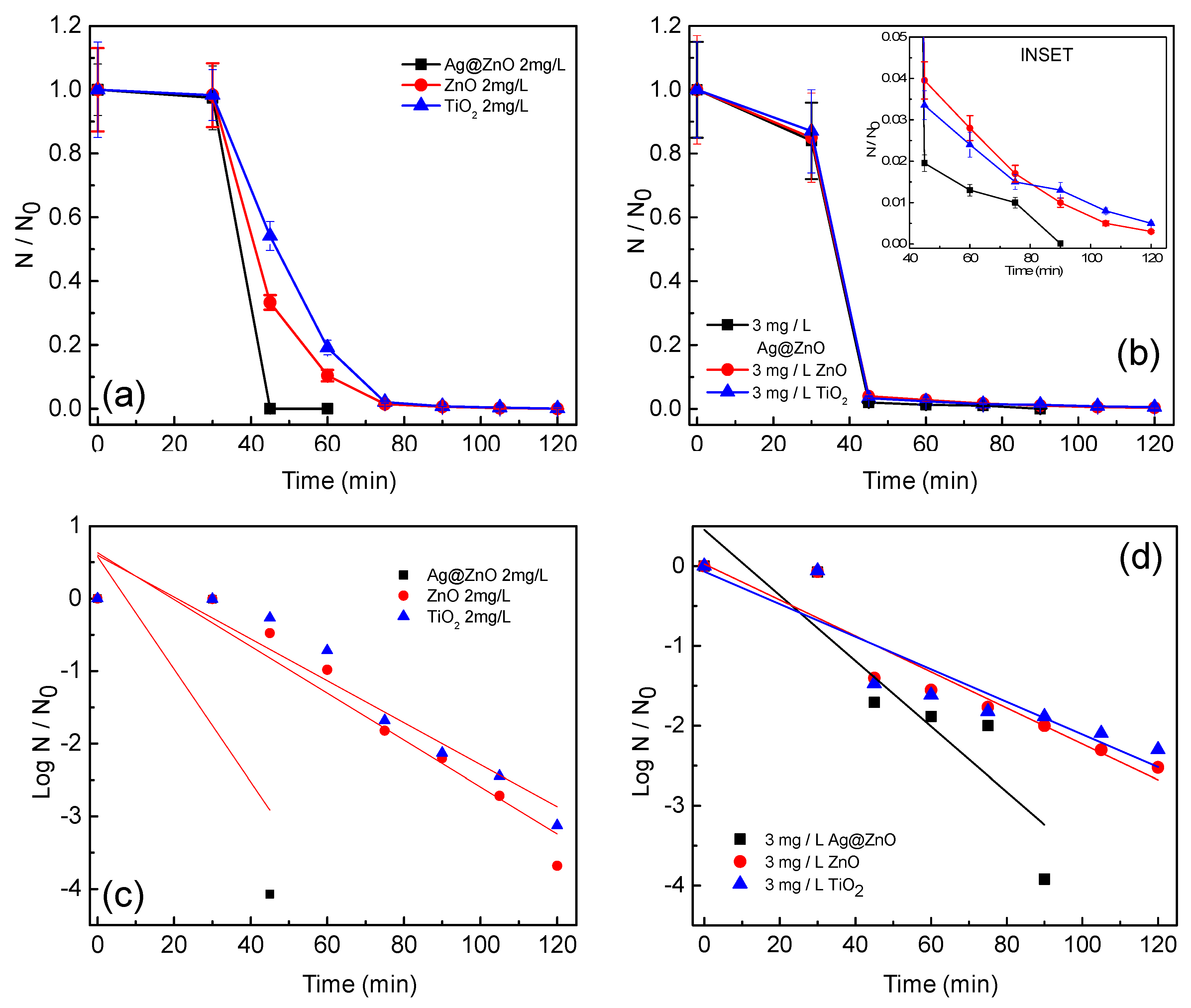
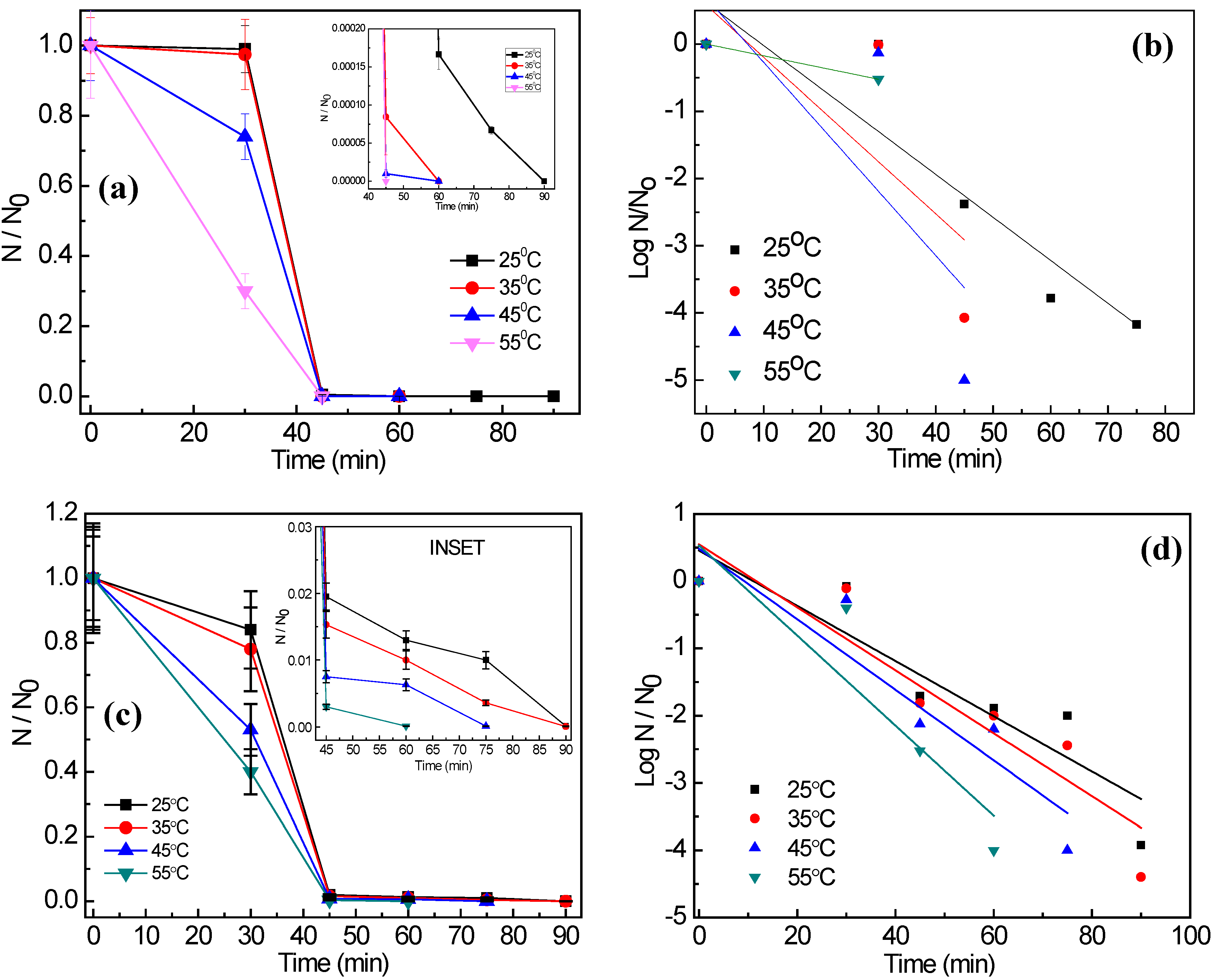
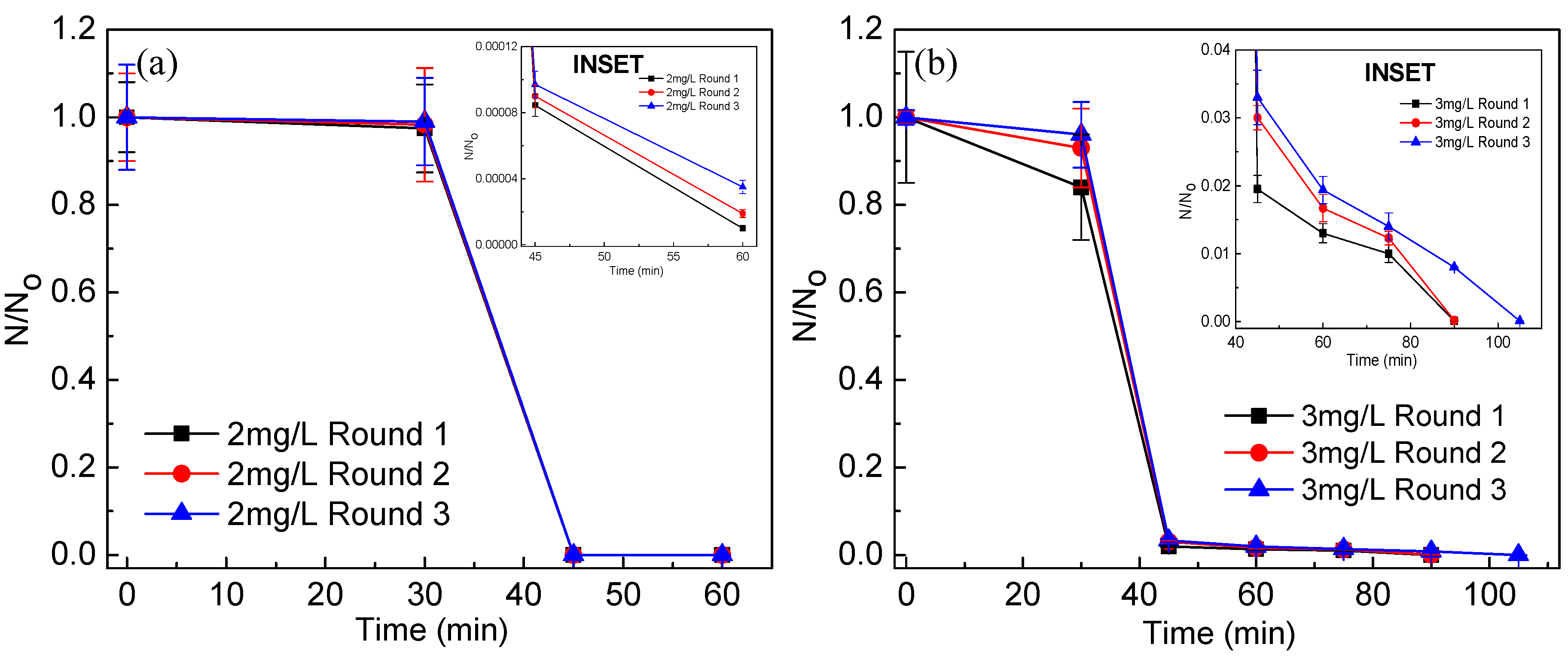
3.2. Photocatalytic Disinfection of Target Pathogens
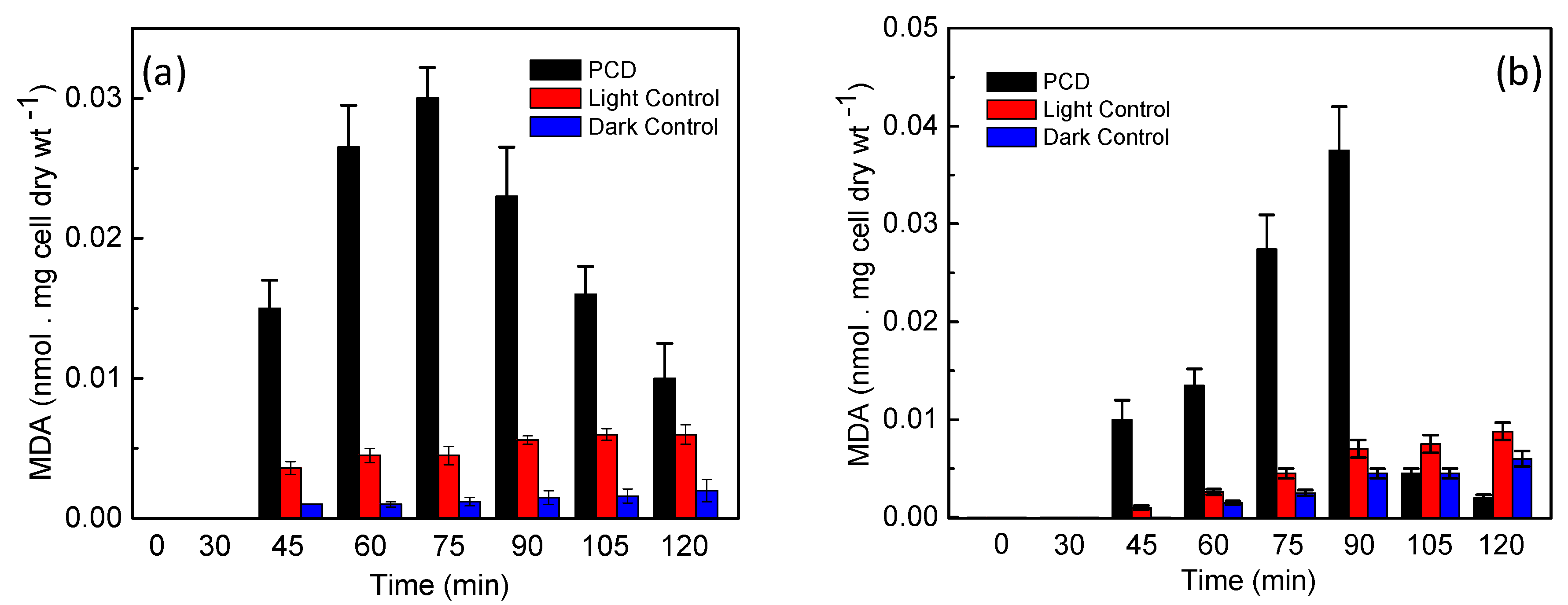
3.3. Determination of MDA to Study the Membrane Lipid Peroxidation
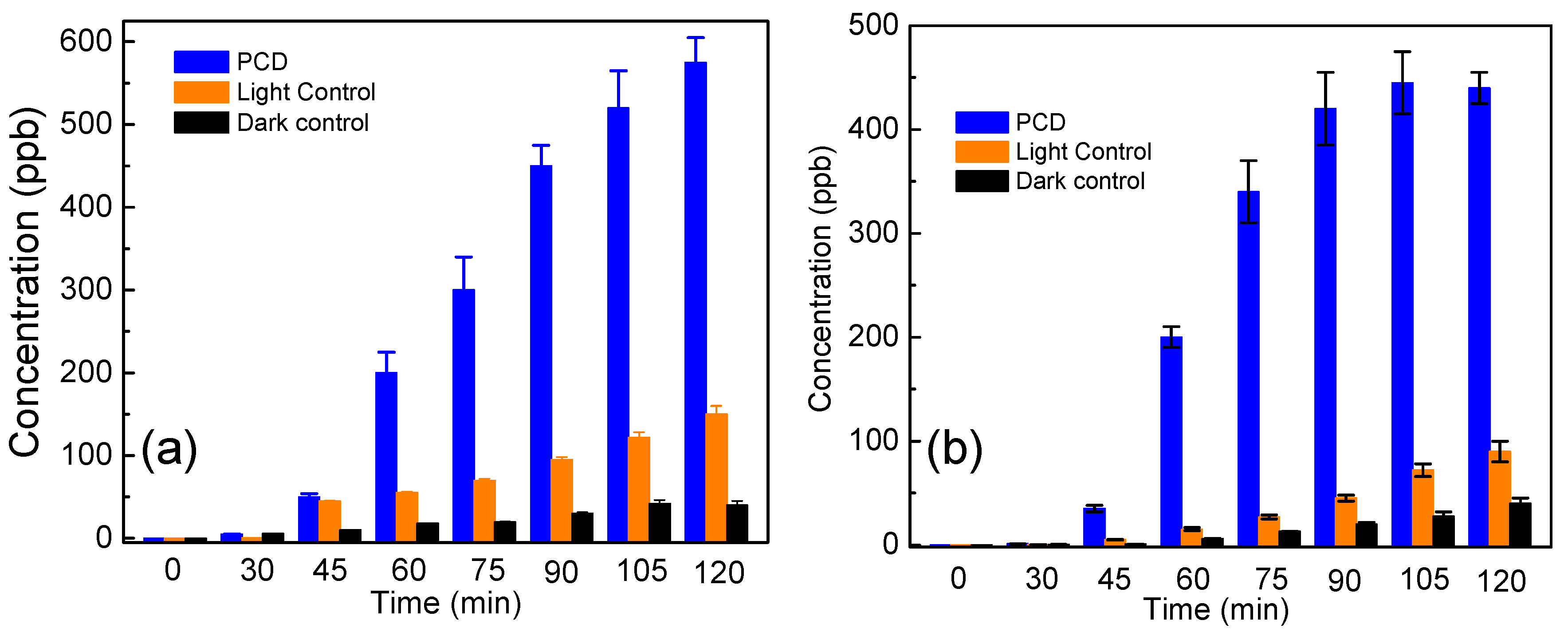
3.4. Analysis of Potassium Ion (K+) to Study the Cell Membrane Damage
3.5. Stability and Reusability of the Catalyst Post Disinfection
3.6. Photocatalytic Disinfection Efficiency in Real Water Systems
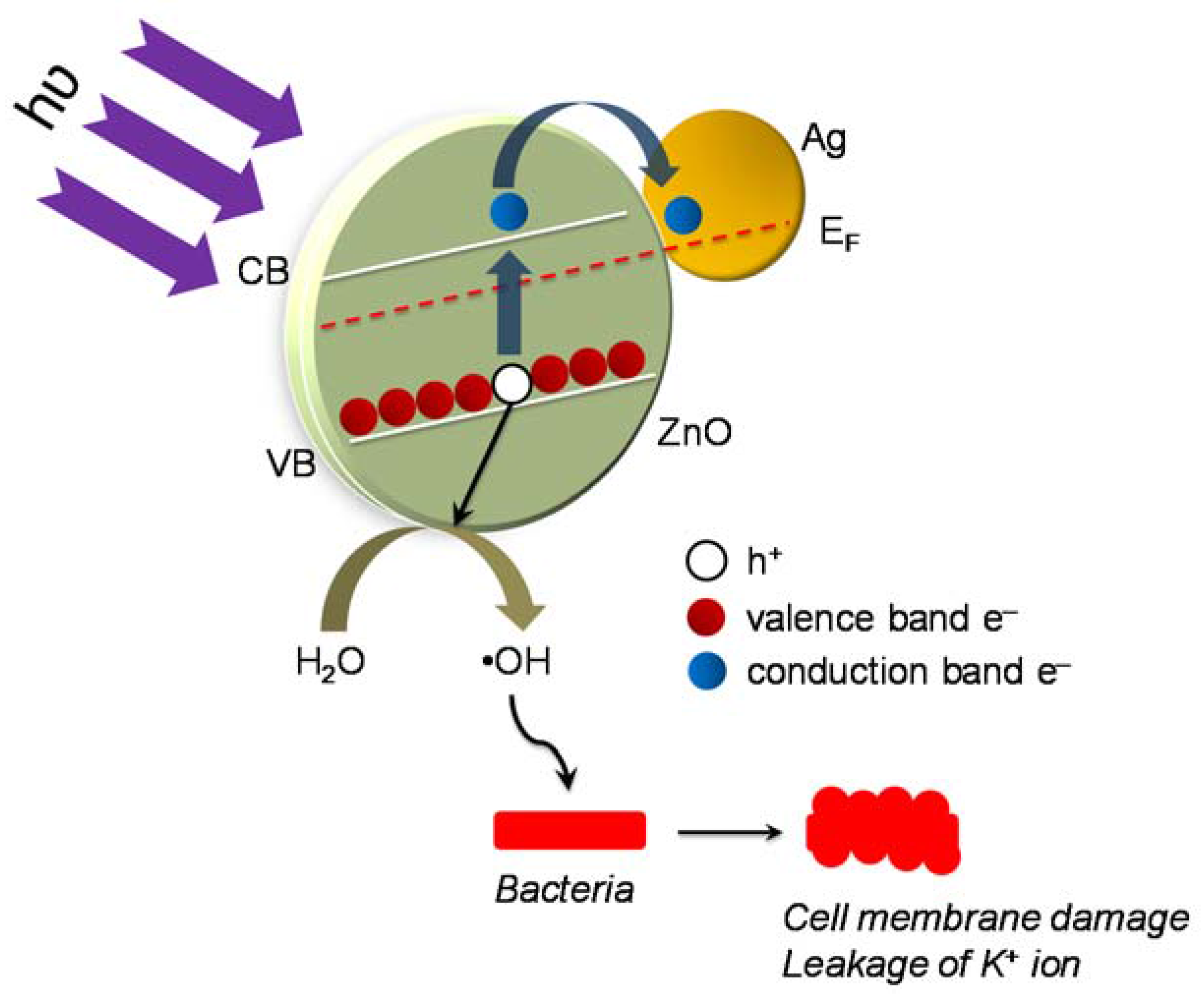
3.7. Proposed Mechanism of Photocatalytic Disinfection
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ashbolt, N.J. Microbial contamination of drinking water and disease outcomes in developing regions. Toxicology 2004, 198, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Adeleye, A.S.; Conway, J.R.; Garner, K.; Huang, Y.; Su, Y.; Keller, A.A. Engineered nanomaterials for water treatment and remediation: Costs, benefits, and applicability. Chem. Eng. J. 2016, 286, 640–662. [Google Scholar] [CrossRef]
- Malato, S.; Fernández-Ibáñez, P.; Maldonado, M.I.; Blanco, J.; Gernjak, W. Decontamination and disinfection of water by solar photocatalysis: Recent overview and trends. Catal. Today 2009, 147, 1–59. [Google Scholar] [CrossRef]
- Tripathy, S.K.; Mishra, A.; Jha, S.K.; Wahab, R.; Al-Khedhairy, A.A. Synthesis of thermally stable monodispersed Au@SnO2 core–shell structure nanoparticles by a sonochemical technique for detection and degradation of acetaldehyde. Anal. Methods 2013, 5, 1456–1462. [Google Scholar] [CrossRef]
- Haldar, K.K.; Sen, T.; Patra, A. Au@ZnO core−shell nanoparticles are efficient energy acceptors with organic dye donors. J. Phys. Chem. C 2008, 112, 11650–11656. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, F.; Meng, A.; Xie, C.; Xing, J. ZnO/Ag micro/nanospheres with enhanced photocatalytic and antibacterial properties synthesized by a novel continuous synthesis method. RSC Adv. 2015, 5, 612–620. [Google Scholar] [CrossRef]
- Ribeiro, M.A.; Cruz, J.M.; Montagnolli, R.N.; Bidoia, E.D.; Lopes, P.R.M. Photocatalytic and photoelectrochemical inactivation of Escherichia coli and Staphylococcus aureus. Water Sci. Technol. Water Supply 2015, 15, 107–113. [Google Scholar] [CrossRef]
- Petti, S.; Messano, G.A. Nano-TiO2-based Photocatalytic disinfection of environmental surfaces contaminated by methicillin-resistant Staphylococcus aureus. J. Hosp. Infect. 2016, 93, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.P.; Venugopal, A.; Subrahmanyam, M. Hydroxyapatite-supported Ag–TiO2 as Escherichia coli disinfection photocatalyst. Water Res. 2007, 41, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, M.E.; Rodríguez, H.B.; San Román, E.; Feldhoff, A.; Grela, M.A. Ag@ZnO core–shell nanoparticles formed by the timely reduction of Ag+ Ions and Zinc acetate hydrolysis in N,N-dimethylformamide: mechanism of growth and photocatalytic properties. J. Phys. Chem. C 2011, 115, 24967–24974. [Google Scholar] [CrossRef]
- Das, S.; Sinha, S.; Suar, M.; Yun, S.I.; Mishra, A.; Tripathy, S.K. Solar-photocatalytic disinfection of Vibrio Cholerae by using Ag@Zno core–shell structure nanocomposites. J. Photochem. Photobiol. B 2015, 142, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Chong, M.N.; Jin, B.; Chow, C.W.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Liu, S.; Xu, Y.J. Recent Progress on metal core@ semiconductor shell nanocomposites as a promising type of photocatalyst. Nanoscale 2012, 4, 2227–2238. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zeiger, B.W.; Suslick, K.S. Sonochemical synthesis of nanomaterials. Chem. Soc. Rev. 2013, 42, 2555–2567. [Google Scholar] [CrossRef] [PubMed]
- Carré, G.; Hamon, E.; Ennahar, S.; Estner, M.; Lett, M.C.; Horvatovich, P.; Andre, P. TiO2 photocatalysis damages lipids and proteins in Escherichia coli. Appl. Environ. Microbiol. 2014, 80, 2573–2581. [Google Scholar] [CrossRef] [PubMed]
- Maness, P.C.; Smolinski, S.; Blake, D.M.; Huang, Z.; Wolfrum, E.J.; Jacoby, W.A. Bactericidal activity of photocatalytic TiO2 reaction: Toward an understanding of Its killing mechanism. Appl. Environ. Microbiol. 1999, 65, 4094–4098. [Google Scholar] [PubMed]
- Esterbauer, H.; Cheeseman, K.H. Determination of aldehydic lipid peroxidation products: Malonaldehyde and 4-hydroxynonenal. Methods Enzymol. 1990, 186, 407–421. [Google Scholar] [PubMed]
- Das, S.; Sinha, S.; Das, B.; Jayabalan, R.; Suar, M.; Mishra, A.; Tripathy, S.K. Disinfection of multidrug resistant Escherichia coli by solar-photocatalysis using Fe-doped ZnO nanoparticles. Sci. Rep. 2017, 7, 104. [Google Scholar] [CrossRef] [PubMed]
- Leung, T.Y.; Chan, C.Y.; Hu, C.; Yu, J.C.; Wong, P.K. Photocatalytic disinfection of marine bacteria using fluorescent light. Water Res. 2008, 42, 4827–4837. [Google Scholar] [CrossRef] [PubMed]
- Chick, H. An investigation of the laws of disinfection. J. Hyg. 1908, 8, 92–158. [Google Scholar] [CrossRef] [PubMed]
- Watson, H.E. A Note on the variation of the rate of disinfection with change in the concentration of the disinfectant. J Hyg. 1908, 8, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Choi, O.; Deng, K.K.; Kim, N.J.; Ross, L.; Surampalli, R.Y.; Hu, Z. The Inhibitory effects of silver nanoparticles, silver ions, and silver chloride colloids on microbial growth. Water Res. 2008, 42, 3066–3074. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, L.; Della Sala, A.; Fiorentino, A.; Puma, G.L. Disinfection of urban wastewater by solar driven and UV lamp–TiO2 photocatalysis: Effect on a multi-drug resistant Escherichia coli strain. Water Res. 2014, 53, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Shang, K.; Ai, S.; Ma, Q.; Tang, T.; Yin, H.; Han, H. Effective photocatalytic disinfection of E. coli and S. aureus using polythiophene/MnO2 nanocomposite photocatalyst under solar light irradiation. Desalination 2011, 278, 173–178. [Google Scholar]
- Mills, A.; Le Hunte, S. An overview of semiconductor photocatalysis. J. Photochem. Photobiol. A 1997, 108, 1–35. [Google Scholar] [CrossRef]
- Kiwi, J.; Nadtochenko, V. Evidence for the mechanism of photocatalytic degradation of the bacterial wall membrane at the TiO2 interface by ATR-FTIR and laser kinetic spectroscopy. Langmuir 2005, 21, 4631–4641. [Google Scholar] [CrossRef] [PubMed]
- Foster, H.A.; Ditta, I.B.; Varghese, S.; Steele, A. Photocatalytic disinfection using titanium dioxide: Spectrum and mechanism of antimicrobial activity. Appl. Environ. Microbiol. 2011, 90, 1847–1868. [Google Scholar] [CrossRef] [PubMed]
- Doran, P.M. Bioprocess Engineering Principles; Academic Press: Cambridge, MA, USA, 1995. [Google Scholar]
- Pulgarin, C.; Kiwi, J.; Nadtochenko, V. Mechanism of photocatalytic bacterial inactivation on TiO2 films involving cell-wall damage and lysis. Appl. Catal. B 2012, 128, 179–183. [Google Scholar] [CrossRef]
- Ennis, H.L. Role of potassium in the regulation of polysome content and protein synthesis in Escherichia coli. Arch. Biochem. Biophys. 1971, 142, 190–200. [Google Scholar] [CrossRef]
- Helali, S.; Polo-López, M.I.; Fernández-Ibáñez, P.; Ohtani, B.; Amano, F.; Malato, S.; Guillard, C. Solar photocatalysis: A green technology for E. coli contaminated water disinfection. Effect of concentration and different types of suspended catalyst. J. Photochem. Photobiol. A 2014, 276, 31–40. [Google Scholar]
- Zhang, L.S.; Wong, K.H.; Yip, H.Y.; Hu, C.; Yu, J.C.; Chan, C.Y.; Wong, P.K. Effective photocatalytic disinfection of E. coli K-12 using AgBr−Ag−Bi2WO6 nanojunction system irradiated by visible light: The role of diffusing hydroxyl radicals. Environ. Sci. Technol. 2010, 44, 1392–1398. [Google Scholar] [PubMed]
- Hu, C.; Guo, J.; Qu, J.; Hu, X. Photocatalytic degradation of pathogenic bacteria with AgI/TiO2 under visible light irradiation. Langmuir 2007, 23, 4982–4987. [Google Scholar]
- Paik, P. Recent advancement in functional core-shell nanoparticles of polymers: Synthesis, physical properties, and applications in medical biotechnology. J. Nanopart. Res. 2013. [CrossRef]
- Chudobova, D.; Dostalova, S.; Ruttkay-Nedecky, B.; Guran, R.; Rodrigo, M.A.M.; Tmejova, K.; Kizek, R. The effect of metal ions on Staphylococcus aureus revealed by biochemical and mass spectrometric analyses. Microbiol. Res. 2015, 170, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Bondarenko, O.; Juganson, K.; Ivask, A.; Kasemets, K.; Mortimer, M.; Kahru, A. Toxicity of Ag, CuO and ZnO nanoparticles to selected environmentally relevant test organisms and mammalian cells in vitro: A critical review. Arch. Toxicol. 2013, 87, 1181–1200. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Gautam, N.; Mishra, A.; Gupta, R. Heavy metals and living systems: An overview. Indian J. Pharmacol. 2011, 43, 246. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Das, S.; Ranjana, N.; Misra, A.J.; Suar, M.; Mishra, A.; Tamhankar, A.J.; Lundborg, C.S.; Tripathy, S.K. Disinfection of the Water Borne Pathogens Escherichia coli and Staphylococcus aureus by Solar Photocatalysis Using Sonochemically Synthesized Reusable Ag@ZnO Core-Shell Nanoparticles. Int. J. Environ. Res. Public Health 2017, 14, 747. https://doi.org/10.3390/ijerph14070747
Das S, Ranjana N, Misra AJ, Suar M, Mishra A, Tamhankar AJ, Lundborg CS, Tripathy SK. Disinfection of the Water Borne Pathogens Escherichia coli and Staphylococcus aureus by Solar Photocatalysis Using Sonochemically Synthesized Reusable Ag@ZnO Core-Shell Nanoparticles. International Journal of Environmental Research and Public Health. 2017; 14(7):747. https://doi.org/10.3390/ijerph14070747
Chicago/Turabian StyleDas, Sourav, Neha Ranjana, Ananyo Jyoti Misra, Mrutyunjay Suar, Amrita Mishra, Ashok J. Tamhankar, Cecilia Stålsby Lundborg, and Suraj K. Tripathy. 2017. "Disinfection of the Water Borne Pathogens Escherichia coli and Staphylococcus aureus by Solar Photocatalysis Using Sonochemically Synthesized Reusable Ag@ZnO Core-Shell Nanoparticles" International Journal of Environmental Research and Public Health 14, no. 7: 747. https://doi.org/10.3390/ijerph14070747