Removal of Arsenic (V) from Aqueous Solutions Using Chitosan–Red Scoria and Chitosan–Pumice Blends
Abstract
:1. Introduction
2. Materials and Methods
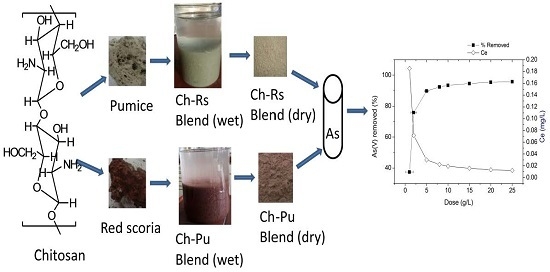
2.1. Adsorbent Preparation
2.2. Adsorbent Characterization
2.2.1. Chemical Composition
2.2.2. Determination of pH and Point of Zero Charge
2.3. Chemicals
2.4. Analytical Procedures
2.5. Batch Arsenic Adsorption Studies
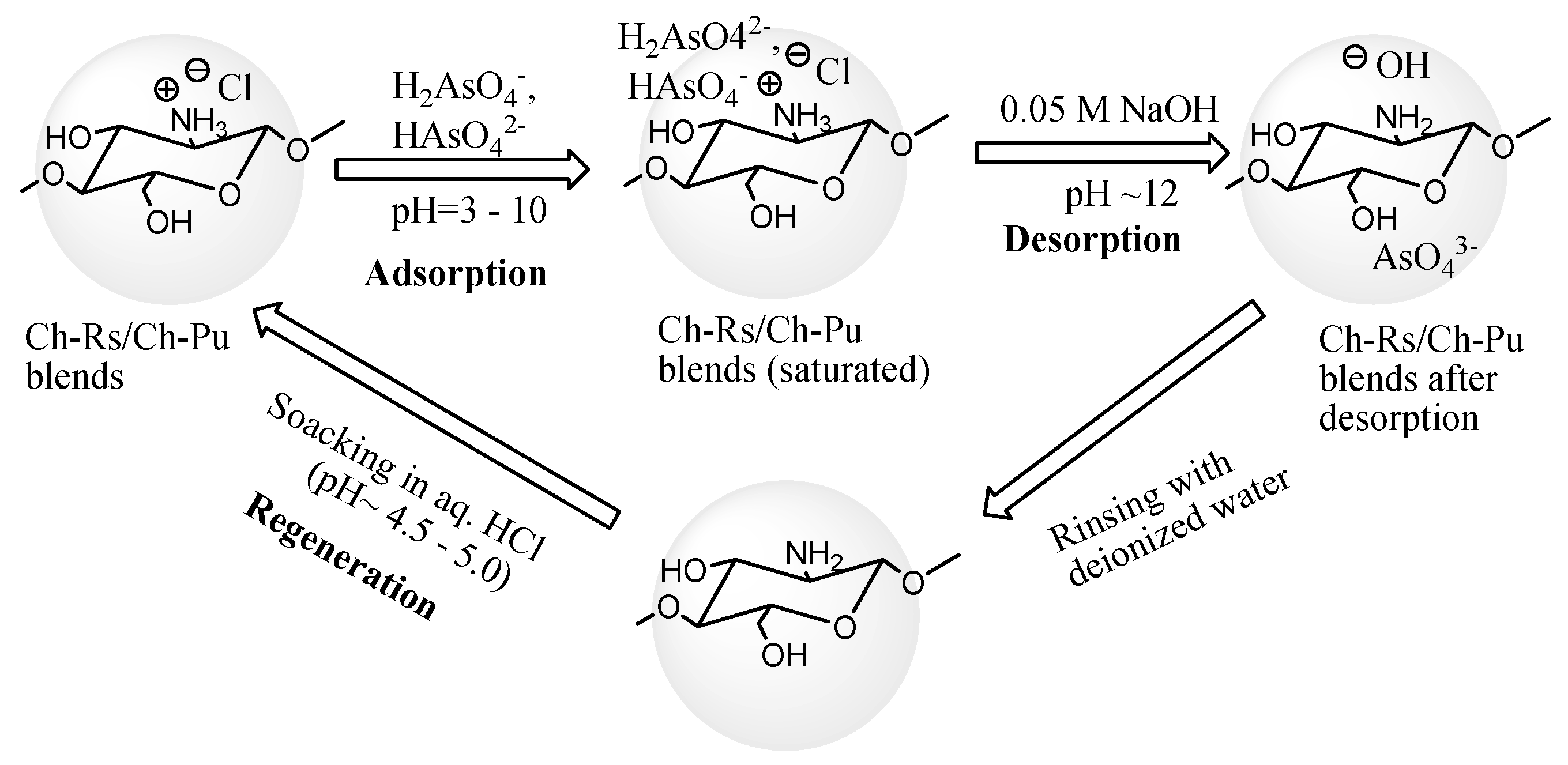
2.6. Regeneration of the Spent Adsorbents
3. Results and Discussion
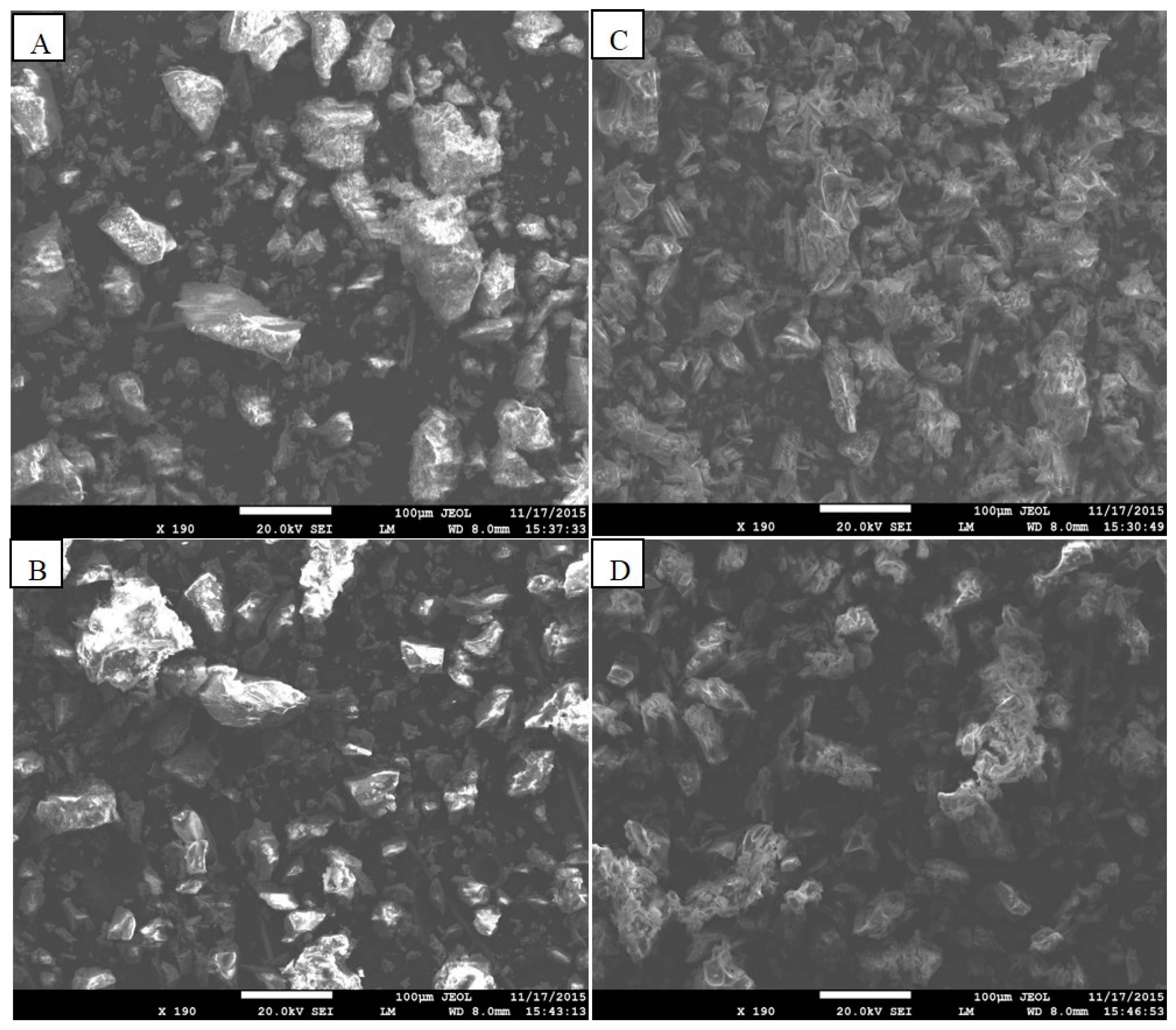
3.1. Adsorbent Characterization
3.1.1. Chitosan Loaded on Rs and Pu
3.1.2. Chemical Composition
3.1.3. pH and Point of Zero Charge
3.2. Adsorption of As (V) on Ch–Rs and Ch–Pu Blends
3.2.1. Preliminary Adsorption Experiments
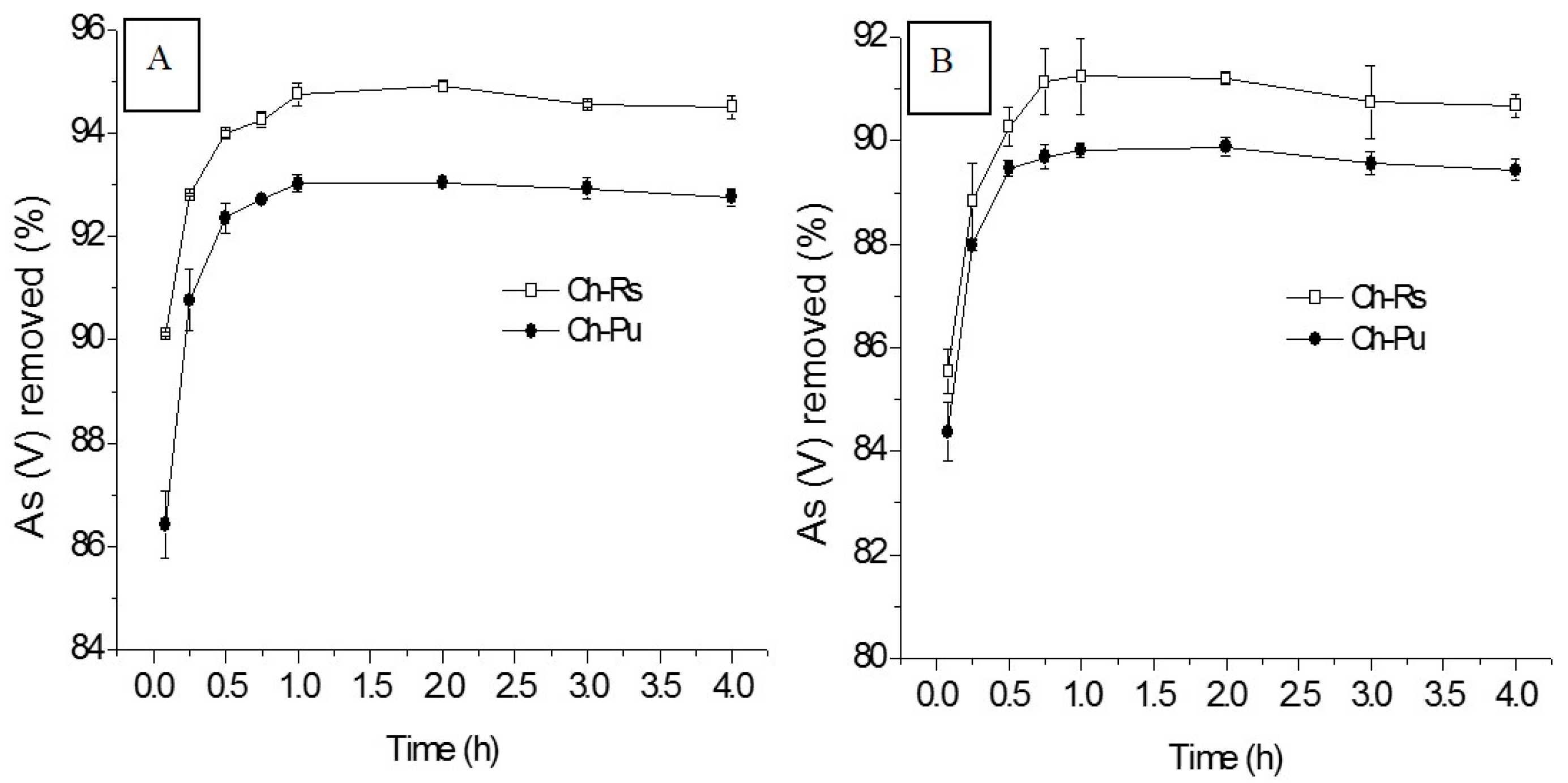
3.2.2. Effect of Contact Time
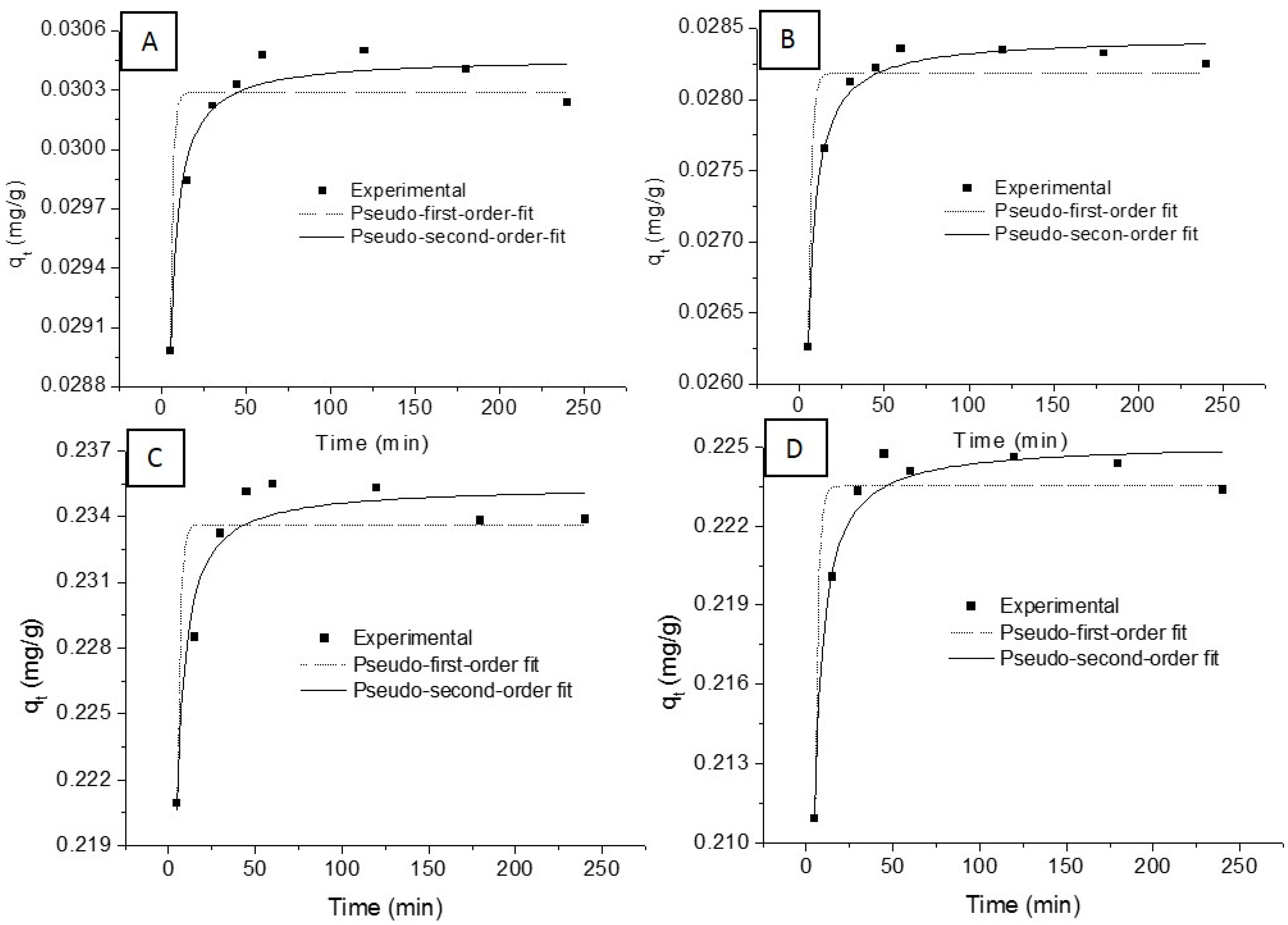
3.2.3. Adsorption Kinetics
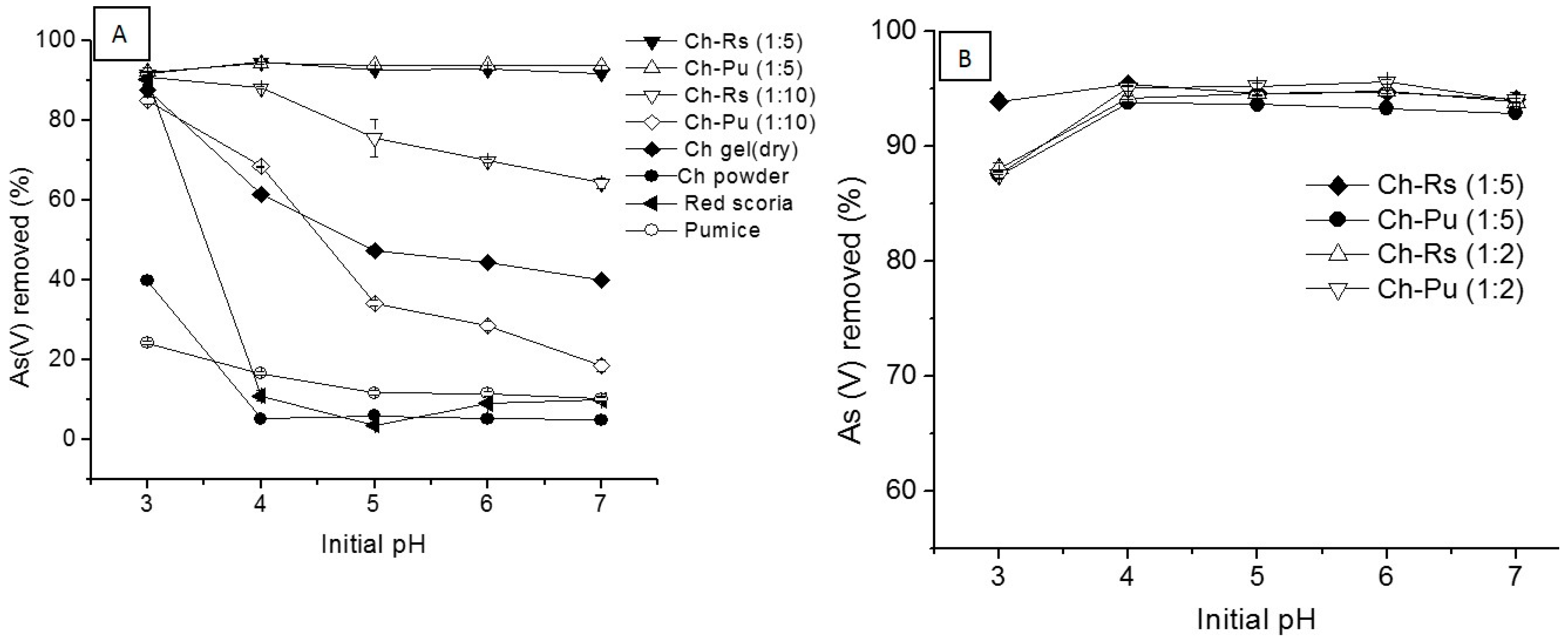
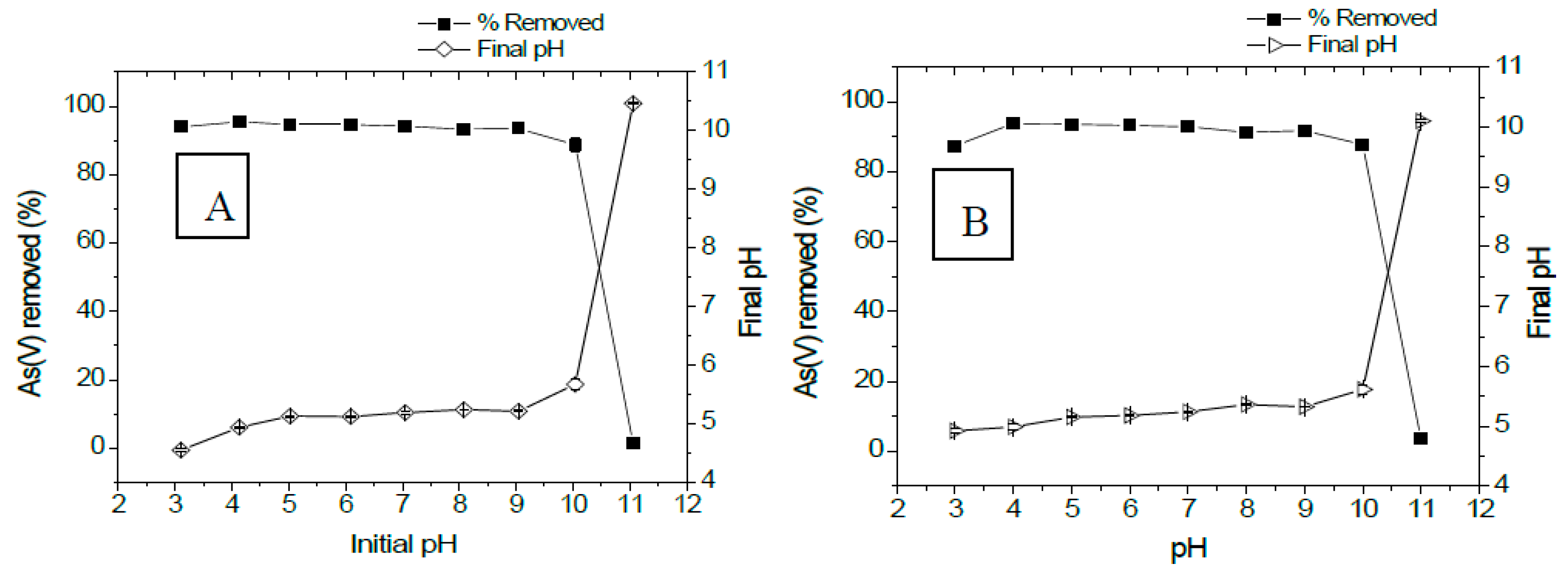
3.2.4. Influence of pH
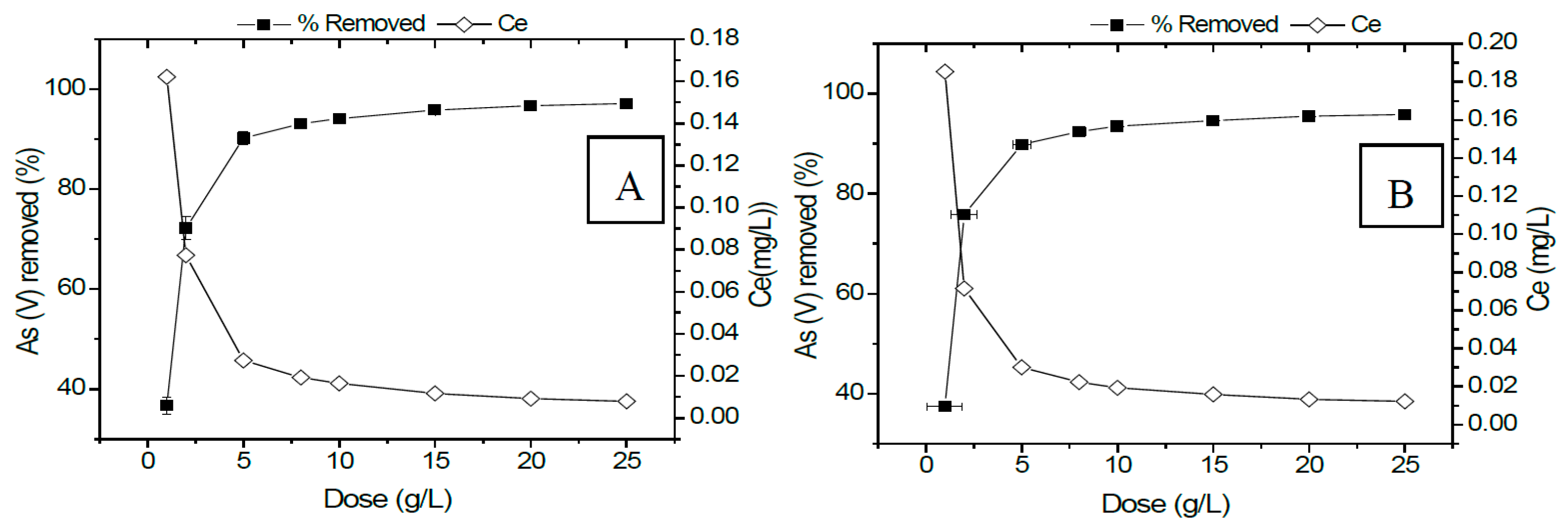
3.2.5. Optimization of Adsorbent Dose
3.2.6. Effect of Initial Concentration
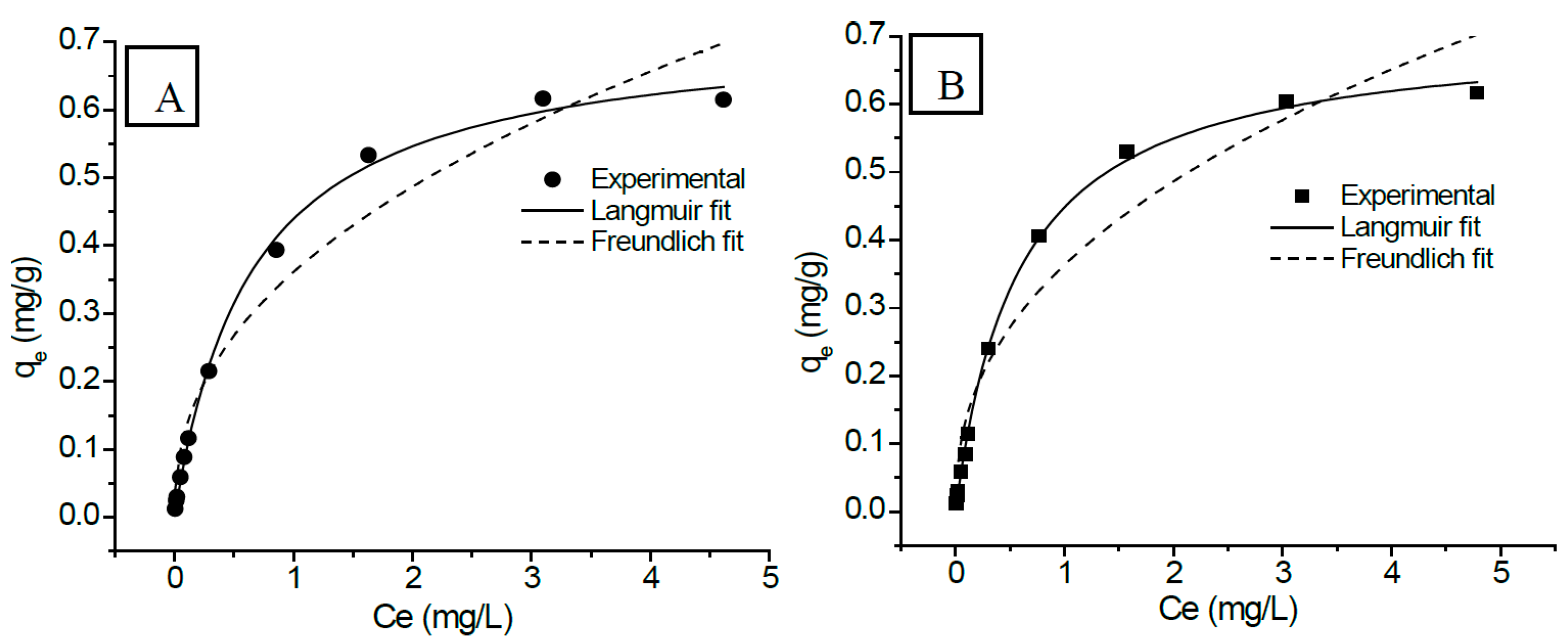
3.2.7. Adsorption Isotherm
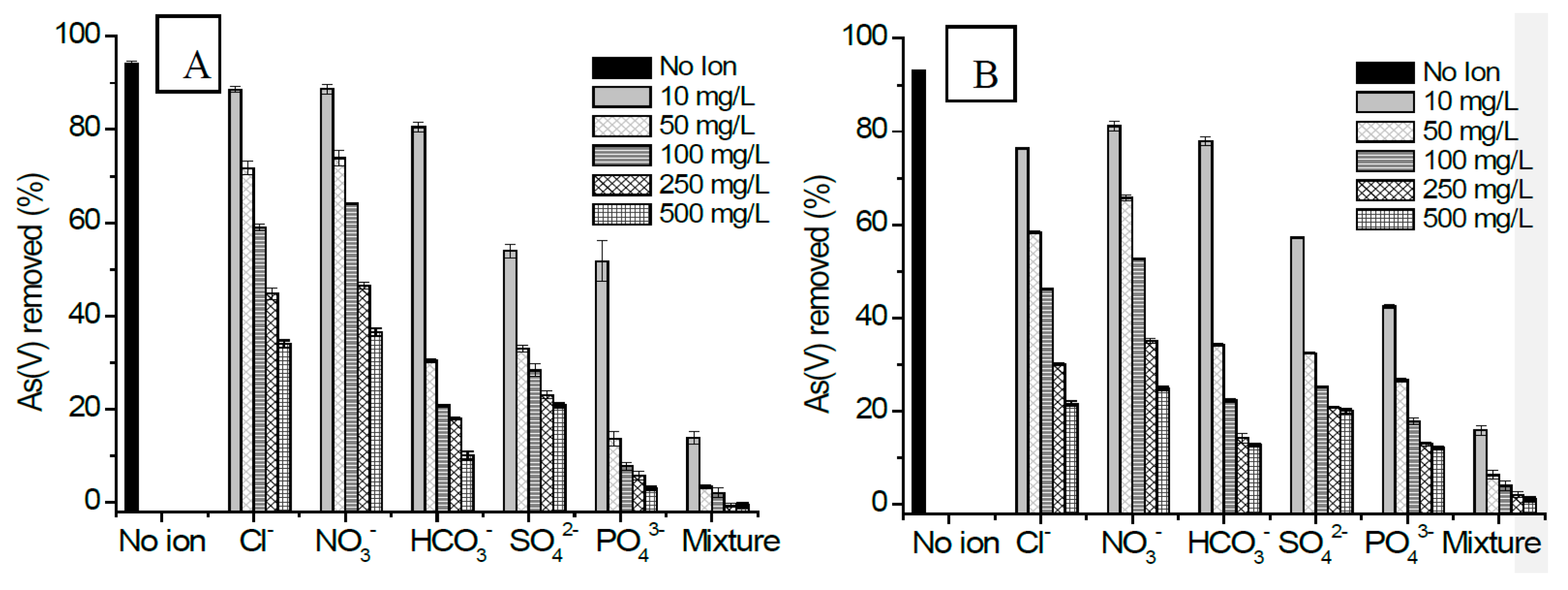
3.2.8. Effect of Co-Existing Anions
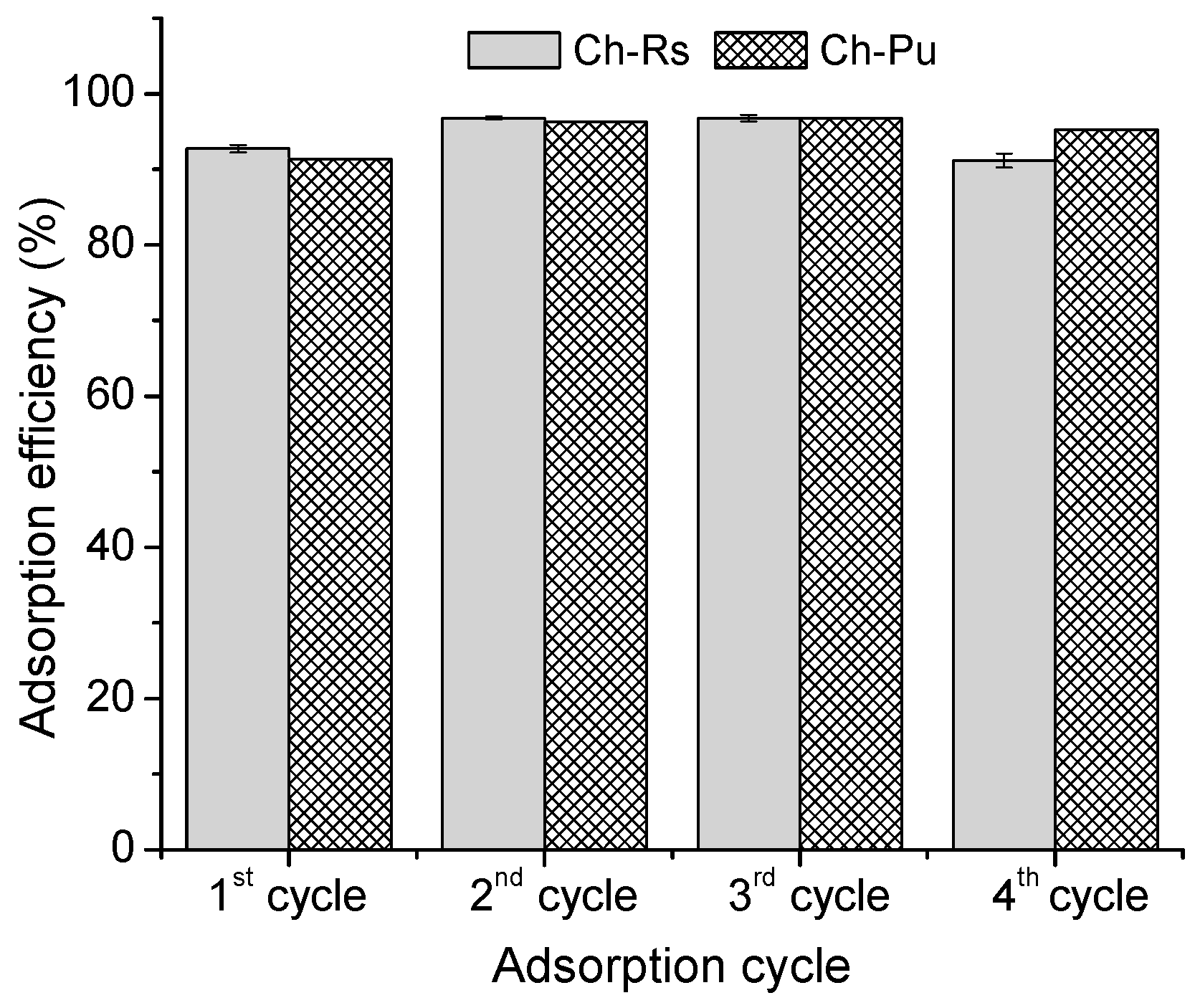
3.2.9. Desorption of As (V) from Ch–Rs and Ch–Pu Surfaces
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhu, J.; Pigna, M.; Cozzolino, V.; Caporale, A.G.; Violante, A. Higher sorption of arsenate versus arsenite on amorphous Al-oxide, effect of ligands. Environ. Chem. Lett. 2013, 11, 289–294. [Google Scholar] [CrossRef]
- Bissen, M.; Frimmel, F.H. Arsenic—A review. Part I: Occurrence, toxicity, speciation, mobility. Acta Hydrochim. Hydrobiol. 2003, 31, 9–18. [Google Scholar] [CrossRef]
- Smedley, P.L.; Kinniburgh, D.G. A review of the source, behaviour and distribution of arsenic in natural waters. Appl. Geochem. 2002, 17, 517–568. [Google Scholar] [CrossRef]
- Smedley, P.L.; Kinniburgh, D. Arsenic in Groundwater and the Environment. In Essentials of Medical Geology; Selinus, O., Ed.; Springer: Dordrecht, The Netherlands, 2013; pp. 279–310. [Google Scholar]
- Styblo, M.; Del Razo, L.M.; Vega, L.; Germolec, D.R.; LeCluyse, E.L.; Hamilton, G.A.; Reed, W.; Wang, C.; Cullen, W.R.; Thomas, D.J. Comparative toxicity of trivalent and pentavalent inorganic and methylated arsenicals in rat and human cells. Arch. Toxicol. 2000, 74, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Ritter, L.; Solomon, K.; Sibley, P.; Hall, K.; Keen, P.; Mattu, G.; Linton, B. Sources, pathways, and relative risks of contaminants in surface water and groundwater: A perspective prepared for the Walkerton inquiry. J. Toxicol. Environ. Health Part A Curr. Issues 2002, 65, 1–142. [Google Scholar]
- Boddu, V.M.; Abburi, K.; Talbott, J.L.; Smith, E.D.; Haasch, R. Removal of arsenic (III) and arsenic (V) from aqueous medium using chitosan-coated biosorbent. Water Res. 2008, 42, 633–642. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. IPCS Environmental Health Criteria 224 Arsenic and Arsenic Compounds. Geneva: International Program on Chemical Safety; WHO: Geneva, Switzerland, 2001. [Google Scholar]
- Wang, J.P.; Wang, S.L.; Lin, Q.; Zhang, L.; Huang, D.; Ng, J.C. Association of arsenic and kidney dysfunction in people with diabetes and validation of its effects in rats. Environ. Int. 2009, 35, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.-H.; Chong, C.-K.; Chen, C.-J.; Tai, T.-Y. Dose-response relationship between peripheral vascular disease and ingested inorganic arsenic among residents in blackfoot disease endemic villages in Taiwan. Atherosclerosis 1996, 120, 125–133. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for Drinking-Water Quality, 3rd ed.; WHO Press: Geneva, Switzerland, 2006; Volume 1, (Suppl. 1), ISBN 924154696. [Google Scholar]
- Jain, C.K.; Ali, I. Arsenic: Occurrence, toxicity and speciation techniques. Water Res. 2000, 34, 4304–4312. [Google Scholar] [CrossRef]
- Danish, M.I.; Qazi, I.A.; Zeb, A.; Habib, A.; Ali Awan, M.; Khan, Z. Arsenic removal from aqueous solution using pure and metal-doped titania nanoparticles coated on glass beads: Adsorption and column studies. J. Nanomater. 2013, 2013, 69. [Google Scholar] [CrossRef]
- Rango, T.; Vengosh, A.; Dwyer, G.; Bianchini, G. Mobilization of arsenic and other naturally occurring contaminants in groundwater of the Main Ethiopian Rift aquifers. Water Res. 2013, 47, 5801–5818. [Google Scholar] [CrossRef] [PubMed]
- Tripathy, S.S.; Raichur, A.M. Enhanced adsorption capacity of activated alumina by impregnation with alum for removal of As (V) from water. Chem. Eng. J. 2008, 138, 179–186. [Google Scholar] [CrossRef]
- Chen, R.; Zhang, Z.; Feng, C.; Lei, Z.; Li, Y.; Li, M.; Shimizu, K.; Sugiura, N. Batch study of arsenate (V) adsorption using Akadama mud: Effect of water mineralization. Appl. Surf. Sci. 2010, 256, 2961–2967. [Google Scholar] [CrossRef]
- Elson, C.M.; Davies, D.H.; Hayes, E.R. Removal of arsenic from contaminated drinking-water by a chitosan-chitin mixture. Water Res. 1980, 14, 1307–1311. [Google Scholar] [CrossRef]
- Kwok, K.C.M.; Lee, V.K.C.; Gerente, C.; McKay, G. Novel model development for sorption of arsenate on chitosan. Chem. Eng. J. 2009, 151, 122–133. [Google Scholar] [CrossRef]
- Gao, Q.; Zhu, H.; Luo, W.-J.; Wang, S.; Zhou, C.-G. Preparation, characterization, and adsorption evaluation of chitosan-functionalized mesoporous composites. Microporous Mesoporous Mater. 2014, 193, 15–26. [Google Scholar] [CrossRef]
- Kamble, S.P.; Jagtap, S.; Labhsetwar, N.K.; Thakare, D.; Godfrey, S.; Devotta, S.; Rayalu, S.S. Defluoridation of drinking water using chitin, chitosan and lanthanum-modified chitosan. Chem. Eng. J. 2007, 129, 173–180. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Sillanpaa, M. Applications of chitin- and chitosan-derivatives for the detoxification of water and wastewater—A short review. Adv. Colloid Interface Sci. 2009, 152, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Kwok, K.C.; Koong, L.F.; Chen, G.; McKay, G. Mechanism of arsenic removal using chitosan and nanochitosan. J. Colloid Interface Sci. 2014, 416, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Gerente, C.; Andres, Y.; McKay, G.; Le Cloirec, P. Removal of arsenic (V) onto chitosan: From sorption mechanism explanation to dynamic water treatment process. Chem. Eng. J. 2010, 158, 593–598. [Google Scholar] [CrossRef]
- Ramesh, A.; Hasegawa, H.; Sugimoto, W.; Maki, T.; Ueda, K. Adsorption of gold (III), platinum (W) and palladium (II) onto glycine modified crosslinked chitosan resin. Bioresour. Technol. 2008, 99, 3801–3809. [Google Scholar] [CrossRef] [PubMed]
- McAfee, B.J.; Gould, W.D.; Nadeau, J.C.; da Costa, A.C.A. Biosorption of metal ions using chitosan, chitin, and biomass of Rhizopus oryzae. Sep. Sci. Technol. 2001, 36, 3207–3222. [Google Scholar] [CrossRef]
- Wang, G.H.; Liu, J.S.; Wang, X.G.; Xie, Z.Y.; Deng, N.S. Adsorption of uranium (VI) from aqueous solution onto cross-linked chitosan. J. Hazard. Mater. 2009, 168, 1053–1058. [Google Scholar] [CrossRef] [PubMed]
- Cheung, W.H.; Szeto, Y.S.; McKay, G. Enhancing the adsorption capacities of acid dyes by chitosan nano particles. Bioresour. Technol. 2009, 100, 1143–1148. [Google Scholar] [CrossRef] [PubMed]
- Auta, M.; Hameed, B.H. Chitosan-clay composite as highly effective and low-cost adsorbent for batch and fixed-bed adsorption of methylene blue. Chem. Eng. J. 2014, 237, 352–361. [Google Scholar] [CrossRef]
- Chen, A.H.; Liu, S.C.; Chen, C.Y.; Chen, C.Y. Comparative adsorption of Cu (II), Zn (II), and Pb (II) ions in aqueous solution on the crosslinked chitosan with epichlorohydrin. J. Hazard. Mater. 2008, 154, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Sureshkumar, M.K.; Radhakrishnan, K.; Nuwar, J.; Pillai, C.G.S. Adsorptive removal of Cr (III) from aqueous solution using tripolyphosphate cross-linked chitosan beads. J. Radioanal. Nucl. Chem. 2011, 289, 275–285. [Google Scholar] [CrossRef]
- Sureshkumar, M.K.; Das, D.; Mallia, M.B.; Gupta, P.C. Adsorption of uranium from aqueous solution using chitosan-tripolyphosphate (CTPP) beads. J. Hazard. Mater. 2010, 184, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Baroni, P.; Vieira, R.S.; Meneghetti, E.; da Silva, M.G.C.; Beppu, M.M. Evaluation of batch adsorption of chromium ions on natural and crosslinked chitosan membranes. J. Hazard. Mater. 2008, 152, 1155–1163. [Google Scholar] [CrossRef] [PubMed]
- Rijith, S.; Anirudhan, T.S.; Sumi, V.S.N.; Shripathi, T. Sorptive potential of glutaraldehyde cross-linked epoxyaminated chitosan for the removal of Pb (II) from aqueous media: Kinetics and thermodynamic profile. Desalin. Water Treat. 2016, 57, 15083–15097. [Google Scholar] [CrossRef]
- Turan, D.; Kocahakimoglu, C.; Boyaci, E.; Sofuoglu, S.C.; Eroglu, A.E. Chitosan-Immobilized Pumice for the Removal of As (V) from Waters. Water Air Soil Pollut. 2014, 225, 1931. [Google Scholar] [CrossRef]
- Sabarudin, A.; Oshita, K.; Oshima, M.; Motomizu, S. Synthesis of chitosan resin possessing 3,4-diamino benzoic acid moiety for the collection/concentration of arsenic and selenium in water samples and their measurement by inductively coupled plasma-mass spectrometry. Anal. Chim. Acta 2005, 542, 207–215. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Wang, D.; Li, H.; Wang, L.; Zhang, L. As (III) Removal from Aqueous Solution Using a-Fe2O3-impregnated Chitosan Beads. In Proceedings of the 2010 IEEE International Conference on Digital Manufacturing and Automation (ICDMA), Changsha, China, 18–20 December 2010; pp. 289–292. [Google Scholar]
- Gang, D.D.; Deng, B.; Lin, L. As (III) removal using an iron-impregnated chitosan sorbent. J. Hazard. Mater. 2010, 182, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xu, W.; Chen, L.; Huang, X.; Liu, J. Preparation and evaluation of magnetic nanoparticles impregnated chitosan beads for arsenic removal from water. Chem. Eng. J. 2014, 251, 25–34. [Google Scholar] [CrossRef]
- Yamani, J.S.; Miller, S.M.; Spaulding, M.L.; Zimmerman, J.B. Enhanced arsenic removal using mixed metal oxide impregnated chitosan beads. Water Res. 2012, 46, 4427–4434. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.M.; Spaulding, M.L.; Zimmerman, J.B. Optimization of capacity and kinetics for a novel bio-based arsenic sorbent, TiO2-impregnated chitosan bead. Water Res. 2011, 45, 5745–5754. [Google Scholar] [CrossRef] [PubMed]
- Dambies, L.; Guibal, E.; Roze, A. Arsenic (V) sorption on molybdate-impregnated chitosan beads. Colloids Surf. A Physicochem. Eng. Asp. 2000, 170, 19–31. [Google Scholar] [CrossRef]
- Gupta, A.; Yunus, M.; Sankararamakrishnan, N. Zerovalent iron encapsulated chitosan nanospheres—A novel adsorbent for the removal of total inorganic Arsenic from aqueous systems. Chemosphere 2012, 86, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Shinde, R.N.; Pandey, A.K.; Acharya, R.; Guin, R.; Das, S.K.; Rajurkar, N.S.; Pujari, P.K. Chitosan-transition metal ions complexes for selective arsenic (V) preconcentration. Water Res. 2013, 47, 3497–3506. [Google Scholar] [CrossRef] [PubMed]
- Boyaci, E.; Eroglu, A.E.; Shahwan, T. Sorption of As (V) from waters using chitosan and chitosan-immobilized sodium silicate prior to atomic spectrometric determination. Talanta 2010, 80, 1452–1460. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Evett, J. Soil Properties—Testing, Measurement, and Evaluation; Banta Book Company: Upper Saddle River, NJ, USA, 2003; ISBN 0-13-093005-9. [Google Scholar]
- Alemayehu, E.; Lennartz, B. Virgin volcanic rocks: Kinetics and equilibrium studies for the adsorption of cadmium from water. J. Hazard. Mater. 2009, 169, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.S.; Yun, S.T.; Lee, J.H.; Kim, S.O.; Jo, H.Y. Removal of divalent heavy metals (Cd, Cu, Pb, and Zn) and arsenic(III) from aqueous solutions using scoria: Kinetics and equilibria of sorption. J. Hazard. Mater. 2010, 174, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Boddu, V.M.; Abburi, K.; Talbott, J.L.; Smith, E.D. Removal of hexavalent chromium from wastewater using a new composite chitosan biosorbent. Environ. Sci. Technol. 2003, 37, 4449–4456. [Google Scholar] [CrossRef] [PubMed]
- Appel, C.; Ma, L.Q.; Rhue, R.D.; Kennelley, E. Point of zero charge determination in soils and minerals via traditional methods and detection of electroacoustic mobility. Geoderma 2003, 113, 77–93. [Google Scholar] [CrossRef]
- Fufa, F.; Alemayehu, E.; Lennartz, B. Defluoridation of groundwater using termite mound. Water Air Soil Pollut. 2013, 224, 1552. [Google Scholar] [CrossRef]
- Sepehr, M.N.; Zarrabi, M.; Kazemian, H.; Amrane, A.; Yaghmaian, K.; Ghaffari, H.R. Removal of hardness agents, calcium and magnesium, by natural and alkaline modified pumice stones in single and binary systems. Appl. Surf. Sci. 2013, 274, 295–305. [Google Scholar] [CrossRef] [Green Version]
- Padilla-Rodriguez, A.; Hernandez-Viezcas, J.A.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L.; Perales-Perez, O.; Roman-Velazquez, F.R. Synthesis of protonated chitosan flakes for the removal of vanadium (III, IV and V) oxyanions from aqueous solutions. Microchem. J. 2015, 118, 1–11. [Google Scholar] [CrossRef]
- Sepehr, M.N.; Amrane, A.; Karimaian, K.A.; Zarrabi, M.; Ghaffari, H.R. Potential of waste pumice and surface modified pumice for hexavalent chromium removal: Characterization, equilibrium, thermodynamic and kinetic study. J. Taiwan Inst. Chem. Eng. 2014, 45, 635–647. [Google Scholar] [CrossRef]
- Padilla-Rodriguez, A.; Hernandez-Viezcas, J.A.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L.; Perales-Perez, O.; Roman-Velazquez, F.R. Adsorption of arsenic (V) oxyanion from aqueous solutions by using protonated chitosan flakes. Sep. Sci. Technol. 2015, 50, 2099–2111. [Google Scholar] [CrossRef]
- Liu, B.; Wang, D.; Yu, G.; Meng, X. Removal of F- from aqueous solution using Zr (IV) impregnated dithiocarbamate modified chitosan beads. Chem. Eng. J. 2013, 228, 224–231. [Google Scholar] [CrossRef]
- Kumar, K.V. Linear and non-linear regression analysis for the sorption kinetics of methylene blue onto activated carbon. J. Hazard. Mater. 2006, 137, 1538–1544. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Li, Q.; Gao, S.; Shang, J.K. Exceptional arsenic adsorption performance of hydrous cerium oxide nanoparticles: Part A. Adsorption capacity and mechanism. Chem. Eng. J. 2012, 185, 127–135. [Google Scholar] [CrossRef]
- Gupta, K.; Ghosh, U.C. Arsenic removal using hydrous nanostructure iron (III)-titanium (IV) binary mixed oxide from aqueous solution. J. Hazard. Mater. 2009, 161, 884–892. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, N.; Sairam Sundaram, C.; Meenakshi, S. Development of multifunctional chitosan beads for fluoride removal. J. Hazard. Mater. 2009, 167, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Zha, F.; Huang, W.Y.; Wang, J.Y.; Chang, Y.; Ding, J.; Ma, J. Kinetic and thermodynamic aspects of arsenate adsorption on aluminum oxide modified palygorskite nanocomposites. Chem. Eng. J. 2013, 215, 579–585. [Google Scholar] [CrossRef]
- Fufa, F.; Alemayehu, E.; Lennartz, B. Sorptive removal of arsenate using termite mound. J. Environ. Manag. 2014, 132, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, C.S.; Viswanathan, N.; Meenakshi, S. Defluoridation chemistry of synthetic hydroxyapatite at nano scale: Equilibrium and kinetic studies. J. Hazard. Mater. 2008, 155, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Yazdani, M.; Tuudjarvi, T.; Bhatnagar, A.; Vahala, R. Adsorptive removal of arsenic (V) from aqueous phase by feldspars: Kinetics, mechanism, and thermodynamic aspects of adsorption. J. Mol. Liq. 2016, 214, 149–156. [Google Scholar] [CrossRef]
- Massoudinejad, M.; Asadi, A.; Vosoughi, M.; Gholami, M.; Karami, M.A. A comprehensive study (kinetic, thermodynamic and equilibrium) of arsenic (V) adsorption using KMnO4 modified clinoptilolite. Korean J. Chem. Eng. 2015, 32, 2078–2086. [Google Scholar] [CrossRef]
- Afzali, D.; Rouhani, M.; Fathirad, F.; Shamspur, T.; Mostafavi, A. Nano-iron oxide coated on sand as a new sorbent for removal of arsenic from drinking water. Desalin. Water Treat. 2016, 57, 13030–13037. [Google Scholar] [CrossRef]
- Hsu, J.C.; Lin, C.J.; Liao, C.H.; Chen, S.T. Removal of As (V) and As (III) by reclaimed iron-oxide coated sands. J. Hazard. Mater. 2008, 153, 817–826. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, S.A.; Ahmed, M.; Siddiqui, S.I.; Ahmed, S. Fe (III)-Sn (IV) mixed binary oxide-coated sand preparation and its use for the removal of As (III) and As (V) from water: Application of isotherm, kinetic and thermodynamics. J. Mol. Liq. 2016, 224, 431–441. [Google Scholar] [CrossRef]
- Mohapatra, D.; Mishra, D.; Chaudhury, G.R.; Das, R.P. Arsenic (V) adsorption mechanism using kaolinite, montmorillonite and illite from aqueous medium. J. Environ. Sci. Health Part A 2007, 42, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Maji, S.K.; Pal, A.; Pal, T. Arsenic removal from aqueous solutions by adsorption on laterite soil. J. Environ. Sci. Health Part A 2007, 42, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, D.; Talat, M.; Hasan, S.H. Biosorption of arsenic from aqueous solution using agricultural residue ‘rice polish’. J. Hazard. Mater. 2009, 166, 1050–1059. [Google Scholar] [CrossRef] [PubMed]
- Yusof, A.M.; Malek, N. Removal of Cr (VI) and As (V) from aqueous solutions by HDTMA-modified zeolite Y. J. Hazard. Mater. 2009, 162, 1019–1024. [Google Scholar] [CrossRef] [PubMed]
- Rahaman, M.S.; Basu, A.; Islam, M.R. The removal of As (III) and As (V) from aqueous solutions by waste materials. Bioresour. Technol. 2008, 99, 2815–2823. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.N.; Chai, L.Y.; Shu, Y.D. Study of arsenic (V) adsorption on bone char from aqueous solution. J. Hazard. Mater. 2008, 160, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Altundogan, H.S.; Altundogan, S.; Tumen, F.; Bildik, M. Arsenic adsorption from aqueous solutions by activated red mud. Waste Manag. 2002, 22, 357–363. [Google Scholar] [CrossRef]
- Altundogan, H.S.; Altundogan, S.; Tumen, F.; Bildik, M. Arsenic removal from aqueous solutions by adsorption on red mud. Waste Manag. 2000, 20, 761–767. [Google Scholar] [CrossRef]
- Yang, J.S.; Lee, J.Y.; Park, Y.T.; Baek, K.; Choi, J. Adsorption of As (III), As (V), Cd (II), Cu (II), and Pb (II) from aqueous solutions by natural muscovite. Sep. Sci. Technol. 2010, 45, 814–823. [Google Scholar] [CrossRef]
- Yang, J.S.; Kwon, M.J.; Park, Y.T.; Choi, J. Adsorption of Arsenic from Aqueous Solutions by Iron Oxide Coated Sand Fabricated with Acid Mine Drainage. Sep. Sci. Technol. 2015, 50, 267–275. [Google Scholar] [CrossRef]
- Nigussie, W.; Zewge, F.; Chandravanshi, B.S. Removal of excess fluoride from water using waste residue from alum manufacturing process. J. Hazard. Mater. 2007, 147, 954–963. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Chung, Y.C. Arsenic removal using a biopolymer chitosan sorbent. J. Environ. Sci. Health Part A 2006, 41, 645–658. [Google Scholar] [CrossRef]


| Elements | Pumice % (wt) | Red Scoria % (wt) | Oxides | Pumice % (wt) | Red Scoria % (wt) |
|---|---|---|---|---|---|
| Si | 27.3 | 18.4 | SiO2 | 69.2 | 42.2 |
| Al | 5.6 | 9.7 | Al2O3 | 11.9 | 18.4 |
| Fe | 3.3 | 8.1 | FeO | 5.8 | 13.0 |
| K | 3.9 | 0.3 | CaO | 0.9 | 11.1 |
| Ca | 0.2 | 6.3 | K2O | 6.3 | 0.6 |
| Na | 1.0 | 2.3 | Na2O | 1.6 | 3.4 |
| Mn | <0.1 | 0.1 | CuO | 1.7 | 1.6 |
| Mg | <0.1 | 2.2 | ZnO | 1.3 | 1.3 |
| Zn | <0.1 | <0.1 | NiO | 1.0 | 1.9 |
| Cr | <0. 1 | <0.1 | MnO | 0.1 | 0.2 |
| Cu | <0. 1 | <0.1 | MgO | 4.1 | |
| Co | <0.1 | <0.1 | TiO2 | 2.3 | |
| Cd | <0.1 | <0.1 | |||
| Ni | <0.1 | <0.1 | |||
| Pb | <0.1 | <0.1 | |||
| As | <0.1 | <0.1 |
| Parameter | Ch–Rs | Ch–Pu | ||||||
|---|---|---|---|---|---|---|---|---|
| Pseudo-First-Order | Pseudo-Second-Order | Pseudo-First-Order | Pseudo-Second-Order | |||||
| C0 (mg/L) | 0.25 | 2.0 | 0.25 | 2.0 | 0.25 | 2.0 | 0.25 | 2.0 |
| qe,exp(mg/g) | 0.032 | 0.234 | 0.032 | 0.234 | 0.028 | 0.224 | 0.028 | 0.224 |
| qe,cal(mg/g) | 0.030 | 0.234 | 0.031 | 0.235 | 0.028 | 0.224 | 0.028 | 0.225 |
| k1 (min−1) | 0.628 | 0.582 | - | - | 0.536 | 0.574 | - | - |
| k2(g/(mg min)) | - | - | 126.88 | 12.72 | - | - | 85.36 | 13.34 |
| V0(mg/(g min)) | - | - | 0.130 | 0.697 | - | - | 0.067 | 0.669 |
| R2 | 0.80706 | 0.77013 | 0.94773 | 0.9303 | 0.8837 | 0.88446 | 0.98627 | 0.96758 |
| χ2 | 4.93 × 10−8 | 5.76 × 10−6 | 1.34 × 10−8 | 1.75 × 10−6 | 5.99 × 10−8 | 2.55 × 10−6 | 7.07 × 10−9 | 7.17 × 10−7 |
| Isotherm | Parameters | Ch–Rs | Ch–Pu |
|---|---|---|---|
| Langmuir | qmax (mg/g) | 0.722 | 0.710 |
| B (L/mg) | 1.551 | 1.712 | |
| RL | 0.06–0.86 | 0.06–0.85 | |
| R2 | 0.997 | 0.999 | |
| χ2 | 8.34 × 10−3 | 8.24 × 10−3 | |
| Freundlich | KF ((mg1−1/n L1/n)/g) | 0.361 | 0.363 |
| n | 2.318 | 2.374 | |
| R2 | 0.953 | 0.938 | |
| χ2 | 2.618 | 2.722 |
| Adsorbent | Initial pH | Qmax (mg/g) | References |
|---|---|---|---|
| Feldspar | 3 | 0.24 | [63] |
| Manganese oxide coated zeolite | 7 | 0.15 | [64] |
| Magnetic iron oxide nanoparticles coated on sand | 7 | 0.29 | [65] |
| Iron-oxide coated sands | 7 | 0.021 | [66] |
| Fe(III)–Sn(IV) mixed oxide-coated sand | 7 | 0.23 | [67] |
| Kaolinite | 0.86 | [68] | |
| Laterite soil | 5.7 | 0.04 | [69] |
| Rice polish | 4 | 0.15 | [70] |
| Modified zeolite Y | 6 | 1.34 | [71] |
| Fish scale | 4 | 0.027 | [72] |
| Bone char | 10 | 1.43 | [73] |
| Red mud | 3.5 | 0.52 | [74] |
| Red mud | 2.3 | 0.51 | [75] |
| Natural Muscovite | 6 | 0.79 | [76] |
| Iron oxide coated sand | 7 | 0.099 | [77] |
| Ch–Rs | 7 | 0.72 | This study |
| Ch–Pu | 7 | 0.71 | This study |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asere, T.G.; Mincke, S.; De Clercq, J.; Verbeken, K.; Tessema, D.A.; Fufa, F.; Stevens, C.V.; Du Laing, G. Removal of Arsenic (V) from Aqueous Solutions Using Chitosan–Red Scoria and Chitosan–Pumice Blends. Int. J. Environ. Res. Public Health 2017, 14, 895. https://doi.org/10.3390/ijerph14080895
Asere TG, Mincke S, De Clercq J, Verbeken K, Tessema DA, Fufa F, Stevens CV, Du Laing G. Removal of Arsenic (V) from Aqueous Solutions Using Chitosan–Red Scoria and Chitosan–Pumice Blends. International Journal of Environmental Research and Public Health. 2017; 14(8):895. https://doi.org/10.3390/ijerph14080895
Chicago/Turabian StyleAsere, Tsegaye Girma, Stein Mincke, Jeriffa De Clercq, Kim Verbeken, Dejene A. Tessema, Fekadu Fufa, Christian V. Stevens, and Gijs Du Laing. 2017. "Removal of Arsenic (V) from Aqueous Solutions Using Chitosan–Red Scoria and Chitosan–Pumice Blends" International Journal of Environmental Research and Public Health 14, no. 8: 895. https://doi.org/10.3390/ijerph14080895