Effects of Visual Stimulation with Bonsai Trees on Adult Male Patients with Spinal Cord Injury
Abstract
:1. Introduction
2. Materials and Methods
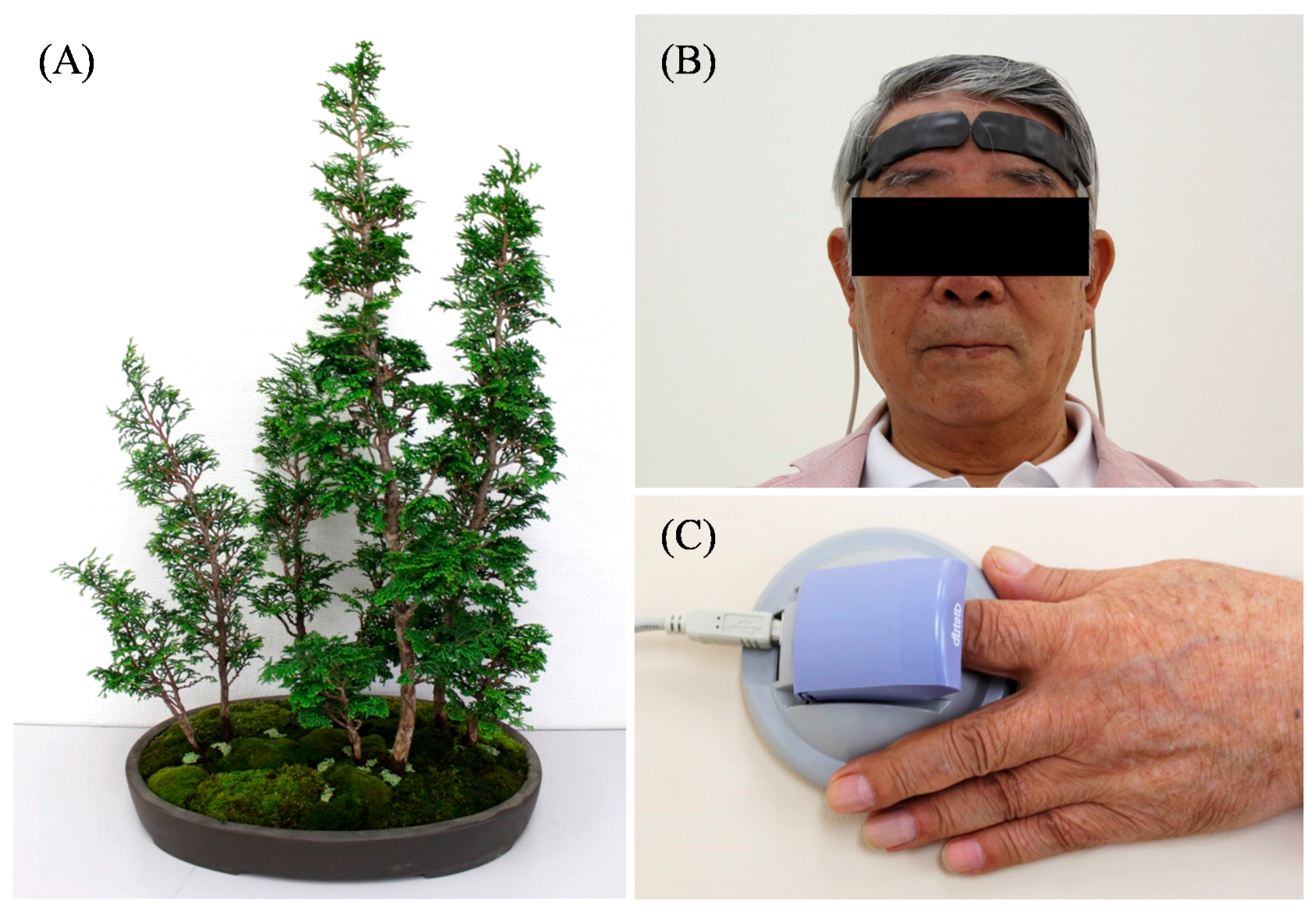
2.1. Experimental Design
2.2. Physiological Indices
2.3. Psychological Indices
2.4. Statistical Analysis
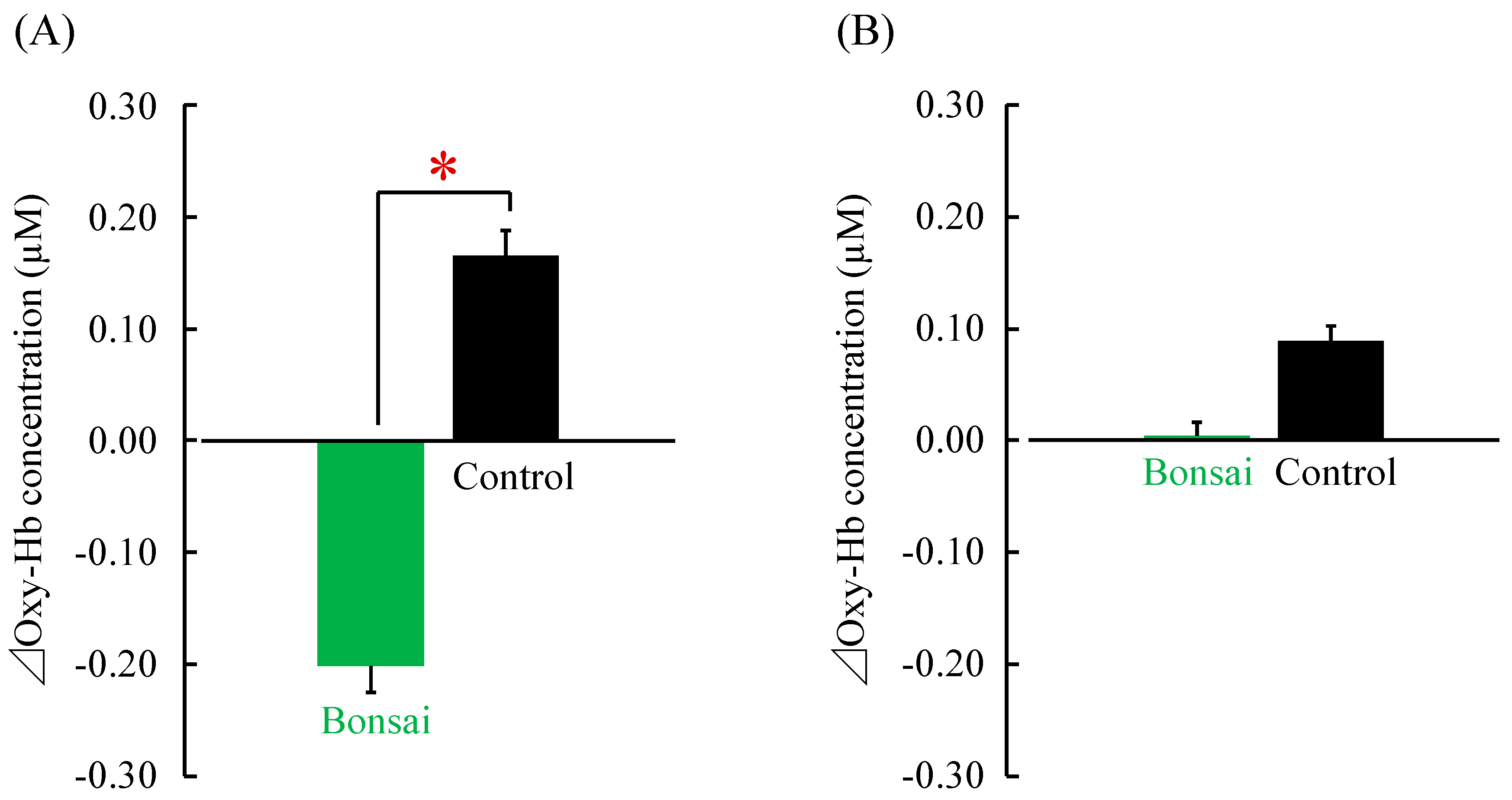
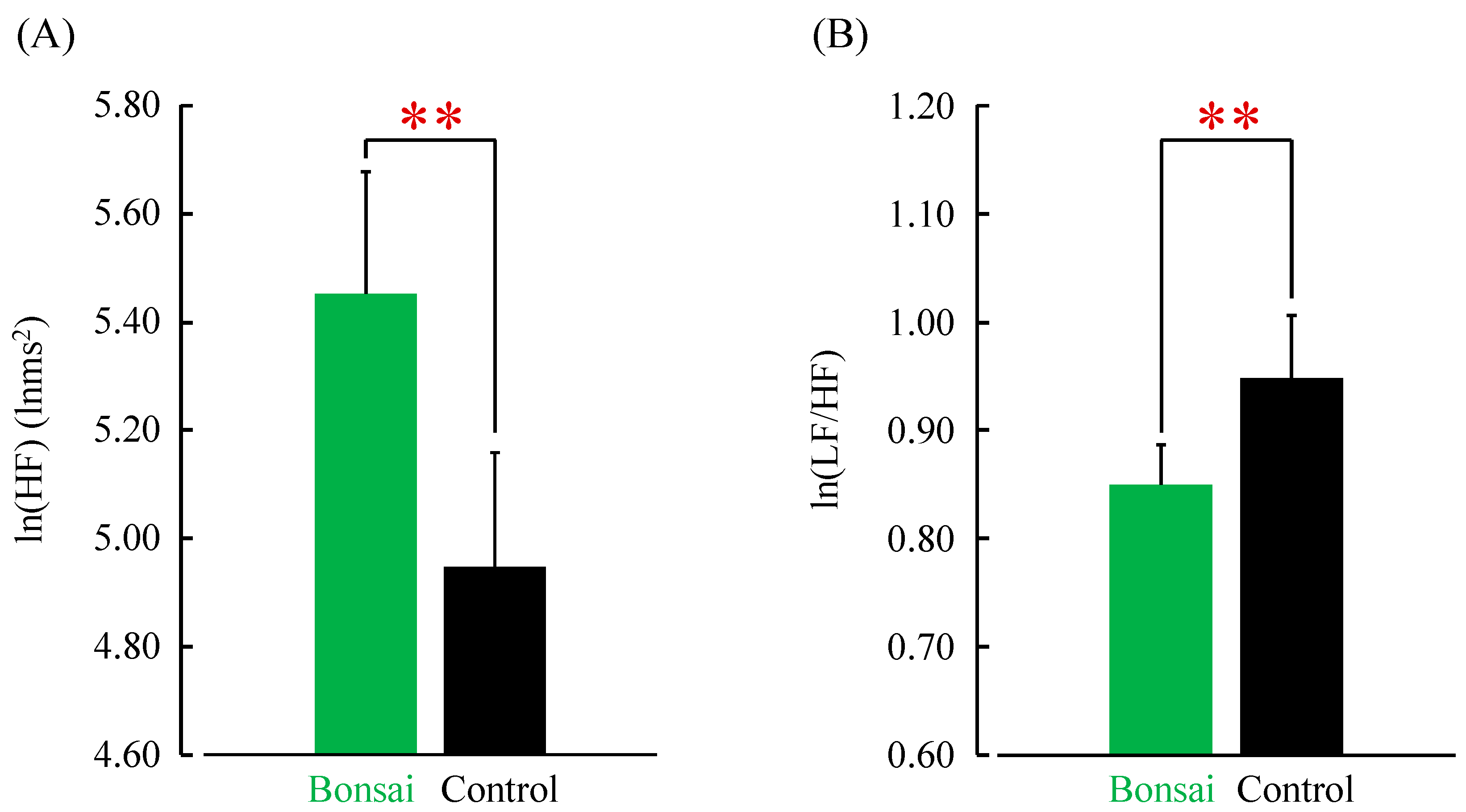
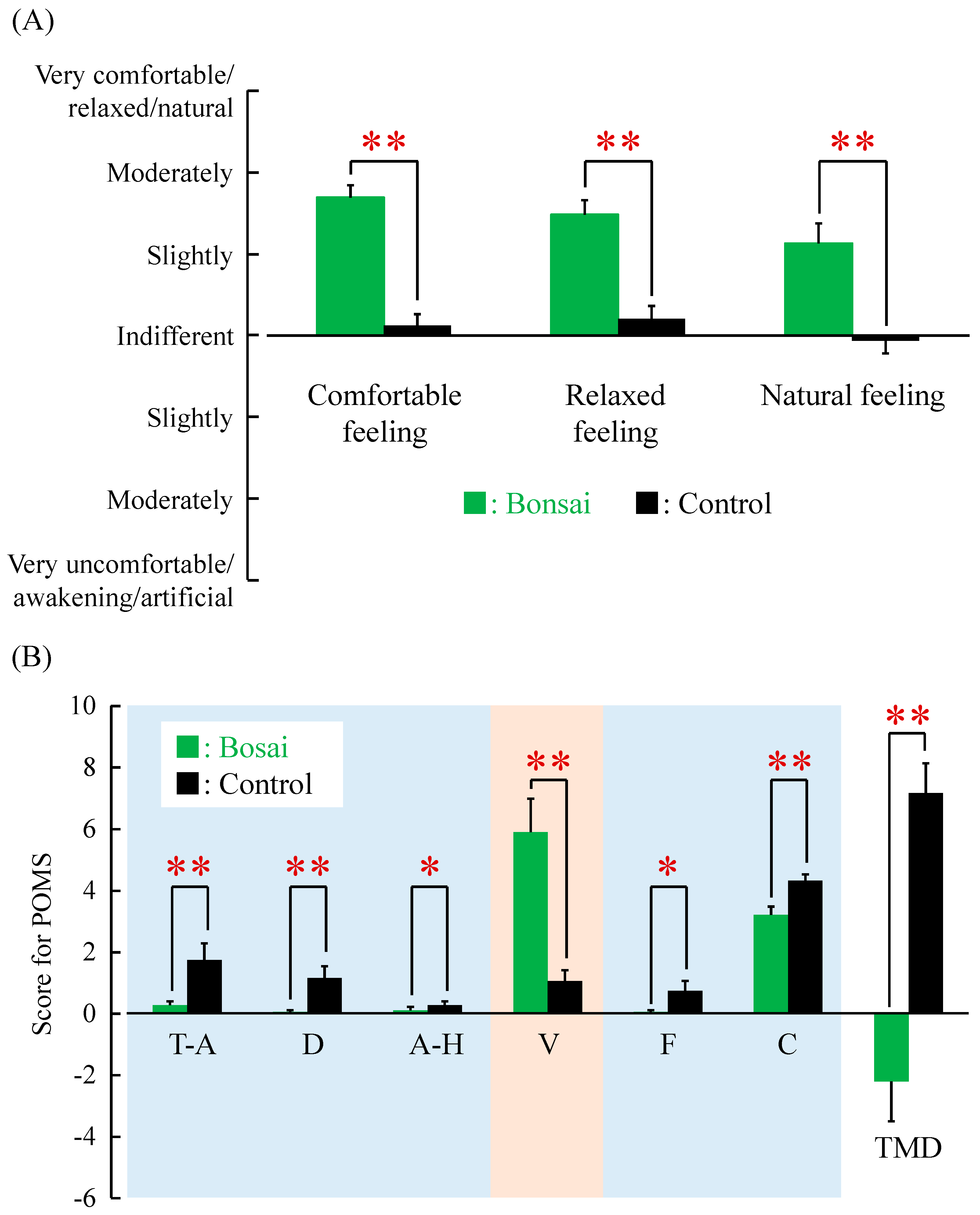
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lederbogen, F.H.; Kirsch, P.; Haddad, L.; Streit, F.; Tost, H.; Schuch, P.; Wüst, S.; Pruessner, J.C.; Rietschel, M.; Deuschle, M.; et al. City living and urban upbringing affect neural social stress processing in humans. Nature 2011, 22, 498–501. [Google Scholar] [CrossRef] [PubMed]
- Annerstedt, M.; Währborg, P. Nature-assisted therapy: Systematic review of controlled and observational studies. Scand. J. Public Health 2011, 39, 371–388. [Google Scholar] [CrossRef] [PubMed]
- Barnicle, T.; Midden, K.S. The effects of a horticulture activity program on the psychological well-being of older people in a long-term care facility. Horttechnology 2003, 13, 81–85. [Google Scholar]
- Lee, Y.; Kim, S. Effects of indoor gardening on sleep, agitation, and cognition in dementia patients—A pilot study. Int. J. Geriatr. Psychiatry 2008, 23, 485–489. [Google Scholar] [CrossRef] [PubMed]
- Park, S.A.; Song, C.; Choi, J.Y.; Son, K.C.; Miyazaki, Y. Foliage plants cause physiological and psychological relaxation-as evidenced by measurements of prefrontal cortex activity and profile of mood states. HortScience 2016, 51, 1308–1312. [Google Scholar] [CrossRef]
- Ikei, H.; Komatsu, M.; Song, C.; Himoro, E.; Miyazaki, Y. The physiological and psychological relaxing effects of viewing rose flowers in office workers. J. Physiol. Anthropol. 2014, 33, 6. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, M.; Aga, M.; Ikei, H.; Namekawa, T.; Miyazaki, Y. Physiological and psychological effects on high school students of viewing real and artificial pansies. Int. J. Environ. Res. Public Health 2015, 12, 2521–2531. [Google Scholar] [CrossRef] [PubMed]
- Van den Berg, A.E.; Custers, M.H.G. Gardening promotes neuroendocrine and affective restoration from stress. Health Psychol. 2011, 16, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Ikei, H.; Miyazaki, Y. Physiological effects of nature therapy: A review of the research in Japan. Int. J. Environ. Res. Public Health 2016, 13, 781. [Google Scholar] [CrossRef] [PubMed]
- Varas-Díaz, G.; Brunetti, E.P.; Rivera-Lillo, G.; Maldonado, P.E. Patients with chronic spinal cord injury exhibit reduced autonomic modulation during an emotion recognition task. Front. Hum. Neurosci. 2017, 11, 59. [Google Scholar] [CrossRef] [PubMed]
- Dryden, D.M.; Saunders, L.D.; Rowe, B.H.; May, L.A.; Yiannakoulias, N.; Svenson, L.W.; Schopflocher, D.P.; Voaklander, D.C. Depression following traumatic spinal cord injury. Neuroepidemiology 2005, 25, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Charlifue, S.; Gerhart, K. Community integration in spinal cord injury of long duration. Neurorehabilitation 2004, 19, 91–101. [Google Scholar] [PubMed]
- Price, G.L.; Kendall, M.; Amsters, D.I.; Pershouse, K.J. Perceived causes of change in function and quality of life for people with long duration spinal cord injury. Clin. Rehabil. 2004, 18, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Depression Following Spinal Cord Injury: A Clinical Practice Guideline for Primary Care Physicians. Available online: http://www.pva.org/CMSPages/GetFile.aspx?guid=cfca724c-8461-40fe-b016-5a57f14a7c72 (accessed on 17 July 2017).
- Donnelly, C.; Eng, J.J.; Hall, J.; Alford, L.; Giachino, R.; Norton, K.; Kerr, D.S. Client-centred assessment and the identification of meaningful treatment goals for individuals with a spinal cord injury. Spinal. Cord. 2004, 42, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Dorstyn, D.; Mathias, J.; Denson, L. Efficacy of cognitive behavior therapy for the management of psychological outcomes following spinal cord injury: A meta-analysis. J. Health Psychol. 2011, 16, 374–391. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Mizuno, T.; Shikayama, T.; Miwa, M. Development of a wireless near infrared tissue oxygen monitor system with high sampling rate. In Digital Holography and Three-Dimensional Imaging; Optical Society of America: Miami, FL, USA, 2012. [Google Scholar]
- Hoshi, Y.; Kobayashi, N.; Tamura, M. Interpretation of near-infrared spectroscopy signals: A study with a newly developed perfused rat brain model. J. Appl. Physiol. 2011, 90, 1657–1662. [Google Scholar]
- Takada, M.; Ebara, T.; Kamijima, M. Heart rate variability assessment in Japanese workers recovered from depressive disorders resulting from job stress: Measurements in the workplace. Int. Arch. Occup. Environ. Health 2010, 83, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Takada, M.; Ebara, T.; Sakai, Y. The acceleration plethysmography system as a new physiological technology for evaluating autonomic modulations. Health Eval. Promot. 2008, 35, 373–377. [Google Scholar] [CrossRef]
- Kanaya, N.; Hirata, N.; Kurosawa, S.; Nakayama, M.; Namiki, A. Differential effects of propofol and sevoflurane on heart rate variability. Anesthesiology 2003, 98, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Camm, A.J.; Malik, M.; Bigger, J.T.; Breithardt, G.; Cerutti, S.; Cohen, R.J.; Coumel, P.; Fallen, E.L.; Kennedy, H.L.; Kleiger, R.E. Heart rate variability: Standards of measurement, physiological interpretation and clinical use. Task force of the European society of cardiology and the North American society of pacing and electrophysiology. Circulation 1996, 93, 1043–1065. [Google Scholar]
- Osgood, C.E.; Suchi, G.J.; Tannenbaum, P. The Measurement of Meaning; University of Illinois Press: Urbana, IL, USA, 1957. [Google Scholar]
- McNair, D.M.; Lorr, M. An analysis of mood in neurotics. J. Abnorm. Psychol. 1964, 69, 620–627. [Google Scholar] [CrossRef] [PubMed]
- McNair, D.M.; Lorr, M.; Droppleman, L. Profile of Mood States Manual; Educational and Industrial Testing Services: San Diego, CA, USA, 1964. [Google Scholar]
- Yokoyama, K. Poms Shortened Version-Manual and Commentary on Cases; Kaneko Syoboh: Tokyo, Japan, 2005. [Google Scholar]
- Park, B.J.; Tsunetsugu, Y.; Kasetani, T.; Hirano, H.; Kagawa, T.; Sato, M.; Miyazaki, Y. Physiological effects of Shinrin-yoku (Taking in the atmosphere of the forest)—Using salivary cortisol and cerebral activity as indicators. J. Physiol. Anthropol. 2007, 26, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, M.; Ikei, H.; Song, C.; Miyazaki, Y. Effects of olfactory stimulation with rose and orange oil on prefrontal cortex activity. Complement. Ther. Med. 2014, 22, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Canli, T.; Desmond, J.E.; Zhao, Z.; Glover, G.; Gabrieli, J.D. Hemispheric asymmetry for emotional stimuli detected with fMRI. Neuroreport 1998, 9, 3233–3239. [Google Scholar] [CrossRef] [PubMed]
- Park, B.J.; Tsunetsugu, Y.; Kasetani, T.; Kagawa, T.; Miyazaki, Y. The physiological effects of Shinrin-yoku (taking in the forest atmosphere or forest bathing): Evidence from field experiments in 24 forests across Japan. Environ. Health Prev. Med. 2010, 15, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Hammell, K.R. Spinal cord injury rehabilitation research: Patient priorities, current deficiencies and potential directions. Disabil. Rehabil. 2010, 32, 1209–1218. [Google Scholar] [CrossRef] [PubMed]
- Gómara-Toldrà, N.; Sliwinski, M.; Dijkers, M.P. Physical therapy after spinal cord injury: A systematic review of treatments focused on participation. J. Spinal Cord Med. 2014, 37, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Ochiai, H.; Ikei, H.; Song, C.; Kobayashi, M.; Takamatsu, A.; Miura, T.; Kagawa, T.; Li, Q.; Kumeda, S.; Imai, M.; et al. Physiological and psychological effects of forest therapy on middle-aged males with high-normal blood pressure. Int. J. Environ. Res. Public Health 2015, 12, 2532–2542. [Google Scholar] [CrossRef] [PubMed]
- Ochiai, H.; Ikei, H.; Song, C.; Kobayashi, M.; Miura, T.; Kagawa, T.; Li, Q.; Kumeda, S.; Imai, M.; Miyazaki, Y. Physiological and psychological effects of a forest therapy program on middle-aged females. Int. J. Environ. Res. Public Health 2015, 12, 15222–15232. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Morimoto, K.; Nakadai, A.; Inagaki, H.; Katsumata, M.; Shimizu, T.; Hirata, Y.; Hirata, K.; Suzuki, H.; Miyazaki, Y.; et al. Forest bathing enhances human natural killer activity and expression of anti-cancer proteins. Int. J. Immunopathol. Pharmacol. 2007, 20, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Morimoto, K.; Kobayashi, M.; Inagaki, H.; Katsumata, M.; Hirata, Y.; Hirata, K.; Suzuki, H.; Li, Y.J.; Wakayama, Y.; et al. Visiting a forest, but not a city, increases human natural killer activity and expression of anti-cancer proteins. Int. J. Immunopathol. Pharmacol. 2008, 21, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Morimoto, K.; Kobayashi, M.; Inagaki, H.; Katsumata, M.; Hirata, Y.; Hirata, K.; Shimizu, T.; Li, Y.J.; Wakayama, Y.; et al. A forest bathing trip increases human natural killer activity and expression of anti-cancer proteins in female subjects. J. Biol. Regul. Homeost. Agents 2008, 22, 45–55. [Google Scholar] [PubMed]
- Park, B.J.; Furuya, K.; Kasetani, T.; Takayama, N.; Kagawa, T.; Miyazaki, Y. Relationship between psychological responses and physical environments in forest settings. Landsc. Urban Plan. 2011, 102, 24–32. [Google Scholar] [CrossRef]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ochiai, H.; Song, C.; Ikei, H.; Imai, M.; Miyazaki, Y. Effects of Visual Stimulation with Bonsai Trees on Adult Male Patients with Spinal Cord Injury. Int. J. Environ. Res. Public Health 2017, 14, 1017. https://doi.org/10.3390/ijerph14091017
Ochiai H, Song C, Ikei H, Imai M, Miyazaki Y. Effects of Visual Stimulation with Bonsai Trees on Adult Male Patients with Spinal Cord Injury. International Journal of Environmental Research and Public Health. 2017; 14(9):1017. https://doi.org/10.3390/ijerph14091017
Chicago/Turabian StyleOchiai, Hiroko, Chorong Song, Harumi Ikei, Michiko Imai, and Yoshifumi Miyazaki. 2017. "Effects of Visual Stimulation with Bonsai Trees on Adult Male Patients with Spinal Cord Injury" International Journal of Environmental Research and Public Health 14, no. 9: 1017. https://doi.org/10.3390/ijerph14091017





