Migration and Accumulation of Octachlorodipropyl Ether in Soil-Tea Systems in Young and Old Tea Gardens
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Instruments
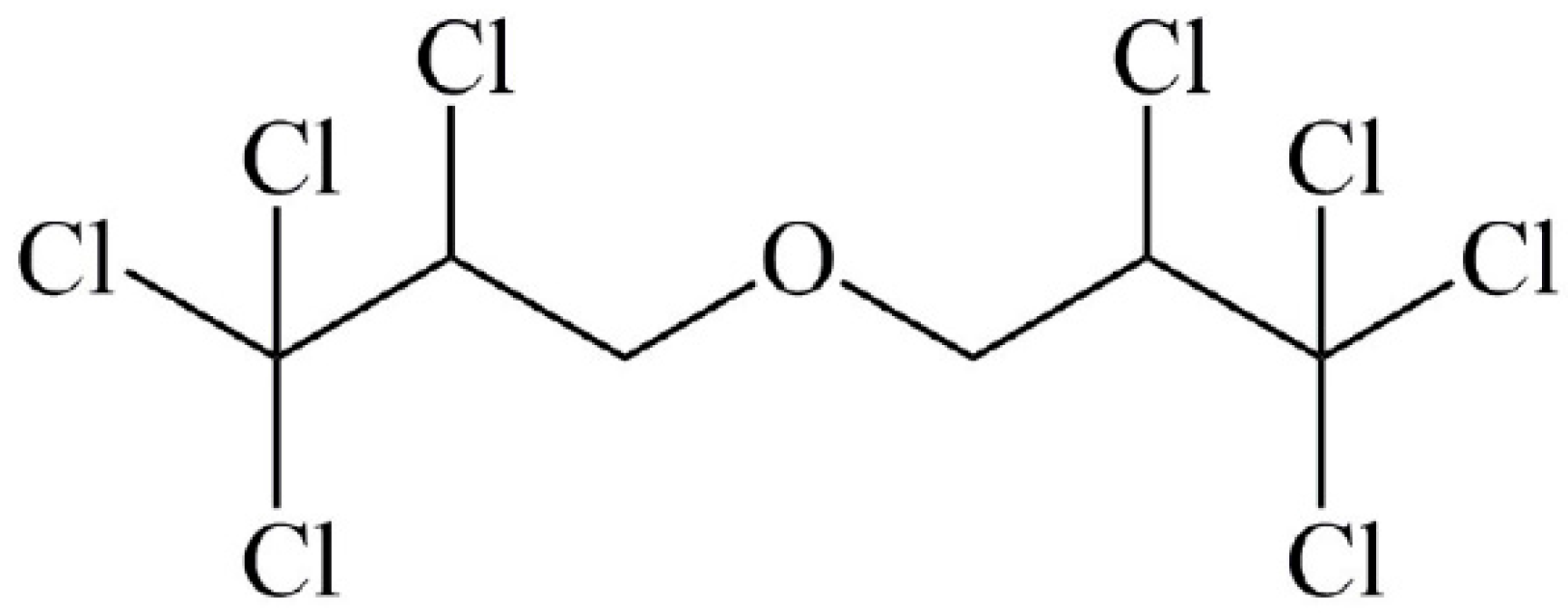
2.2. Preparation of 10% OCDPE Emulsifiable Concentrate (EC)
2.3. Migration and Accumulation Experiment of OCDPE in Soil-Tea Systems
2.4. Sample Extraction and Clean-Up
2.4.1. Fresh Tea Leaf Sample Treatment
2.4.2. Soil Sample Treatment
2.5. Sample Extraction and Clean-Up
2.6. Statistical Analysis
3. Results and Discussion
3.1. Recovery Study
3.2. Migration and Accumulation of the OCDPE in Soil-Tea Systems
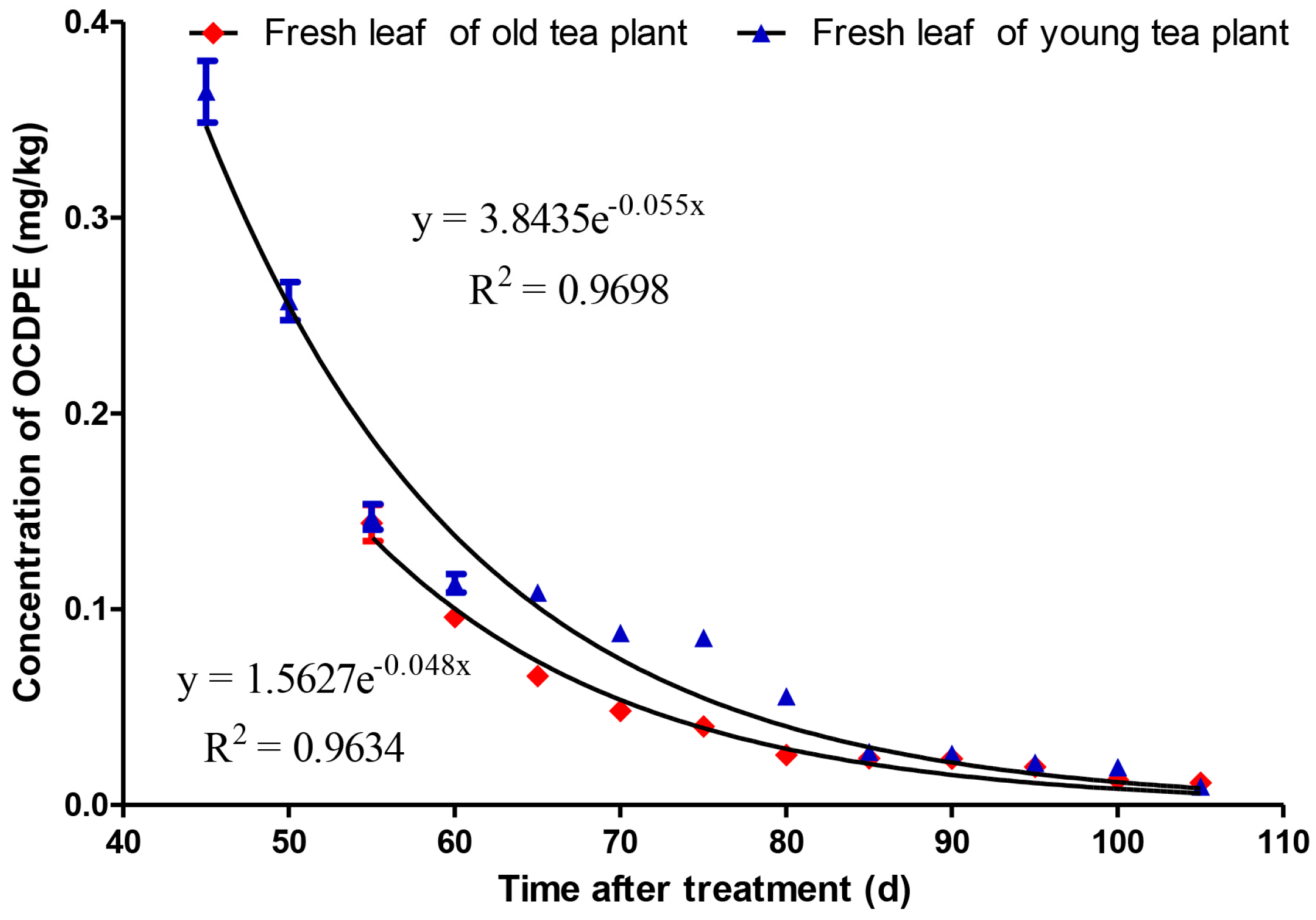
3.3. Accumulation and Dissipation of OCDPE in Fresh Leaves of Tea Plants
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Feng, J.; Tang, H.; Chen, D.Z.; Li, L. Monitoring and risk assessment of pesticide residues in tea samples from China. Hum. Ecol. Risk Assess. 2015, 21, 169–183. [Google Scholar] [CrossRef]
- Oellig, C.; Schwack, W. Planar solid phase extraction clean-up for pesticide residue analysis in tea by liquid chromatography-mass spectrometry. J. Chromatogr. A 2012, 1260, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Huo, F.F.; Tang, H.; Wu, X.; Chen, D.Z.; Zhao, T.; Liu, P.; Li, L. Utilizing a novel sorbent in the solid phase extraction for simultaneous determination of 15 pesticide residues in green tea by GC/MS. J. Chromatogr. B 2016, 1023, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cheung, W.; Leung, D. Determination of pesticide residue transfer rates (percent) from dried tea leaves to brewed tea. J. Agric. Food Chem. 2014, 62, 966–983. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.L.; Tang, H.; Chen, D.Z.; Li, L. A novel method based on mspd for simultaneous determination of 16 pesticide residues in tea by LC-MS/MS. J. Chromatogr. B 2015, 998, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Adolphi, H. Examination of a pyrethrum synergist—Preliminary information. Pyrethrum. Post. 1958, 4, 3–5. [Google Scholar]
- Hayashi, A. Synergistic effect of octachlorodipropylether (S-421). Botyu-Kagaku 1969, 34, 189–192. [Google Scholar]
- Tang, W.W.; Zeng, G.M.; Gong, J.L.; Liu, Y.; Wang, X.Y.; Liu, Y.Y.; Liu, Z.F.; Chen, L.; Zhang, X.R.; Tu, D.Z. Simultaneous adsorption of atrazine and Cu(ii) from wastewater by magnetic multi-walled carbon nanotube. Chem. Eng. J. 2012, 211, 470–478. [Google Scholar] [CrossRef]
- Krieger, R.I.; Dinoff, T.M.; Zhang, X.F. Octachlorodipropyl ether (S-2) mosquito coils are inadequately studied for residential use in Asia and illegal in the United States. Environ. Health Perspect. 2003, 111, 1439–1442. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Xing, J.; Dong, L.; Wu, C. Application of polyphenylmethylsiloxane coated fiber for solid-phase microextraction combined with microwave-assisted extraction for the determination of organochlorine pesticides in Chinese teas. J. Chromatogr. A 2003, 1015, 11–21. [Google Scholar] [CrossRef]
- Yoshida, S.; Taguchi, S.; Kitagawa, M. Isolation of a new organochlorine pollutant 2,3,3,3,2′,3′,3′,3′-octachlorodipropyl ether from fish. Bull. Environ. Contam. Toxicol. 2001, 67, 568–573. [Google Scholar] [CrossRef] [PubMed]
- Mori, Y. Ames mutagenicity of pyrethroid pesticides (tetramethrin, permethrin, empenthrin, and allethrin) and 1,1′-oxibis (2,3,3,3-tetra-chloropropane) as a synergist. Environ. Mutagen. Res. 1998, 20, 29–33. [Google Scholar]
- Huang, Q.; Wang, J.; Ye, C. Detection of octachlorodipropyl ether residue in tea adsorbed from the combustion of the mosquito coils by gas chromatography. Food Sci. Technol. 2010, 7, 085. [Google Scholar]
- Shi, Y.; Liao, M.; Kiyota, H.; Cao, H.; Hua, R.; Tang, F.; Yong, Y. Kinetics of octachlorodipropyl ether photolysis in aqueous solution. J. Pesticide Sci. 2015, 40, 49–54. [Google Scholar] [CrossRef]
- Liao, M.; Shi, Y.H.; Cao, H.Q.; Hua, R.M.; Tang, F.; Wu, X.W.; Tang, J. Dissipation behavior of octachlorodipropyl ether residues during tea planting and brewing process. Environ. Monit. Assess. 2015, 188, 551. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Ye, Q.; Zhang, K.; Li, L.; Wei, S.-M.; Lin, D. Residue dynamics and degradation of s421 in jasmine flower and tea. J. Fujian Agric. For. Univ. 2007, 36. [Google Scholar] [CrossRef]
- Han, Z.; Shi, Y.; Cao, H.; Wu, X.; Tang, F.; Hua, R.; Yue, Y. Degradation of octachlorodipropyl ether in different soils. J. Anhui Agric. Univ. 2011, 5, 024. [Google Scholar]
- Tang, F.; Chen, Z.; Liu, G.; Luo, F.; Lou, Z. Identification of the sources and gas chromatographic determination of octachlorodipropyl ether in tea. Chin. J. Pesticide Sci. 2007, 2. [Google Scholar]
- Zhao, Y.; Jin, Y.; Zhou, X.; Chang, Y.; Li, W.; Yin, L. Determination of residual octachlorodipropyl ether in substitutional tea by capillary gas-chromatography. Sci. Technol. Food Ind. 2008, 5, 093. [Google Scholar]
- Waqas, M.; Khan, S.; Chao, C.; Shamshad, I.; Qamar, Z.; Khan, K. Quantification of pahs and health risk via ingestion of vegetable in Khyber Pakhtunkhwa province, Pakistan. Sci. Total Environ. 2014, 497, 448–458. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Aijun, L.; Zhang, S.Z.; Hu, Q.H.; Zhu, Y.G. Accumulation of polycyclic aromatic hydrocarbons and heavy metals in lettuce grown in the soils contaminated with long-term wastewater irrigation. J. Hazard. Mater. 2008, 152, 506–515. [Google Scholar] [CrossRef] [PubMed]
- Khillare, P.S.; Jyethi, D.S.; Sarkar, S. Health risk assessment of polycyclic aromatic hydrocarbons and heavy metals via dietary intake of vegetables grown in the vicinity of thermal power plants. Food Chem. Toxicol. 2012, 50, 1642–1652. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.H.; Shen, D.Y.; Zhong, D.L.; Mo, R.H.; Ni, Z.L.; Tang, F.B. Time-dependent movement and distribution of chlorpyrifos and its metabolism in bamboo forest under soil surface mulching. J. Agric. Food Chem. 2014, 62, 6565–6570. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.F.; Agassi, M.; Letey, J.; Farmer, W.J.; Nelson, S.D.; Ben-Hur, M. Facilitated transport of napropamide by dissolved organic matter through soil columns. Soil Sci. Soc. Am. J. 2000, 64, 590–594. [Google Scholar] [CrossRef]
- Navarro, S.; Vela, N.; Navarro, G. Review. An overview on the environmental behaviour of pesticide residues in soils. Span. J. Agric. Res. 2007, 5, 357–375. [Google Scholar] [CrossRef]
- Fantke, P.; Gillespie, B.W.; Juraske, R.; Jolliet, O. Estimating half-lives for pesticide dissipation from plants. Environ. Sci. Technol. 2014, 48, 8588–8602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zong, L.; Zhou, J.; Li, C.; Yu, W.; Shan, G. Study on characteristics of absorption and transfer of soil lead in tea plant with different growth period. Int. J. Therm. Sci. 2007, 46, 139–148. [Google Scholar]


| Sample | Spiked Level (mg/kg) | Recovery (%) | RSD a (%) |
|---|---|---|---|
| fresh tea leaf | 0.01 | 92.3 ± 6.51 | 10.2 |
| 0.05 | 84.3 ± 5.84 | 10.4 | |
| 0.5 | 77.7 ± 4.67 | 8.6 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, M.; Shi, Y.-H.; Cao, H.-Q.; Fang, Q.-K.; Xiao, J.-J.; Hua, R.-M. Migration and Accumulation of Octachlorodipropyl Ether in Soil-Tea Systems in Young and Old Tea Gardens. Int. J. Environ. Res. Public Health 2017, 14, 1033. https://doi.org/10.3390/ijerph14091033
Liao M, Shi Y-H, Cao H-Q, Fang Q-K, Xiao J-J, Hua R-M. Migration and Accumulation of Octachlorodipropyl Ether in Soil-Tea Systems in Young and Old Tea Gardens. International Journal of Environmental Research and Public Health. 2017; 14(9):1033. https://doi.org/10.3390/ijerph14091033
Chicago/Turabian StyleLiao, Min, Yan-Hong Shi, Hai-Qun Cao, Qing-Kui Fang, Jin-Jing Xiao, and Ri-Mao Hua. 2017. "Migration and Accumulation of Octachlorodipropyl Ether in Soil-Tea Systems in Young and Old Tea Gardens" International Journal of Environmental Research and Public Health 14, no. 9: 1033. https://doi.org/10.3390/ijerph14091033





