Gingival Pigmentation Affected by Smoking among Different Age Groups: A Quantitative Analysis of Gingival Pigmentation Using Clinical Oral Photographs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Ethical Considerations
2.2. Measurement of the Gingival Color
2.3. Age-Related Differences in the Effect of Smoking Cessation on Gingival Pigmentation
2.4. Statistical Analyses
3. Results
3.1. Subjects
3.2. Smoking-Induced Gingival Pigmentation in Different Age Groups
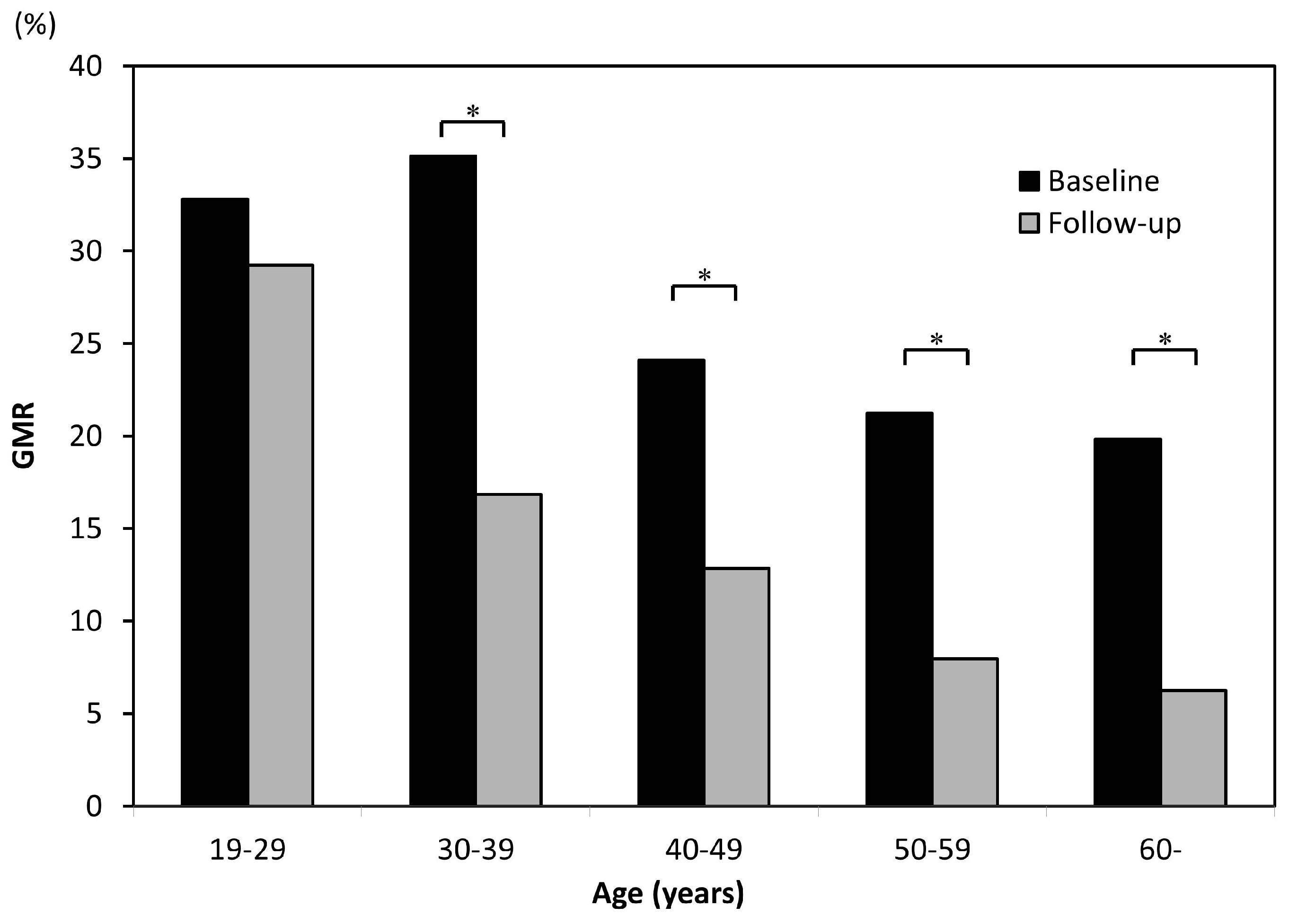
3.3. Age-Related Differences in Decreased Gingival Pigmentation Due to Smoking Cessation
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Katanoda, K.; Marugame, T.; Saika, K.; Satoh, H.; Tajima, K.; Suzuki, T.; Tamakoshi, A.; Tsugane, S.; Sobue, T. Population attributable fraction of mortality associated with tobacco smoking in Japan: A pooled analysis of three large-scale cohort studies. J. Epidemiol. 2008, 18, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Tabuchi, T.; Fujiwara, T.; Nakayama, T.; Miyashiro, I.; Tsukuma, H.; Ozaki, K.; Kondo, N. Maternal and paternal indoor or outdoor smoking and the risk of asthma in their children: A nationwide prospective birth cohort study. Drug Alcohol Depend. 2015, 147, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Miyake, Y. Association between prenatal and postnatal tobacco smoke exposure and allergies in young children. J. Asthma 2011, 48, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, A.; Hanaki, K.; Tomita, K.; Watanabe, M.; Hasagawa, Y.; Okazaki, R.; Igishi, T.; Horimukai, K.; Fukutani, K.; Sugimoto, Y. Environmental tobacco smoke and its effect on the symptoms and medication in children with asthma. Int. J. Environ. Health Res. 2009, 19, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Yolton, K.; Dietrich, K.; Auinger, P.; Lanphear, B.P.; Hornung, R. Exposure to environmental tobacco smoke and cognitive abilities among U.S. children and adolescents. Environ. Health Perspect. 2005, 113, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Hanioka, T.; Tanaka, K.; Ojima, M.; Yuuki, K. Association of melanin pigmentation in the gingiva of children with parents who smoke. Pediatrics 2005, 116, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Miyake, Y.; Arakawa, M.; Sasaki, S.; Ohya, Y. Household smoking and dental caries in schoolchildren: The Ryukyus child health study. BMC Public Health 2010, 10, 335. [Google Scholar] [CrossRef] [PubMed]
- Ciçek, Y.; Ertaş, U. The normal and pathological pigmentation of oral mucous membrane: A review. J. Contemp. Dent. Pract. 2003, 4, 76–86. [Google Scholar] [PubMed]
- Moravej-Salehi, E.; Moravej-Salehi, E.; Hajifattahi, F. Relationship of gingival pigmentation with passive smoking in women. Tanaffos 2015, 14, 107–114. [Google Scholar] [PubMed]
- Hedin, C.A.; Larsson, A. The ultrastructure of the gingival epithelium in smokers’ melanosis. J. Periodontal Res. 1984, 19, 177–190. [Google Scholar] [PubMed]
- Schroeder, H.E. Melanin containing organelles in cells of the human gingiva. J. Periodontal Res. 1969, 4, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Hedin, C.A. Smokers’ melanosis: Occurrence and localization in the attached gingiva. Arch. Dermatol. 1977, 113, 1533–1538. [Google Scholar] [CrossRef] [PubMed]
- Hedin, C.A.; Axéll, T. Oral melanin pigmentation in 467 Thai and Malaysian people with special emphasis on smoker’s melanosis. J. Oral Pathol. Med. 1991, 20, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Hedin, C.A.; Pindborg, J.J.; Axéll, T. Disappearance of smoker’s melanosis after reducing smoking. J. Oral Pathol. Med. 1993, 22, 228–230. [Google Scholar] [CrossRef] [PubMed]
- Ho, D.K.; Ghinea, R.; Herrera, L.J.; Angelov, N.; Paravina, R.D. Color range and color distribution of healthy human gingiva: A prospective clinical study. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lin, J.; Seliger, A.; Gil, M.; da Silva, J.D.; Ishhikawa-Nagai, S. Color effects of gingiva on cervical regions of all-ceramic crowns. J. Esthet. Restor. Dent. 2013, 25, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Ono, T.; Naito, T.; Makino, M.; Sato, M. A color analysis of smoker’s melanosis using a non-contact type dental spectrophotometer. J. Oral Hyg. Health 2015, 2, 160–164. [Google Scholar]
- Kato, T.; Takiuchi, H.; Sugiyama, S.; Makino, M.; Noguchi, S.; Katayama-Ono, T.; Hanioka, T.; Naito, T. Measurement of reduced gingival melanosis after smoking cessation: A novel analysis of gingival pigmentation using clinical oral photographs. Int. J. Environ. Res. Public Health 2016, 13, 598. [Google Scholar] [CrossRef] [PubMed]
- Gilhar, A.; Pillar, T.; David, M.; Eidelman, S. Melanocytes and Langerhans cells in aged versus young skin before and after transplantation onto nude mice. J. Investig. Dermatol. 1991, 96, 210–214. [Google Scholar] [CrossRef] [PubMed]
- Montagna, W.; Carlisle, K. Structural changes in aging human skin. J. Investig. Dermatol. 1979, 73, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Haddad, M.M.; Xu, W.; Medrano, E.E. Aging in epidermal melanocytes: Cell cycle genes and melanins. J. Investig. Dermatol. Symp. Proc. 1998, 3, 36–40. [Google Scholar] [PubMed]
- Fry, L.; Almeyda, J.R. The incidence of buccal pigmentation in caucasoids and negroids in Britain. Br. J. Dermatol. 1968, 80, 244–247. [Google Scholar] [CrossRef] [PubMed]
- Monash, S. Normal pigmentation of the oral mucosa. Arch. Dermatol. 1932, 26, 139–147. [Google Scholar]
- Ando, Y.; Suetaka, T.; Sakuma, I. A statistical investigation of gingival pigmentation. Dent. Abstr. 1956, 1, 749–750. [Google Scholar]
- Axell, T.; Hedin, A. Epidemiologic study of excessive oral melanin pigmentation with special reference to the influence of tobacco habits. Scand. J. Dent. Res. 1982, 90, 434–442. [Google Scholar] [PubMed]
- Marakoğlu, K.; Gürsoy, U.K.; Toker, H.C.; Demirer, S.; Sezer, R.E.; Marakoğlu, I. Smoking status and smoke-related gingival melanin pigmentation in army recruitments. Mil. Med. 2007, 172, 110–113. [Google Scholar] [CrossRef] [PubMed]
- WHO Report on the Global Tobacco Epidemic 2015. Available online: http://www.who.int/tobacco/global_report/2015/en/ (accessed on 6 March 2017).
- Chen, Z.; Peto, R.; Zhou, M.; Iona, A.; Smith, M.; Yang, L.; Guo, Y.; Chen, Y.; Bian, Z.; Lancaster, G.; et al. Contrasting male and female trends in tobacco-attributed mortality in China: Evidence from successive nationwide prospective cohort studies. Lancet 2015, 386, 1447–1456. [Google Scholar] [CrossRef]
- Araki, S.; Murata, K.; Ushio, K.; Sakai, R. Dose-response relationship between tobacco consumption and melanin pigmentation in the attached gingiva. Arch. Environ. Health 1983, 38, 375–378. [Google Scholar] [CrossRef] [PubMed]




| Patient Characteristics | Former Smokers | Current Smokers | Total | p Value |
|---|---|---|---|---|
| Number of subjects | 133 | 126 | 259 | |
| Male/Female | 88/45 | 79/47 | 167/92 | 0.30 |
| Age (years) | 46.8 ± 13.4 | 44.6 ± 13.0 | 45.9 ± 13.2 | 0.60 |
| Duration of smoking (years) | 27.3 ± 12.6 | 27.7 ± 12.4 | 27.5 ± 12.5 | 0.65 |
| GMR (%) | 26.0 ± 18.4 | 27.5 ± 21.1 | 26.7 ± 19.8 | 0.89 |
| Follow-up period (years) | 4.50 ± 2.15 | 3.79 ± 1.17 | 4.15 ± 1.77 | 0.01 |
| Duration of smoking cessation (years) | 3.14 ± 1.95 | - |
| Patient Characteristics | Age of Former Smokers (Years) | Age of Current Smokers (Years) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 19–29 | 30–39 | 40–49 | 50–59 | ≥60 | 19–29 | 30–39 | 40–49 | 50–59 | ≥60 | |
| Number of subjects | 13 | 32 | 29 | 32 | 27 | 17 | 26 | 32 | 36 | 15 |
| Male/Female | 8/5 | 23/9 | 21/8 | 16/16 | 20/7 | 11/6 | 18/8 | 19/13 | 23/13 | 8/7 |
| Duration of smoking (years) | 9.5 | 18.3 | 26.1 | 32.5 | 41.4 | 9.0 | 17.9 | 27.7 | 37.2 | 43.3 |
| Follow-up period (years) | 5.01 | 4.40 | 4.73 | 4.19 | 4.47 | 3.60 | 4.00 | 3.52 | 3.78 | 4.22 |
| Duration of smoking cessation (years) | 2.62 | 2.75 | 3.31 | 3.38 | 3.67 | |||||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kato, T.; Mizutani, S.; Takiuchi, H.; Sugiyama, S.; Hanioka, T.; Naito, T. Gingival Pigmentation Affected by Smoking among Different Age Groups: A Quantitative Analysis of Gingival Pigmentation Using Clinical Oral Photographs. Int. J. Environ. Res. Public Health 2017, 14, 880. https://doi.org/10.3390/ijerph14080880
Kato T, Mizutani S, Takiuchi H, Sugiyama S, Hanioka T, Naito T. Gingival Pigmentation Affected by Smoking among Different Age Groups: A Quantitative Analysis of Gingival Pigmentation Using Clinical Oral Photographs. International Journal of Environmental Research and Public Health. 2017; 14(8):880. https://doi.org/10.3390/ijerph14080880
Chicago/Turabian StyleKato, Tomotaka, Shinsuke Mizutani, Hiroya Takiuchi, Seiichi Sugiyama, Takashi Hanioka, and Toru Naito. 2017. "Gingival Pigmentation Affected by Smoking among Different Age Groups: A Quantitative Analysis of Gingival Pigmentation Using Clinical Oral Photographs" International Journal of Environmental Research and Public Health 14, no. 8: 880. https://doi.org/10.3390/ijerph14080880






