Modeling Nitrogen Dynamics in a Waste Stabilization Pond System Using Flexible Modeling Environment with MCMC
Abstract
:1. Introduction
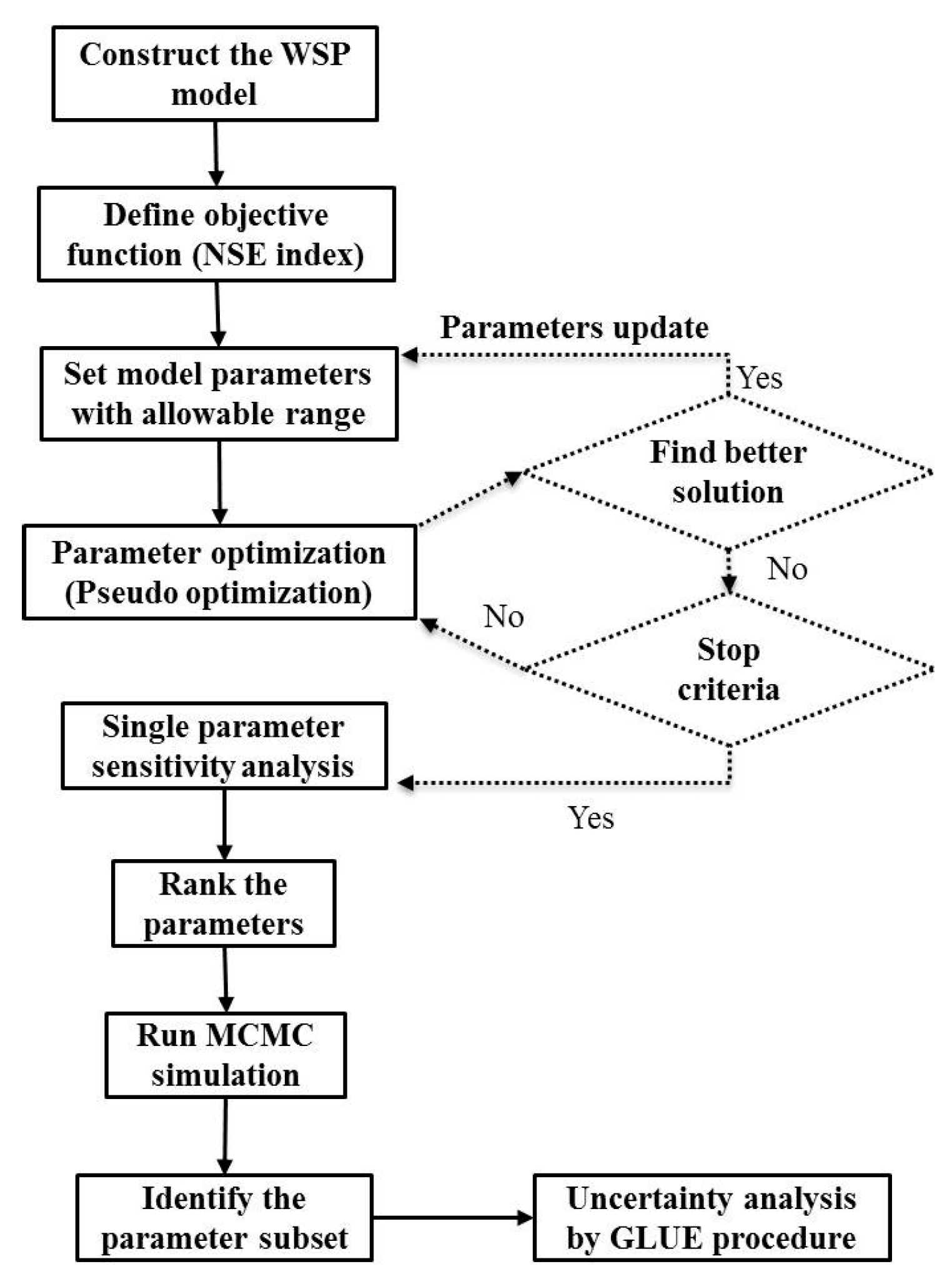
2. Materials and Methods
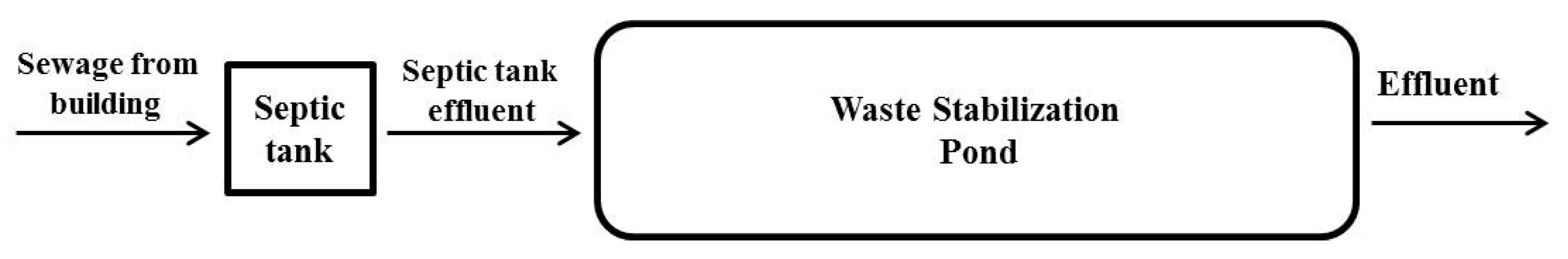
2.1. Site and Experiment Design
2.2. R-FME Functions Adopted in This Study
2.3. GLUE Analysis
2.4. Model Construction
3. Results
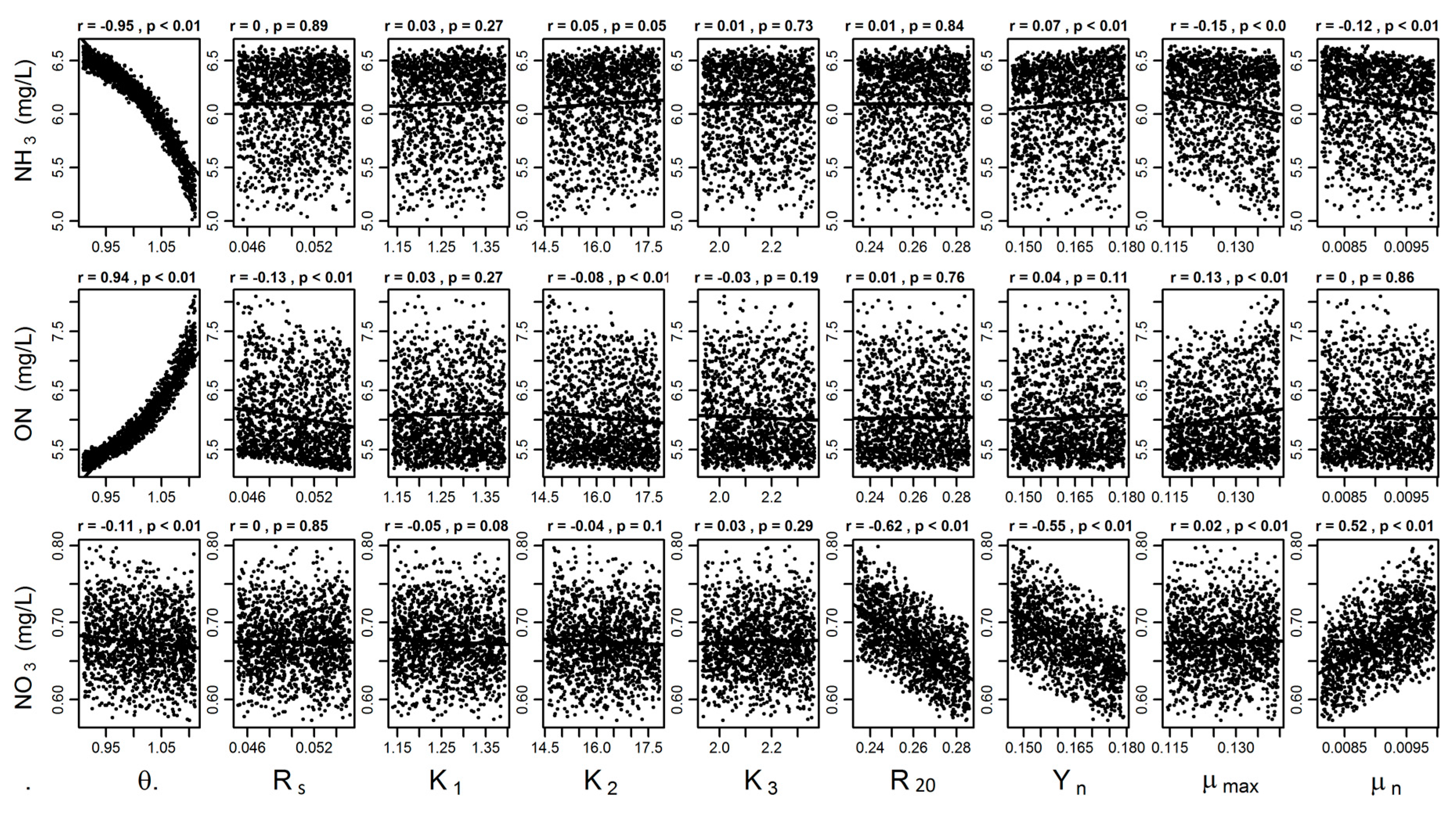
3.1. Local Sensitivity and Parameter Analyses
3.2. Parameter Optimization
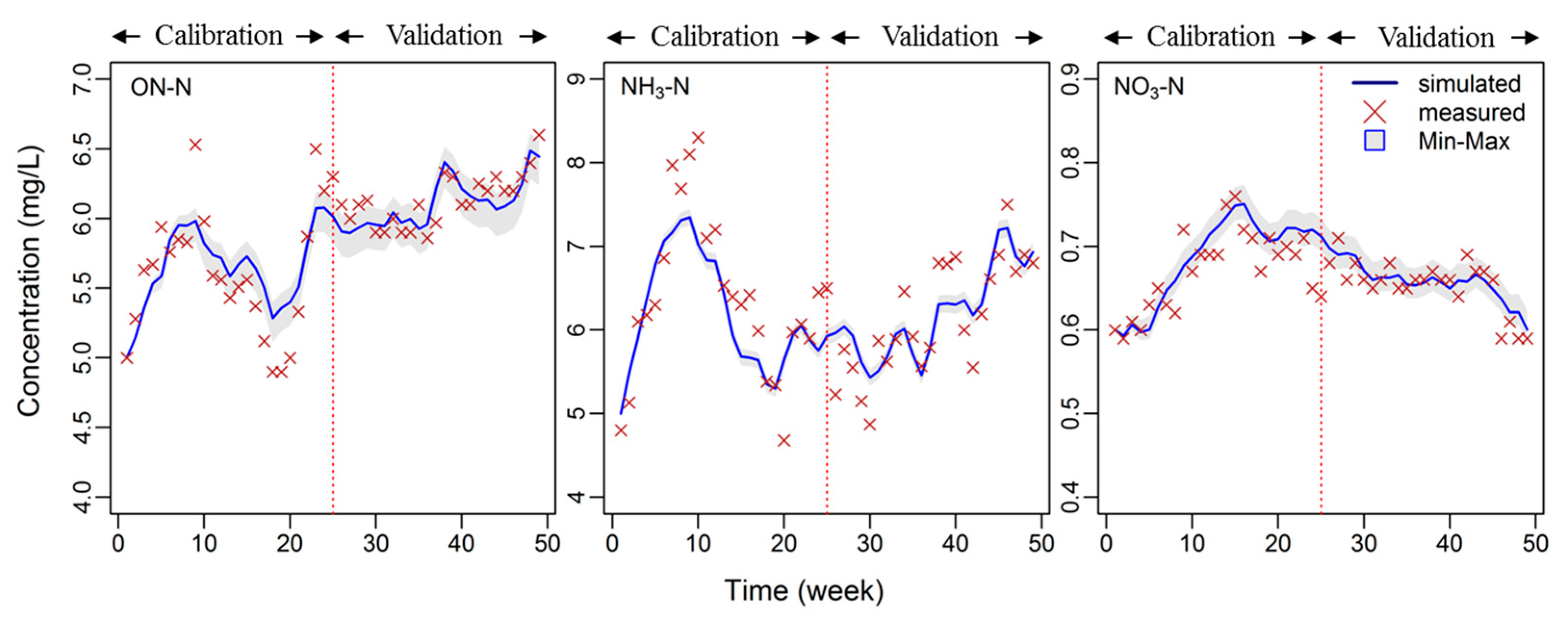
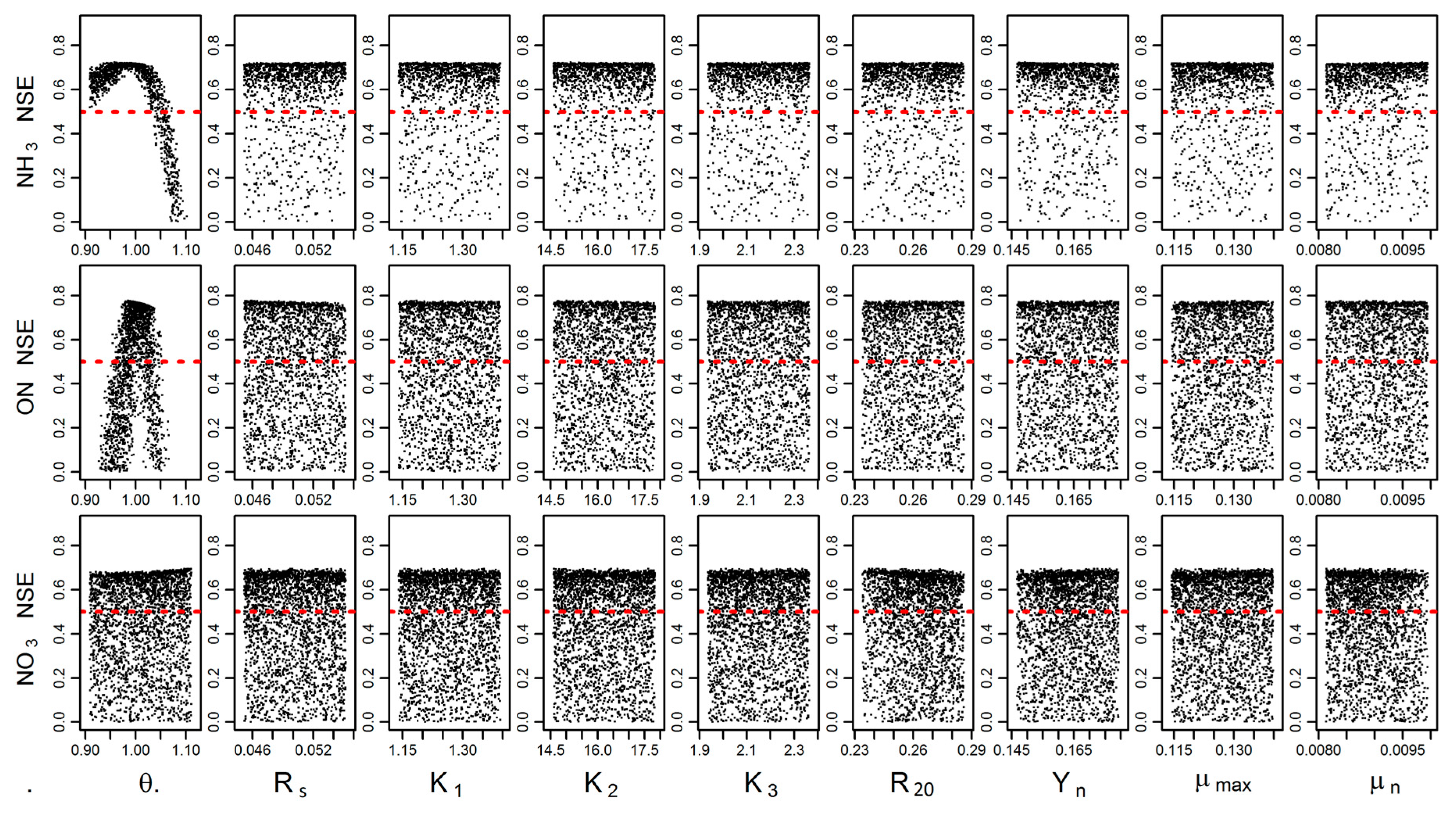
3.3. Model Performance Evaluation
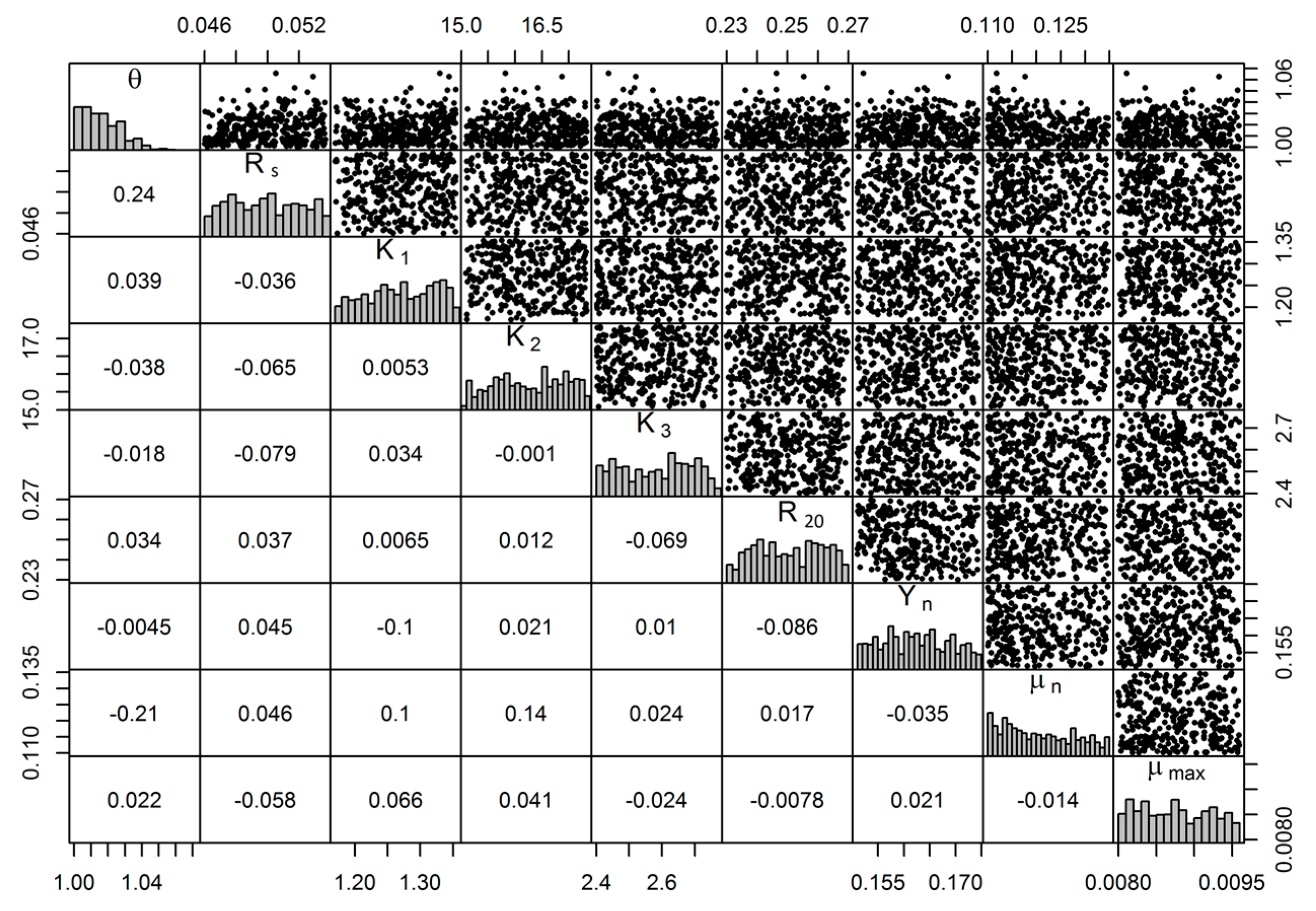
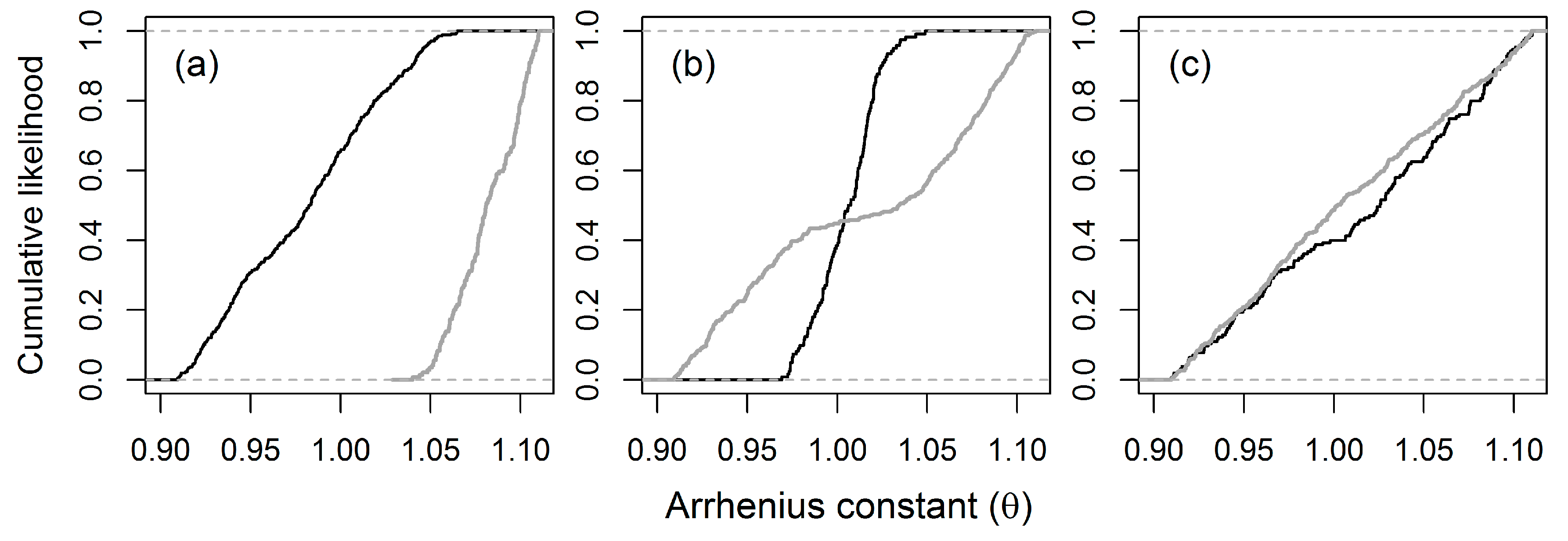
3.4. Uncertainty and Global Sensitivity Analyses
4. Discussion
4.1. Parameter Optimization and Model Performance
4.2. Uncertainty and Global Sensitivity Analyses
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Prisciandaro, M.; Mazziotti di Celso, G.; Musmarra, D.; Zammartino, A. Integrated process scheme for the combined treatment of liquid wastes and municipal wastewaters: A process analysis. Desalin. Water Treat. 2016, 57, 2555–2563. [Google Scholar] [CrossRef]
- Del Nery, V.; Damianovic, M.H.Z.; Pozzi, E.; De Nardi, I.R.; Caldas, V.E.A.; Pires, E.C. Long-term performance and operational strategies of a poultry slaughterhouse waste stabilization pond system in a tropical climate. Resour. Conserv. Recycl. 2013, 71, 7–14. [Google Scholar] [CrossRef]
- Barrington, D.J.; Reichwaldt, E.S.; Ghadouani, A. The use of hydrogen peroxide to remove cyanobacteria and microcystins from waste stabilization ponds and hypereutrophic systems. Ecol. Eng. 2013, 50, 86–94. [Google Scholar] [CrossRef]
- Chaturvedi, M.K.M.; Langote, S.D.; Kumar, D.; Asolekar, S.R. Significance and estimation of oxygen mass transfer coefficient in simulated waste stabilization pond. Ecol. Eng. 2014, 73, 331–334. [Google Scholar] [CrossRef]
- Ouedraogo, F.R.; Zhang, J.; Cornejo, P.K.; Zhang, Q.; Mihelcic, J.R.; Tejada-Martinez, A.E. Impact of sludge layer geometry on the hydraulic performance of a waste stabilization pond. Water Res. 2016, 99, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Beran, B.; Kargi, F. A dynamic mathematical model for wastewater stabilization ponds. Ecol. Model. 2005, 181, 39–57. [Google Scholar] [CrossRef]
- Craggs, R.; Park, J.; Heubeck, S.; Sutherland, D. High rate algal pond systems for low-energy wastewater treatment, nutrient recovery and energy production. N. Z. J. Bot. 2014, 52, 60–73. [Google Scholar] [CrossRef]
- Powell, N.; Shilton, A.N.; Pratt, S.; Chisti, Y. Factors influencing luxury uptake of phosphorus by microalgae in waste stabilization ponds. Environ. Sci. Technol. 2008, 42, 5958–5962. [Google Scholar] [CrossRef] [PubMed]
- Mahapatra, D.M.; Chanakya, H.N.; Ramachandra, T.V. Treatment efficacy of algae-based sewage treatment plants. Environ. Monit. Assess. 2013, 185, 7145–7164. [Google Scholar] [CrossRef] [PubMed]
- Lancia, A.; Musmarra, D.; Pepe, F.; Prisciandaro, M. Model of oxygen absorption into calcium sulfite solutions. Chem. Eng. J. 1997, 66, 123–129. [Google Scholar] [CrossRef]
- Mayo, A.W.; Abbas, M. Removal mechanisms of nitrogen in waste stabilization ponds. Phys. Chem. Earth Parts A/B/C 2014, 72, 77–82. [Google Scholar] [CrossRef]
- Bello, M.; Ranganathan, P.; Brennan, F. Dynamic modelling of microalgae cultivation process in high rate algal wastewater pond. Algal Res. 2016, 24, 457–466. [Google Scholar] [CrossRef]
- Fritz, J.J.; Middleton, A.C.; Meredith, D.D. Dynamic process modeling of wastewater stabilization ponds. J. Water Pollut. Control Fed. 1979, 2724–2743. [Google Scholar]
- Mayo, A.W. Nitrogen mass balance in waste stabilization ponds at the University of Dar es Salaam, Tanzania. Afr. J. Environ. Sci. Technol. 2013, 7, 836–845. [Google Scholar]
- Ragush, C.M.; Schmidt, J.J.; Krkosek, W.H.; Gagnon, G.A.; Truelstrup-Hansen, L.; Jamieson, R.C. Performance of municipal waste stabilization ponds in the Canadian Arctic. Ecol. Eng. 2015, 83, 413–421. [Google Scholar] [CrossRef]
- Møller, C.C.; Weisser, J.J.; Msigala, S.; Mdegela, R.; Jørgensen, S.E.; Styrishave, B. Modelling antibiotics transport in a waste stabilization pond system in Tanzania. Ecol. Model. 2016, 319, 137–146. [Google Scholar] [CrossRef]
- Ochoa, M.P.; Estrada, V.; Hoch, P.M. Wastewater stabilization ponds system: Parametric and dynamic global sensitivity analysis. Ind. Eng. Chem. Res. 2016, 55, 11403–11416. [Google Scholar] [CrossRef]
- Sarkar, S.; Tribedi, P.; Ghoah, P.; Saha, T.; Sil, A.K. Sequential changes of microbial community composition during biological wastewater treatment in single unit waste stabilization system. Waste Biomass Valoriz. 2016, 7, 483–493. [Google Scholar] [CrossRef]
- Mayo, A.W.; Hanai, E.E. Modeling phytoremediation of nitrogen-polluted water using water hyacinth (Eichhornia crassipes). Phys. Chem. Earth Parts A/B/C 2016. [Google Scholar] [CrossRef]
- Sah, L.; Rousseau, D.P.L.; Hooijmans, C.M. Numerical modelling of waste stabilization ponds: Where do we stand? Water Air Soil Pollut. 2012, 223, 3155–3171. [Google Scholar] [CrossRef]
- Mayo, A.W.; Hanai, E.E. Dynamics of nitrogen transformation and removal in a pilot high rate pond. J. Water Resour. Prot. 2014, 6, 433. [Google Scholar] [CrossRef]
- Escalas-Cañellas, A.; Ábrego-Góngora, C.J.; Barajas-López, M.G.; Houweling, D.; Comeau, Y. A time series model for influent temperature estimation: Application to dynamic temperature modelling of an aerated lagoon. Water Res. 2008, 42, 2551–2562. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Liu, S.; Huang, Z.; Yan, W. Parameter optimization, sensitivity, and uncertainty analysis of an ecosystem model at a forest flux tower site in the United States. J. Adv. Model. Earth Syst. 2014, 6, 405–419. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, S.; Li, Z.; Dahal, D.; Young, C.J.; Schmidt, G.L.; Liu, J.; Davis, B.; Sohl, T.L.; Werner, J.M. Development of a generic auto-calibration package for regional ecological modeling and application in the Central Plains of the United States. Ecol. Inform. 2014, 19, 35–46. [Google Scholar] [CrossRef]
- Soetaert, K.; Petzoldt, T. Inverse modelling, sensitivity and monte carlo analysis in R using package FME. J. Stat. Softw. 2010, 33, 1–28. [Google Scholar] [CrossRef]
- Joseph, J.F.; Guillaume, J.H.A. Using a parallelized MCMC algorithm in R to identify appropriate likelihood functions for SWAT. Environ. Model. Softw. 2013, 46, 292–298. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, S. Automating calibration, sensitivity and uncertainty analysis of complex models using the R package Flexible Modeling Environment (FME): SWAT as an example. Environ. Model. Softw. 2012, 31, 99–109. [Google Scholar] [CrossRef]
- Vigiak, O.; Beverly, C.; Roberts, A.; Thayalakumaran, T.; Dickson, M.; McInnes, J.; Borselli, L. Detecting changes in sediment sources in drought periods: The Latrobe River case study. Environ. Model. Softw. 2016, 85, 42–55. [Google Scholar] [CrossRef]
- Jin, X.; Xu, C.-Y.; Zhang, Q.; Singh, V.P. Parameter and modeling uncertainty simulated by GLUE and a formal Bayesian method for a conceptual hydrological model. J. Hydrol. 2010, 383, 147–155. [Google Scholar] [CrossRef]
- Li, L.; Xu, C.-Y.; Engeland, K. Development and comparison in uncertainty assessment based Bayesian modularization method in hydrological modeling. J. Hydrol. 2013, 486, 384–394. [Google Scholar] [CrossRef]
- Beven, K.; Binley, A. GLUE: 20 years on. Hydrol. Process. 2014, 28, 5897–5918. [Google Scholar] [CrossRef]
- Li, B.; Liang, Z.; He, Y.; Hu, L.; Zhao, W.; Acharya, K. Comparison of parameter uncertainty analysis techniques for a TOPMODEL application. Stoch. Environ. Res. Risk Assess. 2016, 31, 1–15. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, S. Improvement of the R-SWAT-FME framework to support multiple variables and multi-objective functions. Sci. Total Environ. 2014, 466, 455–466. [Google Scholar] [CrossRef] [PubMed]
- Vrugt, J.A. Markov chain Monte Carlo simulation using the DREAM software package: Theory, concepts, and MATLAB implementation. Environ. Model. Softw. 2016, 75, 273–316. [Google Scholar] [CrossRef]
- Beven, K.; Binley, A. The future of distributed models: Model calibration and uncertainty prediction. Hydrol. Process. 1992, 6, 279–298. [Google Scholar] [CrossRef]
- Huang, C.-W.; Lin, Y.-P.; Chiang, L.-C.; Wang, Y.-C. Using CV-GLUE procedure in analysis of wetland model predictive uncertainty. J. Environ. Manag. 2014, 140, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Xu, C.-Y. The comparison of sensitivity analysis of hydrological uncertainty estimates by GLUE and Bayesian method under the impact of precipitation errors. Stoch. Environ. Res. Risk Assess. 2014, 28, 491–504. [Google Scholar] [CrossRef]
- Rankinen, K.; Karvonen, T.; Butterfield, D. An application of the GLUE methodology for estimating the parameters of the INCA-N model. Sci. Total Environ. 2006, 365, 123–139. [Google Scholar] [CrossRef] [PubMed]
- Alazzy, A.A.; Lü, H.; Zhu, Y. Assessing the uncertainty of the Xinanjiang rainfall-runoff model: Effect of the likelihood function choice on the GLUE method. J. Hydrol. Eng. 2015, 20, 4015016. [Google Scholar] [CrossRef]
- Knighton, J.; Lennon, E.; Bastidas, L.; White, E. Stormwater detention system parameter sensitivity and uncertainty analysis using SWMM. J. Hydrol. Eng. 2016, 21, 5016014. [Google Scholar] [CrossRef]
- Soetaert, K.; Herman, P. FEMME, a flexible environment for mathematically modelling the environment. Ecol. Model. 2002, 151, 177–193. [Google Scholar] [CrossRef]
- Rice, E.W.; Bridgewater, L.; Association, A.P.H. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2012. [Google Scholar]
- Wang, Y.-C.; Lin, Y.-P.; Huang, C.-W.; Chiang, L.-C.; Chu, H.-J.; Ou, W.-S. A system dynamic model and sensitivity analysis for simulating domestic pollution removal in a free-water surface constructed wetland. Water Air Soil Pollut. 2012, 223, 2719–2742. [Google Scholar] [CrossRef]
- Beven, K.; Freer, J. Equifinality, data assimilation, and uncertainty estimation in mechanistic modelling of complex environmental systems using the GLUE methodology. J. Hydrol. 2001, 249, 11–29. [Google Scholar] [CrossRef]
- Nash, J.E.; Sutcliffe, J.V. River flow forecasting through conceptual models part I—A discussion of principles. J. Hydrol. 1970, 10, 282–290. [Google Scholar] [CrossRef]
- Ferrara, R.A.; Avci, C.B. Nitrogen dynamics in waste stabilization ponds. J. (Water Pollut. Control Fed.) 1982, 54, 361–369. [Google Scholar]
- Senzia, M.A.; Mayo, A.W.; Mbwette, T.S.A.; Katima, J.H.Y.; Jørgensen, S.E. Modelling nitrogen transformation and removal in primary facultative ponds. Ecol. Model. 2002, 154, 207–215. [Google Scholar] [CrossRef]
- Stratton, F.E. Ammonia nitrogen losses from streams. J. Sanit. Eng. Div. 1968, 94, 1085–1092. [Google Scholar]
- Day, N.K.; Hall, R.O., Jr. Ammonium uptake kinetics and nitrification in mountain streams. Freshw. Sci. 2017, 36, 41–54. [Google Scholar] [CrossRef]
- Trang, N.T.D.; Konnerup, D.; Schierup, H.-H.; Chiem, N.H.; Brix, H. Kinetics of pollutant removal from domestic wastewater in a tropical horizontal subsurface flow constructed wetland system: Effects of hydraulic loading rate. Ecol. Eng. 2010, 36, 527–535. [Google Scholar] [CrossRef]
- Merino-Solís, M.L.; Villegas, E.; de Anda, J.; López-López, A. The effect of the hydraulic retention time on the performance of an ecological wastewater treatment system: An anaerobic filter with a constructed wetland. Water 2015, 7, 1149–1163. [Google Scholar] [CrossRef]
- Keffala, C.; Galleguillos, M.; Ghrabi, A.; Vasel, J.-L. Investigation of nitrification and denitrification in the sediment of wastewater stabilization ponds. Water Air Soil Pollut. 2011, 219, 389–399. [Google Scholar] [CrossRef]
- Pauer, J.J.; Auer, M.T. Nitrification in the water column and sediment of a hypereutrophic lake and adjoining river system. Water Res. 2000, 34, 1247–1254. [Google Scholar] [CrossRef]
- Di Toro, D.M.; O’Connor, D.J.; Thomann, R.V. A dynamic model of the phytoplankton population in the Sacramento San Joaquin Delta. Adv. Chem. Ser. 1971, 106, 131–180. [Google Scholar]
- Ferrara, R.A.; Harleman, D.R.F. Dynamic nutrient cycle model for waste stabilization ponds. J. Environ. Eng. Div. Proc. Am. Soc. Civ. Eng. 1980, 106, 37–54. [Google Scholar]
- Baca, R.G.; Arnett, R.C. A Limnological Model for Eutrophic Lakes and Impoundments; Battelle, Inc. Pacific Northwest Lab.: Richland, WA, USA, 1976; pp. 89–95. [Google Scholar]
- Mayo, A.W.; Mutamba, J. Modelling nitrogen removal in a coupled HRP and unplanted horizontal flow subsurface gravel bed constructed wetland. Phys. Chem. Earth Parts A/B/C 2005, 30, 673–679. [Google Scholar] [CrossRef]
- Charley, R.C.; Hooper, D.G.; McLee, A.G. Nitrification kinetics in activated sludge at various temperatures and dissolved oxygen concentrations. Water Res. 1980, 14, 1387–1396. [Google Scholar] [CrossRef]
- Wang, H.; Wang, C.; Wang, Y.; Gao, X.; Yu, C. Bayesian forecasting and uncertainty quantifying of stream flows using Metropolis—Hastings Markov Chain Monte Carlo algorithm. J. Hydrol. 2017, 549, 476–483. [Google Scholar] [CrossRef]
- Choi, H.J.; Lee, S.M. Effect of the N/P ratio on biomass productivity and nutrient removal from municipal wastewater. Bioprocess Biosyst. Eng. 2015, 38, 761–766. [Google Scholar] [CrossRef] [PubMed]
- Reed, S.C. Nitrogen removal in wastewater stabilization ponds. J. (Water Pollut. Control Fed.) 1985, 54, 39–45. [Google Scholar]
- Pano, A.; Middlebrooks, E.J. Ammonia nitrogen removal in facultative wastewater stabilization ponds. J. (Water Pollut. Control Fed.) 1982, 54, 344–351. [Google Scholar]
- Valero, M.A.C.; Mara, D.D. Nitrogen removal via ammonia volatilization in maturation ponds. Water Sci. Technol. 2007, 55, 87–92. [Google Scholar] [CrossRef]
- Cai, T.; Park, S.Y.; Li, Y. Nutrient recovery from wastewater streams by microalgae: Status and prospects. Renew. Sustain. Energy Rev. 2013, 19, 360–369. [Google Scholar] [CrossRef]
- Gonzalez, L.E.; Bashan, Y. Increased growth of the microalga chlorella vulgariswhen coimmobilized and cocultured in alginate beads with the plant-growth-promoting bacterium azospirillum brasilense. Appl. Environ. Microbiol. 2000, 66, 1527–1531. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Su, H.; Zhong, Y.; Zhang, C.; Shen, Z.; Sang, W.; Yan, G.; Zhou, X. The effect of bacterial contamination on the heterotrophic cultivation of Chlorella pyrenoidosa in wastewater from the production of soybean products. Water Res. 2012, 46, 5509–5516. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Wang, Z.; Shu, Q.; Takala, J.; Hiltunen, E.; Feng, P.; Yuan, Z. Nutrient removal and biodiesel production by integration of freshwater algae cultivation with piggery wastewater treatment. Water Res. 2013, 47, 4294–4302. [Google Scholar] [CrossRef] [PubMed]
- Zak, S.K.; Beven, K.J. Equifinality, sensitivity and predictive uncertainty in the estimation of critical loads. Sci. Total Environ. 1999, 236, 191–214. [Google Scholar] [CrossRef]
| Process | Model | Source Literature |
|---|---|---|
| Mineralization | [47,54] | |
| Nitrification | [54] | |
| Sedimentation | [46,47] | |
| Denitrification | R20 NO3-N | [46] |
| Volatilization | [46] | |
| Microorganisms growth 1 | [13,55] | |
| Microorganisms growth 2 | [55] |
| Parameter | Description | Literature Value | Calibrated Value | References | L1 * | L2 | Rank a |
|---|---|---|---|---|---|---|---|
| θ | Arrhenius constant | 1.01 to 1.09 | 1.01 | [56] | 1341.9 | 171.0 | 1 |
| Rs | ON. Sedimentation constant (d−1) | 0.015 | 0.050 | [46,47] | 48.1 | 6.4 | 7 |
| K1 | Nitrosom. half sat. const. (mg/L) | 0.13 to 0.15 | 1.26 | [57] | 1157.9 | 151.9 | 2 |
| K2 | Ammonium. half sat. const. (mg/L) | 18.0 | 16.20 | [55] | 870.6 | 115.5 | 3 |
| K3 | Nitrate. half sat. const. (mg/L) | 2.0 | 2.0 | 0 | 0 | 9 | |
| R20 | denitrification constant (d−1) | 0.0 to 1.0 | 0.26 | [56] | 274.6 | 37.3 | 4 |
| Yn | Yield coeff. Nitrosom. (g microbes/g N) | 0.13 | 0.163 | [58] | 177.4 | 23.1 | 5 |
| µn | Nitrosom. growth rate (d−1) | 0.008 | 0.009 | 144.2 | 18.1 | 6 | |
| µmax | maximum growth rate at 20 °C (d−1) | 0.1 to 0.77 | 0.13 | [21,55] | 9.5 | 1.3 | 8 |
| Water Quality | Nash-Sutcliffe Coefficient (NSE) | Correlation Constant (r) | ||
|---|---|---|---|---|
| Calibration | Validation | Calibration | Validation | |
| ON | 0.66 | 0.53 | 0.84 | 0.76 |
| NH3 | 0.70 | 0.69 | 0.87 | 0.83 |
| NO3 | 0.58 | 0.64 | 0.85 | 0.81 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mukhtar, H.; Lin, Y.-P.; Shipin, O.V.; Petway, J.R. Modeling Nitrogen Dynamics in a Waste Stabilization Pond System Using Flexible Modeling Environment with MCMC. Int. J. Environ. Res. Public Health 2017, 14, 765. https://doi.org/10.3390/ijerph14070765
Mukhtar H, Lin Y-P, Shipin OV, Petway JR. Modeling Nitrogen Dynamics in a Waste Stabilization Pond System Using Flexible Modeling Environment with MCMC. International Journal of Environmental Research and Public Health. 2017; 14(7):765. https://doi.org/10.3390/ijerph14070765
Chicago/Turabian StyleMukhtar, Hussnain, Yu-Pin Lin, Oleg V. Shipin, and Joy R. Petway. 2017. "Modeling Nitrogen Dynamics in a Waste Stabilization Pond System Using Flexible Modeling Environment with MCMC" International Journal of Environmental Research and Public Health 14, no. 7: 765. https://doi.org/10.3390/ijerph14070765






