Evaluation of a Pilot Implementation to Integrate Alcohol-Related Care within Primary Care
Abstract
:1. Introduction
2. Materials and Methods
2.1. Setting
2.2. The SPARC Program
2.3. SPARC Implementation Strategies
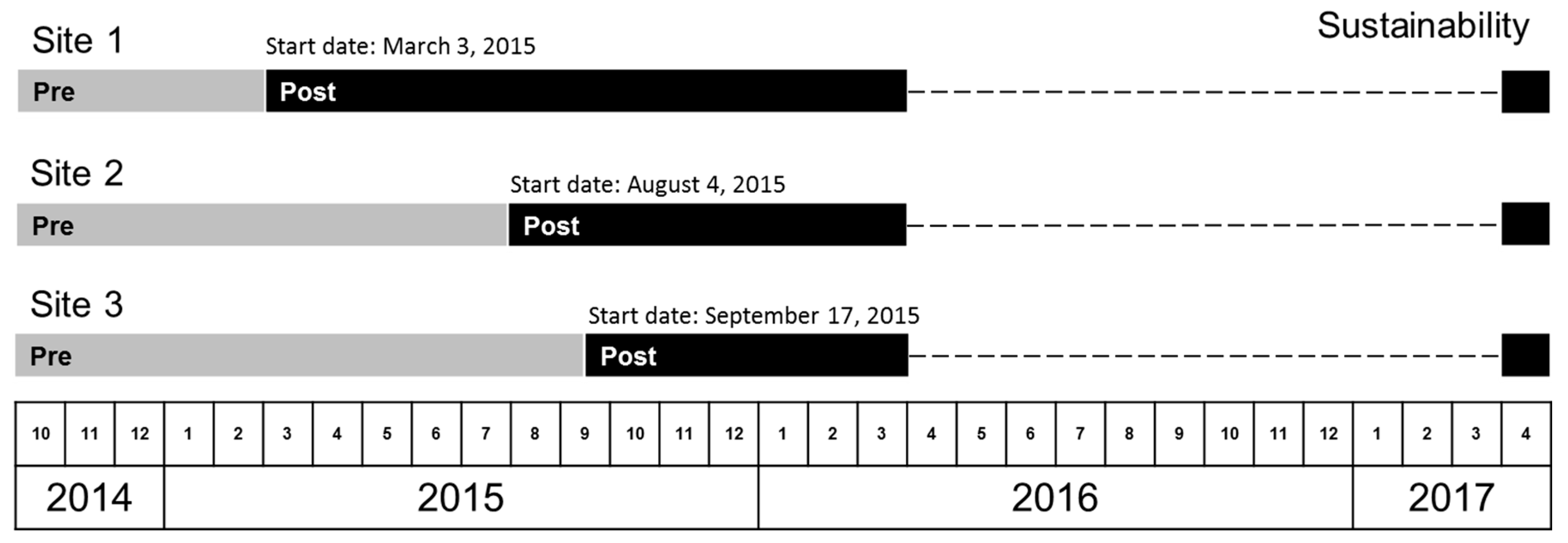
2.4. Planned Timeline of Implementation
2.5. Quantitative Metrics
2.5.1. Alcohol Screening
2.5.2. Assessment for DSM-5 AUD Symptoms
2.5.3. New AUD Diagnosis and Treatment
2.6. Statistical Analysis
2.6.1. Descriptive Analyses
2.6.2. Time Series Analyses
2.6.3. Pre-Versus Post-Implementation Analyses
2.6.4. Sustainability
2.7. Implementation-Focused Formative Evaluation
3. Results
3.1. Pilot Sites Selected by Health System Leaders
3.2. Study Sample
3.3. Quantitative Comparison of Care before versus after Implementation
3.3.1. Alcohol Screening
3.3.2. Assessment for DSM-5 AUD Symptoms
3.3.3. New AUD Diagnosis and Treatment
3.3.4. Sensitivity Analyses
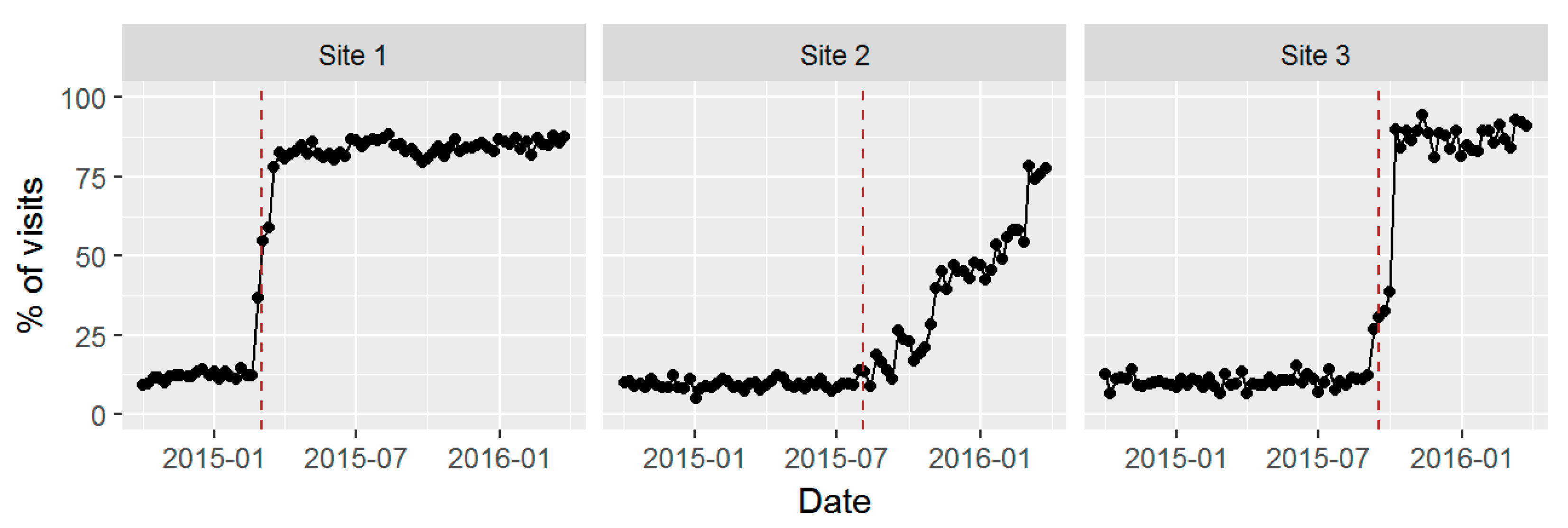
3.4. Sustained Screening after Active Support for Implementation Ended
3.5. Results of Formative Evaluation
3.5.1. Implementing BHI Did Not Lengthen Patient Visit Time
3.5.2. Need for Active Practice Facilitation
3.5.3. Immense Value of Using Stories to Increase Engagement
3.5.4. Implementing SPARC Clinical Care in the Context of BHI May Have Facilitated Adoption
3.5.5. Training Social Workers to Manage Addictions in PC before BHI Implementation
3.5.6. Development of an Alcohol Video to Address Stigma
3.5.7. Anecdotal Increases in Staff Satisfaction
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rehm, J.; Mathers, C.; Popova, S.; Thavorncharoensap, M.; Teerawattananon, Y.; Patra, J. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet 2009, 373, 2223–2233. [Google Scholar] [CrossRef]
- Saitz, R. Clinical practice. Unhealthy alcohol use. N. Engl. J. Med. 2005, 352, 596–607. [Google Scholar] [CrossRef] [PubMed]
- Bryson, C.L.; Au, D.H.; Sun, H.; Williams, E.C.; Kivlahan, D.R.; Bradley, K.A. Alcohol screening scores and medication nonadherence. Ann. Intern. Med. 2008, 149, 795–804. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.T.; Karter, A.J.; Warton, E.M.; Doan, J.U.; Weisner, C.M. The relationship between alcohol consumption and glycemic control among patients with diabetes: The Kaiser Permanente Northern California Diabetes Registry. J. Gen. Intern. Med. 2008, 23, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Jonas, D.E.; Garbutt, J.C.; Amick, H.R.; Brown, J.M.; Brownley, K.A.; Council, C.L.; Viera, A.J.; Wilkins, T.M.; Schwartz, C.J.; Richmond, E.M.; et al. Behavioral counseling after screening for alcohol misuse in primary care: A systematic review and meta-analysis for the U.S. Preventive Services Task force. Ann. Intern. Med. 2012, 157, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Moyer, V.A. Preventive services task force, screening and behavioral counseling interventions in primary care to reduce alcohol misuse: U.S. preventive services task force recommendation statement. Ann. Intern. Med. 2013, 159, 210–218. [Google Scholar] [PubMed]
- Latimer, N.; Guillaume, L.; Goyder, E.; Chilcott, J.; Payne, N. Alcohol Use Disorders—Preventing Harmful Drinking. Screening and Brief Interventions: Cost Effectiveness Review. Available online: https://www.google.com.hk/url?sa=t&rct=j&q=&esrc=s&source=web&cd=3&ved=0ahUKEwjC8v-BrYPWAhXMKcAKHTNYBrEQFgg7MAI&url=https%3A%2F%2Farms.evidence.nhs.uk%2Fresources%2Fhub%2F1033186%2Fattachment&usg=AFQjCNGSYH_4_4YDuGWELWDZfTjcbc_8Ow (accessed on 9 September 2010).
- Fleming, M.F.; Barry, K.L.; Manwell, L.B.; Johnson, K.; London, R. Brief physician advice for problem alcohol drinkers. A randomized controlled trial in community-based primary care practices. JAMA 1997, 277, 1039–1045. [Google Scholar] [CrossRef] [PubMed]
- Fleming, M.F.; Mundt, M.P.; French, M.T.; Manwell, L.B.; Stauffacher, E.A.; Barry, K.L. Benefit-cost analysis of brief physician advice with problem drinkers in primary care settings. Med. Care 2000, 38, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Fleming, M.F.; Mundt, M.P.; French, M.T.; Manwell, L.B.; Stauffacher, E.A.; Barry, K.L. Brief physician advice for problem drinkers: Long-term efficacy and benefit-cost analysis. Alcohol Clin. Exp. Res. 2002, 26, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Maciosek, M.V.; Coffield, A.B.; Edwards, N.M.; Flottemesch, T.J.; Goodman, M.J.; Solberg, L.I. Priorities among effective clinical preventive services results of a systematic review and analysis. Am. J. Prev. Med. 2006, 31, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Solberg, L.I.; Maciosek, M.V.; Edwards, N.M. Primary care intervention to reduce alcohol misuse ranking its health impact and cost effectiveness. Am. J. Prev. Med. 2008, 34, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Jonas, D.E.; Amick, H.R.; Feltner, C.; Bobashev, G.; Thomas, K.; Wines, R.; Kim, M.M.; Shanahan, E.; Gass, C.E.; Rowe, C.J.; et al. Pharmacotherapy for adults with alcohol use disorders in outpatient settings: A systematic review and meta-analysis. JAMA 2014, 311, 1889–1900. [Google Scholar] [CrossRef] [PubMed]
- Bouza, C.; Angeles, M.; Munoz, A.; Amate, J.M. Efficacy and safety of naltrexone and acamprosate in the treatment of alcohol dependence: A systematic review. Addiction 2004, 99, 811–828. [Google Scholar] [PubMed]
- Berglund, M. A better widget? Three lessons for improving addiction treatment from a meta-analytical study. Addiction 2005, 100, 742–750. [Google Scholar] [CrossRef] [PubMed]
- Rosner, S.; Hackl-Herrwerth, A.; Leucht, S.; Lehert, P.; Vecchi, S.; Soyka, M. Acamprosate for alcohol dependence. Cochrane Database Syst. Rev. 2010. [Google Scholar] [CrossRef]
- Rosner, S.; Hackl-Herrwerth, A.; Leucht, S.; Vecchi, S.; Srisurapanont, M.; Soyka, M. Opioid antagonists for alcohol dependence. Cochrane Database Syst. Rev. 2010. [Google Scholar] [CrossRef]
- Dunn, C.; Deroo, L.; Rivara, F.P. The use of brief interventions adapted from motivational interviewing across behavioral domains: A systematic review. Addiction 2001, 96, 1725–1742. [Google Scholar] [CrossRef] [PubMed]
- Mann, K.V. The role of educational theory in continuing medical education: Has it helped us? J. Contin. Educ. Health Prof. 2004, 24 (Suppl. 1), S22–S30. [Google Scholar] [CrossRef] [PubMed]
- Glasner-Edwards, S.; Rawson, R. Evidence-based practices in addiction treatment: Review and recommendations for public policy. Health Policy 2010, 97, 93–104. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Clinical Effectiveness. In Alcohol-Use Disorders: Diagnosis, Assessment and Managment of Harmful Drinking and Alcohol Dependence; UK National Health Service (NHS): London, UK, 2011.
- VA Office of Quality and Performance VA/DoD Clinical Practice Guideline for the Management of Substance Use Disorders. Version 2.0. Available online: http://www.healthquality.va.gov/guidelines/mh/ sud/index.asp (accessed on 11 April 2014).
- National Quality Forum. National Voluntary Consensus Standards for the Treatment of Substance Use Conditions: Evidence-Based Treatment Practices; National Quality Forum: Washington, DC, USA, 2007. [Google Scholar]
- Glass, J.E.; Bohnert, K.M.; Brown, R.L. Alcohol screening and intervention among United States adults who attend ambulatory healthcare. J. Gen. Intern. Med. 2016, 31, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Oslin, D.W.; Lynch, K.G.; Maisto, S.A.; Lantinga, L.J.; McKay, J.R.; Possemato, K.; Ingram, E.; Wierzbicki, M. A randomized clinical trial of alcohol care management delivered in Department of Veterans Affairs primary care clinics versus specialty addiction treatment. J. Gen. Intern. Med. 2014, 29, 162–168. [Google Scholar] [CrossRef] [PubMed]
- McGlynn, E.A.; Asch, S.M.; Adams, J.L.; Keesey, J.; Hicks, J.; DeCristofaro, A.; Kerr, E.A. The quality of health care delivered to adults in the United States. N. Engl. J. Med. 2003, 348, 2635–2645. [Google Scholar] [CrossRef] [PubMed]
- Willenbring, M.L.; Massey, S.H.; Gardner, M.B. Helping patients who drink too much: An evidence-based guide for primary care clinicians. Am. Fam. Physician 2009, 80, 44–50. [Google Scholar] [PubMed]
- Watkins, K.; Pincus, H.A.; Tanielian, T.L.; Lloyd, J. Using the chronic care model to improve treatment of alcohol use disorders in primary care settings. J. Stud. Alcohol 2003, 64, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Saitz, R.; Larson, M.J.; Labelle, C.; Richardson, J.; Samet, J.H. The case for chronic disease management for addiction. J. Addict. Med. 2008, 2, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Bradley, K.A.; Kivlahan, D.R. Bringing patient-centered care to patients with alcohol use disorders. JAMA 2014, 311, 1861–1862. [Google Scholar] [CrossRef] [PubMed]
- Bradley, K.A.; Williams, E.C.; Achtmeyer, C.E.; Volpp, B.; Collins, B.J.; Kivlahan, D.R. Implementation of evidence-based alcohol screening in the Veterans Health Administration. Am. J. Manag. Care 2006, 12, 597–606. [Google Scholar] [PubMed]
- Lapham, G.T.; Achtmeyer, C.E.; Williams, E.C.; Hawkins, E.J.; Kivlahan, D.R.; Bradley, K.A. Increased documented brief alcohol interventions with a performance measure and electronic decision support. Med. Care 2012, 50, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Mertens, J.R.; Chi, F.W.; Weisner, C.M.; Satre, D.D.; Ross, T.B.; Allen, S.; Pating, D.; Campbell, C.I.; Lu, Y.W.; Sterling, S.A. Physician versus non-physician delivery of alcohol screening, brief intervention and referral to treatment in adult primary care: The ADVISe cluster randomized controlled implementation trial. Addict. Sci. Clin. Pract. 2015, 10, 26. [Google Scholar] [CrossRef] [PubMed]
- Chi, F.W.; Weisner, C.M.; Mertens, J.R.; Ross, T.B.; Sterling, S.A. Alcohol brief intervention in primary care: Blood pressure outcomes in hypertensive patients. J. Subst. Abuse Treat. 2017, 77, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Williams, E.C.; Johnson, M.L.; Lapham, G.T.; Caldeiro, R.M.; Chew, L.; Fletcher, G.S.; McCormick, K.A.; Weppner, W.G.; Bradley, K.A. Strategies to implement alcohol screening and brief intervention in primary care settings: A structured literature review. Psychol. Addict. Behav. 2011, 25, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Glass, J.E.; Kristjansson, S.D.; Bucholz, K.K. Perceived alcohol stigma: Factor structure and construct validation. Alcohol. Clin. Exp. Res. 2013, 37 (Suppl. 1), E237–E246. [Google Scholar] [CrossRef] [PubMed]
- Williams, E.C.; Achtmeyer, C.E.; Young, J.P.; Rittmueller, S.E.; Ludman, E.J.; Lapham, G.T.; Lee, A.K.; Chavez, L.J.; Berger, D.; Bradley, K.A. Local implementation of alcohol screening and brief intervention at five veterans health administration primary care clinics: Perspectives of clinical and administrative staff. J. Subst. Abuse Treat. 2016, 60, 27–35. [Google Scholar] [CrossRef] [PubMed]
- McCormick, K.A.; Cochran, N.E.; Back, A.L.; Merrill, J.O.; Williams, E.C.; Bradley, K.A. How primary care providers talk to patients about alcohol: A qualitative study. J Gen Intern Med. 2006, 21, 966–972, PMCID:1831591. [Google Scholar] [CrossRef]
- Spandorfer, J.M.; Israel, Y.; Turner, B.J. Primary care physicians’ views on screening and management of alcohol abuse: Inconsistencies with national guidelines. J. Fam. Pract. 1999, 48, 899–902. [Google Scholar] [PubMed]
- Bradley, K.A.; Williams, E.C. Implementation of SBI in Clinical Settings Using Quality Improvement Principles. In Principles of Addiction Medicine, 5th ed.; Ries, R.K., Miller, S.C., Fiellin, D.A., Saitz, R., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013. [Google Scholar]
- Bradley, K.A.; Lapham, G.T.; Hawkins, E.J.; Achtmeyer, C.E.; Williams, E.C.; Thomas, R.M.; Kivlahan, D.R. Quality concerns with routine alcohol screening in VA clinical settings. J. Gen. Intern. Med. 2011, 26, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Chavez, L.J.; Williams, E.C.; Lapham, G.T.; Rubinsky, A.D.; Kivlahan, D.R.; Bradley, K.A. Changes in patient-reported alcohol-related advice following veterans health administration implementation of brief alcohol interventions. J. Stud. Alcohol. Drugs 2016, 77, 500–508. [Google Scholar] [CrossRef] [PubMed]
- Berger, D.; Lapham, G.T.; Shortreed, S.; Hawkins, E.J.; Rubinsky, A.D.; Williams, E.C.; Achtmeyer, C.E.; Kivlahan, D.R.; Bradley, K.A. Increasing rates of documented alcohol advice: More advice or just more documentation? J. Gen. Intern. Med. 2017, in press. [Google Scholar]
- Reynolds, C.F.; Frank, E. US Preventive services task force recommendation statement on screening for depression in adults: Not good enough. JAMA Psychiatry 2016, 73, 189–190. [Google Scholar] [CrossRef] [PubMed]
- Kroenke, K.; Spitzer, R.L. The PHQ-9: A new depression diagnostic and severity measure. Psychiatr. Ann. 2002, 32, 509–515. [Google Scholar] [CrossRef]
- Lowe, B.; Schenkel, I.; Carney-Doebbeling, C.; Gobel, C. Responsiveness of the PHQ-9 to psychopharmacological depression treatment. Psychosomatics 2006, 47, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Bradley, K.A.; DeBenedetti, A.F.; Volk, R.J.; Williams, E.C.; Frank, D.; Kivlahan, D.R. AUDIT-C as a brief screen for alcohol misuse in primary care. Alcohol Clin. Exp. Res. 2007, 31, 1208–1217. [Google Scholar] [CrossRef] [PubMed]
- Lapham, G.; Lee, A.; Caldeiro, R.; McCarty, D.; Browne, K.; Walker, D.; Kivlahan, D.; Bradley, K. Frequency of cannabis use among primary care patients in Washington state where use is legal. J. Am. Board Fam. Med. 2017, in press. [Google Scholar]
- Smith, P.C.; Schmidt, S.M.; Allensworth-Davies, D.; Saitz, R. A single-question screening test for drug use in primary care. Arch. Intern. Med. 2010, 170, 1155–1160. [Google Scholar] [CrossRef] [PubMed]
- National Institute on Alcohol Abuse and Alcoholism. Helping Patients Who Drink Too Much: A Clinician’s Guide (Updated 2005 Edition); NIH Publication 07-3769; National Institutes of Health, U.S. Department of Health and Human Services: Washington, DC, USA, 2005.
- Whitlock, E.P.; Polen, M.R.; Green, C.A.; Orleans, T.; Klein, J. Behavioral counseling interventions in primary care to reduce risky/harmful alcohol use by adults: A summary of the evidence for the U.S. Preventive Services Task Force. Ann. Intern. Med. 2004, 140, 557–568. [Google Scholar] [CrossRef] [PubMed]
- National Committee for Quality Assurance. HEDIS 2011 Technical Specifications; National Committee for Quality Assurance: Washington, DC, USA, 2011; Volume 2. [Google Scholar]
- Bradley, K.A. Preparing Clinicians and Patients for Shared Decision-making about Alcohol Use Disorders: Development of an Entertaining Video (The Mike Evans Video Project); Group Health Development Fund, Group Health Research Institute: Seattle, WA, USA, 31 December 2014–1 January 2014.
- Bradley, K.A.; Caldeiro, R. Alcohol and Drug Use Disorders: 3 Visits in 30 Days (The 3:30 Project); Partnership for Innovation, Group Health Research Institute: Seattle, WA, USA, 1 July 2014–30 June 2015.
- Whooley, M.A.; Avins, A.L.; Miranda, J.; Browner, W.S. Case finding instruments for depression. Two questions are as good as many. J. Gen. Intern. Med. 1997, 12, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Rubinsky, A.D.; Dawson, D.A.; Williams, E.C.; Kivlahan, D.R.; Bradley, K.A. AUDIT-C scores as a scaled marker of mean daily drinking, alcohol use disorder severity, and probability of alcohol dependence in a U.S. general population sample of drinkers. Alcohol Clin. Exp. Res. 2013, 37, 1380–1390. [Google Scholar] [CrossRef] [PubMed]
- Rubinsky, A.D.; Kivlahan, D.R.; Volk, R.J.; Maynard, C.; Bradley, K.A. Estimating risk of alcohol dependence using alcohol screening scores. Drug Alcohol Depend. 2010, 108, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.A.; Lee, A.; Vinson, D.; Seale, J.P. Use of AUDIT-based measures to identify unhealthy alcohol use and alcohol dependence in primary care: A validation study. Alcohol Clin. Exp. Res. 2013, 37 (Suppl. 1), E253–E259. [Google Scholar] [CrossRef] [PubMed]
- Hasin, D.S.; O’Brien, C.P.; Auriacombe, M.; Borges, G.; Bucholz, K.; Budney, A.; Compton, W.M.; Crowley, T.; Ling, W.; Petry, N.M.; et al. DSM-5 criteria for substance use disorders: Recommendations and rationale. Am. J. Psychiatry 2013, 170, 834–851. [Google Scholar] [CrossRef] [PubMed]
- Bradley, K.A.; Chavez, L.J.; Lapham, G.T.; Williams, E.C.; Achtmeyer, C.E.; Rubinsky, A.D.; Hawkins, E.J.; Saitz, R.; Kivlahan, D.R. When quality indicators undermine quality: Bias in a quality indicator of follow-up for alcohol misuse. Psychiatr. Serv. 2013, 64, 1018–1025. [Google Scholar] [CrossRef] [PubMed]
- Zeger, S.L.; Liang, K.Y. Longitudinal data analysis for discrete and continuous outcomes. Biometrics 1986, 42, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Stetler, C.B.; Legro, M.W.; Wallace, C.M.; Bowman, C.; Guihan, M.; Hagedorn, H.; Kimmel, B.; Sharp, N.D.; Smith, J.L. The role of formative evaluation in implementation research and the QUERI experience. J. Gen. Intern. Med. 2006, 21 (Suppl. S2), S1–S8. [Google Scholar] [CrossRef] [PubMed]
- King, N. Template Analysis. In Qualitative Methods and Analysis in Organizational Research; Symon, G., Cassell, C., Eds.; Sage Publications: London, UK, 1998; pp. 118–134. [Google Scholar]
- Greenhalgh, T.; Robert, G.; Macfarlane, F.; Bate, P.; Kyriakidou, O. Diffusion of innovations in service organizations: Systematic review and recommendations. Milbank Q. 2004, 82, 581–629. [Google Scholar] [CrossRef] [PubMed]
- Batalden, P.B.; Nelson, E.C.; Edwards, W.H.; Godfrey, M.M.; Mohr, J.J. Microsystems in health care: Part 9. Developing small clinical units to attain peak performance. Jt. Comm. J. Qual. Saf. 2003, 29, 575–585. [Google Scholar] [CrossRef]
- Elwyn, G.; Dehlendorf, C.; Epstein, R.M.; Marrin, K.; White, J.; Frosch, D.L. Shared decision making and motivational interviewing: Achieving patient-centered care across the spectrum of health care problems. Ann. Fam. Med. 2014, 12, 270–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wallace, P.; Cutler, S.; Haines, A. Randomised controlled trial of general practitioner intervention in patients with excessive alcohol consumption. BMJ 1988, 297, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Coulehan, J.L.; Zettler-Segal, M.; Block, M.; McClelland, M.; Schulberg, H.C. Recognition of alcoholism and substance abuse in primary care patients. Arch. Intern. Med. 1987, 147, 349–352. [Google Scholar] [CrossRef] [PubMed]
- Buchsbaum, D.G.; Buchanan, R.G.; Poses, R.M.; Schnoll, S.H.; Lawton, M.J. Physician detection of drinking problems in patients attending a general medicine practice. J. Gen. Intern. Med. 1992, 7, 517–521. [Google Scholar] [CrossRef] [PubMed]
- Barnes, H.N.; Aronson, M.D.; Delbanco, T.L. Alcoholism—A Guide for the Primary Care Physician; Springer: New York, NY, USA, 1987; pp. 1–231. [Google Scholar]
- Buchsbaum, D.G.; Buchanan, R.G.; Lawton, M.J.; Elswick, R.K., Jr.; Schnoll, S.H. A program of screening and prompting improves short-term physician counseling of dependent and nondependent harmful drinkers. Arch. Intern. Med. 1993, 153, 1573–1577. [Google Scholar] [CrossRef] [PubMed]
- Grant, B.F.; Goldstein, R.B.; Saha, T.D.; Chou, S.P.; Jung, J.; Zhang, H.; Pickering, R.P.; Ruan, W.J.; Smith, S.M.; Huang, B.; et al. Epidemiology of DSM-5 alcohol use disorder: Results from the national epidemiologic survey on alcohol and related conditions III. JAMA Psychiatry 2015, 72, 757–766. [Google Scholar] [CrossRef] [PubMed]


| Strategy #1: Enabling Primary Care (PC) Teams to Offer High-Quality Alcohol-Related Care |
| Components: |
|
| Strategy #2: Supporting PC Teams through Electronic Health Record (EHR) Decision Support |
| Components: |
|
| Strategy #3: Systematic Monitoring and Feedback of Performance Measures Including Alcohol Screening and AUD Assessment Rates |
| Components: |
|
| Measure | Pre (%) | Post (%) | p Value a |
|---|---|---|---|
| (N = 32,295) | (N = 39,599) | ||
| Male * | 38.0 | 40.1 | < 0.0001 |
| Race ** | < 0.0001 | ||
| Asian | 5.9 | 5.1 | |
| Black | 2.4 | 2.3 | |
| Other/Multiracial | 6.9 | 6.6 | |
| White | 82.1 | 83.1 | |
| Unknown | 2.8 | 2.9 | |
| Hispanic * | 0.022 | ||
| No | 92.2 | 92.1 | |
| Yes | 5.1 | 4.9 | |
| Unknown | 2.7 | 3 | |
| Age ***, mean (IQR) | 54.5 (40, 68) | 55.4 (42, 68) | < 0.0001 |
| Measure | Pre (%) | Post (%) | p Value * |
|---|---|---|---|
| (N = 32,295) | (N = 39,599) | ||
| Screened for unhealthy alcohol use | 8.9 | 62 | <0.0001 |
| Positive screen | 2.2 | 17 | <0.0001 |
| High risk unhealthy alcohol use | 0.31 | 1.4 | <0.0001 |
| Assessed for AUDs | 0.012 | 0.75 | <0.0001 |
| AUD (2 + symptoms) | 0.0062 | 0.40 | <0.0001 |
| New AUD diagnosis ** | 0.39 | 0.58 | 0.0002 |
| AUD treatment among patients with new AUDs ** | |||
| Within 14 days | 0.065 | 0.10 | 0.083 |
| Within 30 days | 0.087 | 0.14 | 0.034 |
| Within 90 days | 0.110 | 0.18 | 0.024 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bobb, J.F.; Lee, A.K.; Lapham, G.T.; Oliver, M.; Ludman, E.; Achtmeyer, C.; Parrish, R.; Caldeiro, R.M.; Lozano, P.; Richards, J.E.; et al. Evaluation of a Pilot Implementation to Integrate Alcohol-Related Care within Primary Care. Int. J. Environ. Res. Public Health 2017, 14, 1030. https://doi.org/10.3390/ijerph14091030
Bobb JF, Lee AK, Lapham GT, Oliver M, Ludman E, Achtmeyer C, Parrish R, Caldeiro RM, Lozano P, Richards JE, et al. Evaluation of a Pilot Implementation to Integrate Alcohol-Related Care within Primary Care. International Journal of Environmental Research and Public Health. 2017; 14(9):1030. https://doi.org/10.3390/ijerph14091030
Chicago/Turabian StyleBobb, Jennifer F., Amy K. Lee, Gwen T. Lapham, Malia Oliver, Evette Ludman, Carol Achtmeyer, Rebecca Parrish, Ryan M. Caldeiro, Paula Lozano, Julie E. Richards, and et al. 2017. "Evaluation of a Pilot Implementation to Integrate Alcohol-Related Care within Primary Care" International Journal of Environmental Research and Public Health 14, no. 9: 1030. https://doi.org/10.3390/ijerph14091030




