Work-Related Noise Exposure in a Cohort of Patients with Chronic Tinnitus: Analysis of Demographic and Audiological Characteristics
Abstract
:1. Introduction
2. Materials and Methods
Statistics
3. Results
3.1. Demographics, Family History and Comorbidities
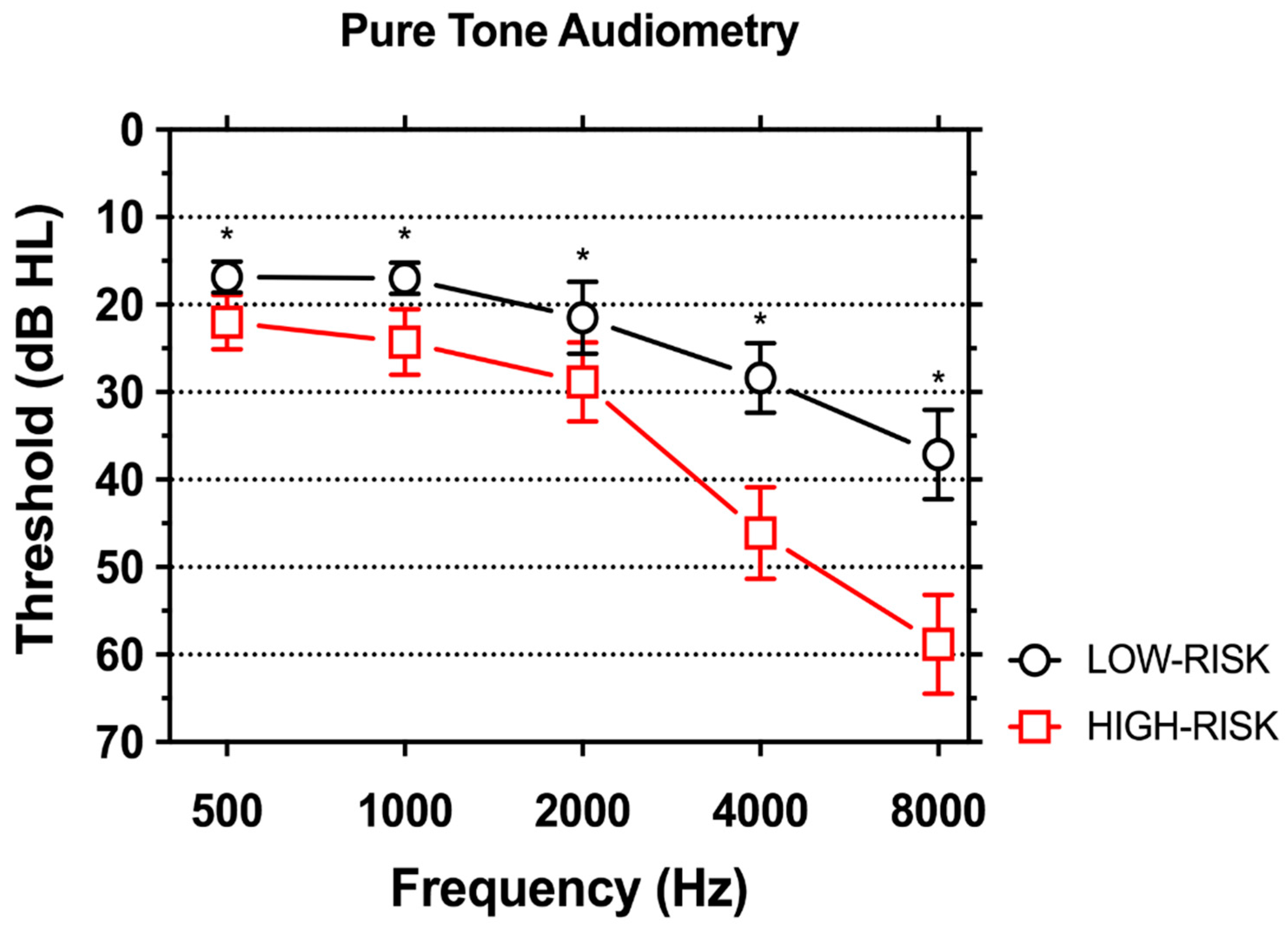
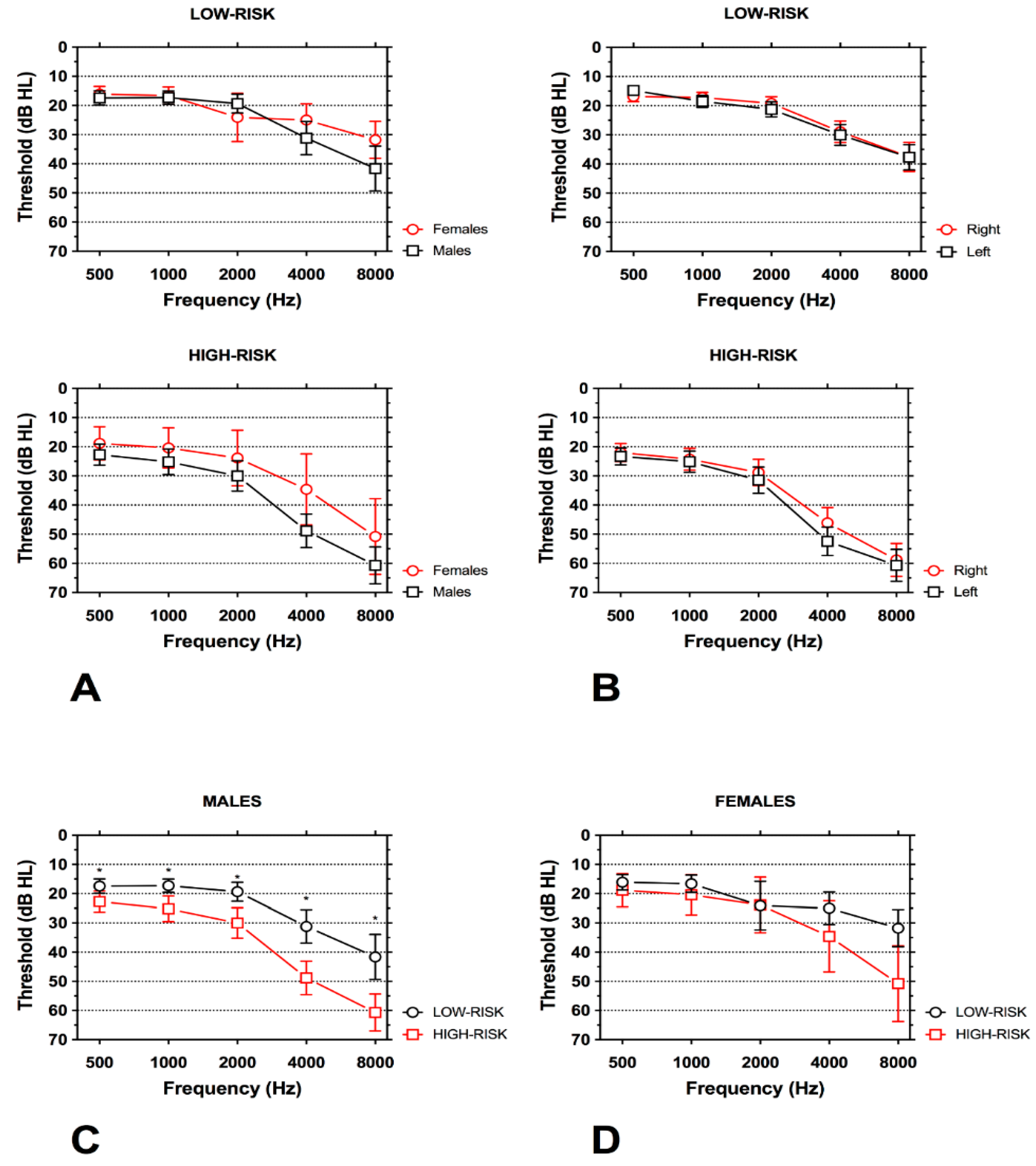
3.2. Hearing Loss
3.3. Tinnitus Characteristics and Self-Administered Questionnaires Scores
3.4. Differences among Occupations
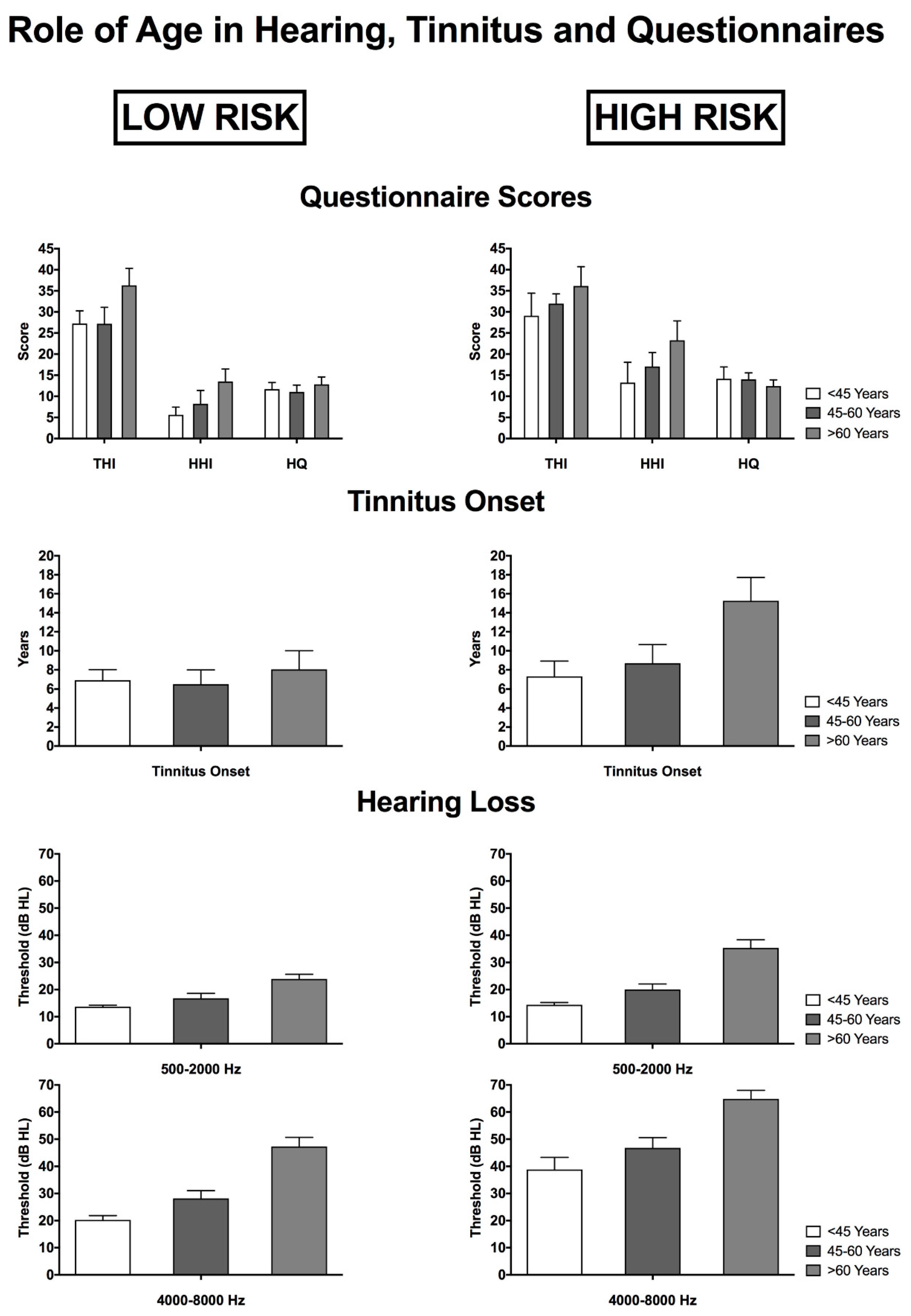
3.5. The Role of Age in Relation to Tinnitus, Hearing Characteristics and Questionnaire Scores
4. Discussion
4.1. Main Differences for Gender, Age, Family History and Comorbidities
4.2. Characteristics of Hearing Loss in Subjects at High-and Low-Risk for Work-Related Hearing Loss
4.3. Tinnitus Characteristics: Laterality, Pitch, Annoyance
4.4. Study Limitations
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Attias, J.; Horovitz, G.; El-Hatib, N.; Nageris, B. Detection and clinical diagnosis of noise-induced hearing loss by otoacoustic emissions. Noise Health 2001, 3, 19–31. [Google Scholar] [PubMed]
- Nelson, D.I.; Nelson, R.Y.; Concha-Barrientos, M.; Fingerhut, M. The global burden of occupational noise-induced hearing loss. Am. J. Ind. Med. 2005, 48, 446–458. [Google Scholar] [CrossRef] [PubMed]
- Tikka, C.; Verbeek, J.H.; Kateman, E.; Morata, T.C.; Dreschler, W.A.; Ferrite, S. Interventions to prevent occupational noise-induced hearing loss. Cochrane Database Syst. Rev. 2017. [Google Scholar] [CrossRef]
- Basner, M.; Babisch, W.; Davis, A.; Brink, M.; Clark, C.; Janssen, S.; Stansfeld, S. Auditory and non-auditory effects of noise on health. Lancet 2014, 383, 1325–1332. [Google Scholar] [CrossRef]
- Masterson, E.A.; Tak, S.; Themann, C.L.; Wall, D.K.; Groenewold, M.R.; Deddens, J.A.; Calvert, G.M. Prevalence of hearing loss in the United States by industry. Am. J. Ind. Med. 2013, 56, 670–681. [Google Scholar] [CrossRef] [PubMed]
- Spongr, V.P.; Flood, D.G.; Frisina, R.D.; Salvi, R.J. Quantitative measures of hair cell loss in CBA and C57BL/6 mice throughout their life spans. J. Acoust. Soc. Am. 1997, 101, 3546–3553. [Google Scholar] [CrossRef] [PubMed]
- Forge, A.; Schacht, J. Aminoglycoside antibiotics. Audiol. Neurootol. 2000, 5, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.D.; Decker, B.; Krishnan Muthaiah, V.P.; Sheppard, A.; Salvi, R. Prolonged noise exposure-induced auditory threshold shifts in rats. Hear. Res. 2014, 317, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Le Prell, C.G.; Yamashita, D.; Minami, S.B.; Yamasoba, T.; Miller, J.M. Mechanisms of noise-induced hearing loss indicate multiple methods of prevention. Hear. Res. 2007, 226, 22–43. [Google Scholar] [CrossRef] [PubMed]
- Henderson, D.; Bielefeld, E.C.; Harris, K.C.; Hu, B.H. The role of oxidative stress in noise-induced hearing loss. Ear Hear. 2006, 27, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Fetoni, A.R.; Garzaro, M.; Ralli, M.; Landolfo, V.; Sensini, M.; Pecorari, G.; Mordente, A.; Paludetti, G.; Giordano, C. The monitoring role of otoacoustic emissions and oxidative stress markers in the protective effects of antioxidant administration in noise-exposed subjects: A pilot study. Med. Sci. Monit. 2009, 15, PR1-8. [Google Scholar] [PubMed]
- Fetoni, A.R.; Mancuso, C.; Eramo, S.L.; Ralli, M.; Piacentini, R.; Barone, E.; Paludetti, G.; Troiani, D. In vivo protective effect of ferulic acid against noise-induced hearing loss in the guinea-pig. Neuroscience 2010, 169, 1575–1588. [Google Scholar] [CrossRef] [PubMed]
- Fetoni, A.R.; Ralli, M.; Sergi, B.; Parrilla, C.; Troiani, D.; Paludetti, G. Protective effects of N-acetylcysteine on noise-induced hearing loss in guinea pigs. Acta Otorhinolaryngol. Ital. 2009, 29, 70–75. [Google Scholar] [PubMed]
- Fetoni, A.R.; Ralli, R.; Sergi, B.; Parrilla, C.; Troiani, D.; Paludetti, G. Protective properties of antioxidant drugs in noise-induced hearing loss in the guinea pig. Audiol. Med. 2009, 6, 271–277. [Google Scholar] [CrossRef]
- Shore, S.E.; Roberts, L.E.; Langguth, B. Maladaptive plasticity in tinnitus—Triggers, mechanisms and treatment. Nat. Rev. Neurol. 2016, 12, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Ralli, M.; Altissimi, G.; Turchetta, R.; Mazzei, F.; Salviati, M.; Cianfrone, F.; Orlando, M.P.; Testugini, V.; Cianfrone, G. Somatosensory Tinnitus: Correlation between Cranio-Cervico-Mandibular Disorder History and Somatic Modulation. Audiol. Neurootol. 2016, 21, 372–382. [Google Scholar] [CrossRef] [PubMed]
- Ralli, M.; Greco, A.; Turchetta, R.; Altissimi, G.; de Vincentiis, M.; Cianfrone, G. Somatosensory tinnitus: Current evidence and future perspectives. J. Int. Med. Res. 2017, 45, 933–947. [Google Scholar] [CrossRef] [PubMed]
- Ralli, M.; Lobarinas, E.; Fetoni, A.R.; Stolzberg, D.; Paludetti, G.; Salvi, R. Comparison of salicylate-and quinine-induced tinnitus in rats: Development, time course, and evaluation of audiologic correlates. Otol. Neurotol. 2010, 31, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, A.; Hayes, S.H.; Chen, G.D.; Ralli, M.; Salvi, R. Review of salicylate-induced hearing loss, neurotoxicity, tinnitus and neuropathophysiology. Acta Otorhinolaryngol. Ital. 2014, 34, 79–93. [Google Scholar] [PubMed]
- Cianfrone, G.; Mazzei, F.; Salviati, M.; Turchetta, R.; Orlando, M.P.; Testugini, V.; Carchiolo, L.; Cianfrone, F.; Altissimi, G. Tinnitus Holistic Simplified Classification (THoSC): A New Assessment for Subjective Tinnitus, With Diagnostic and Therapeutic Implications. Ann. Otol. Rhinol. Laryngol. 2015, 124, 550–560. [Google Scholar] [CrossRef] [PubMed]
- Salviati, M.; Macri, F.; Terlizzi, S.; Melcore, C.; Provenzano, A.; Capparelli, E.; Altissimi, G.; Cianfrone, G. The Tinnitus Handicap Inventory as a screening test for psychiatric comorbidity in patients with tinnitus. Psychosomatics 2013, 54, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Ralli, M.; Troiani, D.; Podda, M.V.; Paciello, F.; Eramo, S.L.; de Corso, E.; Salvi, R.; Paludetti, G.; Fetoni, A.R. The effect of the NMDA channel blocker memantine on salicylate-induced tinnitus in rats. Acta Otorhinolaryngol. Ital. 2014, 34, 198–204. [Google Scholar] [PubMed]
- Ralli, M.; Altissimi, G.; Stadio, D.A.; Mazzei, F.; Turchetta, R.; Cianfrone, G. Relationship between hearing function and myasthenia gravis: A contemporary review. J. Int. Med. Res. 2016. [Google Scholar] [CrossRef] [PubMed]
- Di Stadio, A.; Ralli, M. Systemic Lupus Erythematosus and hearing disorders: Literature review and meta-analysis of clinical and temporal bone findings. J. Int. Med. Res. 2017. [Google Scholar] [CrossRef] [PubMed]
- Shargorodsky, J.; Curhan, G.C.; Farwell, W.R. Prevalence and characteristics of tinnitus among U.S. adults. Am. J. Med. 2010, 123, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.; Smith, P.A.; Booth, M.; Martin, M. Diagnosing Patients with Age-Related Hearing Loss and Tinnitus: Supporting GP Clinical Engagement through Innovation and Pathway Redesign in Audiology Services. Int. J. Otolaryngol. 2012. [Google Scholar] [CrossRef] [PubMed]
- Nondahl, D.M.; Cruickshanks, K.J.; Wiley, T.L.; Klein, R.; Klein, B.E.; Tweed, T.S. Prevalence and 5-year incidence of tinnitus among older adults: The epidemiology of hearing loss study. J. Am. Acad. Audiol. 2002, 13, 323–331. [Google Scholar] [PubMed]
- Lin, Y.H.; Chen, C.Y.; Lu, S.Y. Physical discomfort and psychosocial job stress among male and female operators at telecommunication call centers in Taiwan. Appl. Ergon. 2009, 40, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Palmer, K.T.; Griffin, M.J.; Syddall, H.E.; Davis, A.; Pannett, B.; Coggon, D. Occupational exposure to noise and the attributable burden of hearing difficulties in Great Britain. Occup. Environ. Med. 2002, 59, 634–639. [Google Scholar] [CrossRef] [PubMed]
- Ricci, S.; Massoni, F.; Di Meo, M.; Petrone, L.; Canitano, N.; Ippoliti, F.; Cinti, M.E. Correlation among measures of stress, indicators of biohumoral nature and medico-legal considerations. Riv. Psichiatr. 2013, 48, 113–120. [Google Scholar] [PubMed]
- Mrena, R.; Savolainen, S.; Kuokkanen, J.T.; Ylikoski, J. Characteristics of tinnitus induced by acute acoustic trauma: a long-term follow-up. Audiol. Neurootol. 2002, 7, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Nageris, B.I.; Attias, J.; Raveh, E. Test-retest tinnitus characteristics in patients with noise-induced hearing loss. Am. J. Otolaryngol. 2010, 31, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Abbate, C.; Concetto, G.; Fortunato, M.; Brecciaroli, R.; Tringali, M.A.; Beninato, G.; D’Arrigo, G.; Domenico, G. Influence of environmental factors on the evolution of industrial noise-induced hearing loss. Environ. Monit. Assess. 2005, 107, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Masterson, E.A.; Bushnell, P.T.; Themann, C.L.; Morata, T.C. Hearing Impairment Among Noise-Exposed Workers-United States, 2003–2012. MMWR Morb. Mortal. Wkly. Rep. 2016, 65, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Rosler, G. Progression of hearing loss caused by occupational noise. Scand. Audiol. 1994, 23, 13–37. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.D.; Chen, Y.; McDonald, J.C.; Cherry, N.M. Surveillance for work-related hearing loss in the UK: OSSA and OPRA 1997–2000. Occup. Med. (Lond.) 2002, 52, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Muhr, P.; Mansson, B.; Hellstrom, P.A. A study of hearing changes among military conscripts in the Swedish Army. Int. J. Audiol. 2006, 45, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Kurmis, A.P.; Apps, S.A. Occupationally-acquired noise-induced hearing loss: A senseless workplace hazard. Int. J. Occup. Med. Environ. Health 2007, 20, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Alamgir, H.; Turner, C.A.; Wong, N.J.; Cooper, S.P.; Betancourt, J.A.; Henry, J.; Senchak, A.J.; Hammill, T.L.; Packer, M.D. The impact of hearing impairment and noise-induced hearing injury on quality of life in the active-duty military population: Challenges to the study of this issue. Mil. Med. Res. 2016, 3, 11. [Google Scholar] [CrossRef] [PubMed]
- Yong, J.S.; Wang, D.Y. Impact of noise on hearing in the military. Mil. Med. Res. 2015, 2, 6. [Google Scholar] [CrossRef] [PubMed]
- Heupa, A.B.; Goncalves, C.G.; Coifman, H. Effects of impact noise on the hearing of military personnel. Braz. J. Otorhinolaryngol. 2011, 77, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Wells, T.S.; Seelig, A.D.; Ryan, M.A.; Jones, J.M.; Hooper, T.I.; Jacobson, I.G.; Boyko, E.J. Hearing loss associated with U.S. military combat deployment. Noise Health 2015, 17, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Masterson, E.A.; Sweeney, M.H.; Deddens, J.A.; Themann, C.L.; Wall, D.K. Prevalence of workers with shifts in hearing by industry: A comparison of OSHA and NIOSH Hearing Shift Criteria. J. Occup. Environ. Med. 2014, 56, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Ishii, E.K.; Talbott, E.O. Race/ethnicity differences in the prevalence of noise-induced hearing loss in a group of metal fabricating workers. J. Occup. Environ. Med. 1998, 40, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Reilly, M.J.; Rosenman, K.D.; Kalinowski, D.J. Occupational noise-induced hearing loss surveillance in Michigan. J. Occup. Environ. Med. 1998, 40, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Tantranont, K.; Codchanak, N. Predictors of Hearing Protection Use Among Industrial Workers. Workplace Health Saf. 2017, 65, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, A.S.; Cardoso, M.R. Hearing loss among workers exposed to road traffic noise in the city of Sao Paulo in Brazil. Auris Nasus Larynx 2005, 32, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, A.; Etemadinezhad, S.; Charati, J.Y.; Mohamadiyan, M. Noise-induced hearing loss in bus and truck drivers in Mazandaran province, 2011. Int. J. Occup. Saf. Ergon. 2016, 22, 193–198. [Google Scholar] [CrossRef] [PubMed]
- McBride, D.I. Noise-induced hearing loss and hearing conservation in mining. Occup. Med. (Lond.) 2004, 54, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Scott, D.F.; Grayson, R.L.; Metz, E.A. Disease and illness in U.S. mining, 1983–2001. J. Occup. Environ. Med. 2004, 46, 1272–1277. [Google Scholar] [PubMed]
- Schmuziger, N.; Patscheke, J.; Probst, R. Hearing in nonprofessional pop/rock musicians. Ear Hear. 2006, 27, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Pouryaghoub, G.; Mehrdad, R.; Pourhosein, S. Noise-Induced hearing loss among professional musicians. J. Occup. Health 2017, 59, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Halevi-Katz, D.N.; Yaakobi, E.; Putter-Katz, H. Exposure to music and noise-induced hearing loss (NIHL) among professional pop/rock/jazz musicians. Noise Health 2015, 17, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Rubak, T.; Kock, S.A.; Koefoed-Nielsen, B.; Bonde, J.P.; Kolstad, H.A. The risk of noise-induced hearing loss in the Danish workforce. Noise Health 2006, 8, 80–87. [Google Scholar] [PubMed]
- Lie, A.; Skogstad, M.; Johnsen, T.S.; Engdahl, B.; Tambs, K. Noise-induced hearing loss in a longitudinal study of Norwegian railway workers. BMJ Open 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Seixas, N.S.; Goldman, B.; Sheppard, L.; Neitzel, R.; Norton, S.; Kujawa, S.G. Prospective noise induced changes to hearing among construction industry apprentices. Occup. Environ. Med. 2005, 62, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Hessel, P.A. Hearing loss among construction workers in Edmonton, Alberta, Canada. J. Occup. Environ. Med. 2000, 42, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Van der Molen, H.F.; de Vries, S.C.; Stocks, S.J.; Warning, J.; Frings-Dresen, M.H. Incidence rates of occupational diseases in the Dutch construction sector, 2010–2014. Occup. Environ. Med. 2016, 73, 350–352. [Google Scholar] [CrossRef] [PubMed]
- Mrena, R.; Ylikoski, M.; Makitie, A.; Pirvola, U.; Ylikoski, J. Occupational noise-induced hearing loss reports and tinnitus in Finland. Acta Otolaryngol. 2007, 127, 729–735. [Google Scholar] [CrossRef] [PubMed]
- Engdahl, B.; Tambs, K. Occupation and the risk of hearing impairment—Results from the Nord-Trondelag study on hearing loss. Scand. J. Work Environ. Health 2010, 36, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Passi, S.; Ralli, G.; Capparelli, E.; Mammone, A.; Scacciatelli, D.; Cianfrone, G. The THI questionnaire: Psychometric data for reliability and validity of the Italian version. Int. Tinnitus. J. 2008, 14, 26–33. [Google Scholar] [PubMed]
- Ventry, I.M.; Weinstein, B.E. The hearing handicap inventory for the elderly: A new tool. Ear Hear. 1982, 3, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Khalfa, S.; Dubal, S.; Veuillet, E.; Perez-Diaz, F.; Jouvent, R.; Collet, L. Psychometric normalization of a hyperacusis questionnaire. ORL J. Otorhinolaryngol. Relat. Spec. 2002, 64, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Nelting, M.; Rienhoff, N.K.; Hesse, G.; Lamparter, U. The assessment of subjective distress related to hyperacusis with a self-rating questionnaire on hypersensitivity to sound. Laryngorhinootologie 2002, 81, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Engdahl, B.; Krog, N.H.; Kvestad, E.; Hoffman, H.J.; Tambs, K. Occupation and the risk of bothersome tinnitus: Results from a prospective cohort study (HUNT). BMJ Open 2012, 2. [Google Scholar] [CrossRef] [PubMed]
- Frederiksen, T.W.; Ramlau-Hansen, C.H.; Stokholm, Z.A.; Grynderup, M.B.; Hansen, A.M.; Lund, S.P.; Kristiansen, J.; Vestergaard, J.M.; Bonde, J.P.; Kolstad, H.A. Occupational noise exposure, psychosocial working conditions and the risk of tinnitus. Int. Arch. Occup. Environ. Health 2017, 90, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Boger, M.E.; Sampaio, A.L.L.; Oliveira, C. Analysis of Hearing and Tinnitus in Workers Exposed to Occupational Noise. Int. Tinnitus. J. 2017, 20, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Flores, L.S.; Teixeira, A.R.; Rosito, L.P.; Seimetz, B.M.; Dall’Igna, C. Pitch and Loudness from Tinnitus in Individuals with Noise-induced Hearing Loss. Int. Arch. Otorhinolaryngol. 2016, 20, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Mazurek, B.; Olze, H.; Haupt, H.; Szczepek, A.J. The more the worse: The grade of noise-induced hearing loss associates with the severity of tinnitus. Int. J. Environ. Res. Public Health 2010, 7, 3071–3079. [Google Scholar] [CrossRef] [PubMed]
- Le, T.N.; Straatman, L.V.; Lea, J.; Westerberg, B. Current insights in noise-induced hearing loss: A literature review of the underlying mechanism, pathophysiology, asymmetry, and management options. J. Otolaryngol. Head Neck. Surg. 2017, 46, 41. [Google Scholar] [CrossRef] [PubMed]
- Rosati, M.V.; Tomei, F.; Loreti, B.; Casale, T.; Cianfrone, G.; Altissimi, G.; Tomei, G.; Bernardini, A.; Di Marzio, A.; Sacco, C.; et al. Distortion-product otoacoustic emissions in workers exposed to urban stressors. Arch. Environ. Occup. Health 2017. [Google Scholar] [CrossRef] [PubMed]
- Sereda, M.; Hall, D.A.; Bosnyak, D.J.; Edmondson-Jones, M.; Roberts, L.E.; Adjamian, P.; Palmer, A.R. Re-examining the relationship between audiometric profile and tinnitus pitch. Int. J. Audiol. 2011, 50, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Schecklmann, M.; Vielsmeier, V.; Steffens, T.; Landgrebe, M.; Langguth, B.; Kleinjung, T. Relationship between Audiometric slope and tinnitus pitch in tinnitus patients: Insights into the mechanisms of tinnitus generation. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sha, S.H.; Schacht, J. Emerging therapeutic interventions against noise-induced hearing loss. Expert Opin. Investig. Drugs 2017, 26, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Abreu-Silva, R.S.; Rincon, D.; Horimoto, A.R.; Sguillar, A.P.; Ricardo, L.A.; Kimura, L.; Batissoco, A.C.; Auricchio, M.T.; Otto, P.A.; Mingroni-Netto, R.C. The search of a genetic basis for noise-induced hearing loss (NIHL). Ann. Hum. Biol. 2011, 38, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Ohlemiller, K.K.; McFadden, S.L.; Ding, D.L.; Lear, P.M.; Ho, Y.S. Targeted mutation of the gene for cellular glutathione peroxidase (Gpx1) increases noise-induced hearing loss in mice. J. Assoc. Res. Otolaryngol. 2000, 1, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.R.; Newlander, J.K.; Ling, X.; Cortopassi, G.A.; Krieg, E.F.; Erway, L.C. Genetic basis for susceptibility to noise-induced hearing loss in mice. Hear. Res. 2001, 155, 82–90. [Google Scholar] [CrossRef]
- Daniel, E. Noise and hearing loss: A review. J. Sch. Health 2007, 77, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Pankova, V.B.; Preobrazhenskaya, E.A.; Fedina, I.N. The occupational risk of hearing impairment associated with cardiovascular pathologies in the subjects engaged in ‘noisy’ industries. Vestn. Otorinolaringol. 2016, 81, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Tomei, F.; Fantini, S.; Tomao, E.; Baccolo, T.P.; Rosati, M.V. Hypertension and chronic exposure to noise. Arch. Environ. Health 2000, 55, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Munzel, T.; Daiber, A.; Steven, S.; Tran, L.P.; Ullmann, E.; Kossmann, S.; Schmidt, F.P.; Oelze, M.; Xia, N.; Li, H.; et al. Effects of noise on vascular function, oxidative stress, and inflammation: Mechanistic insight from studies in mice. Eur. Heart J. 2017. [Google Scholar] [CrossRef] [PubMed]
- Lusk, S.L.; Hagerty, B.M.; Gillespie, B.; Caruso, C.C. Chronic effects of workplace noise on blood pressure and heart rate. Arch. Environ. Health 2002, 57, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Samelli, A.G.; Santos, I.S.; Moreira, R.R.; Rabelo, C.M.; Rolim, L.P.; Bensenor, I.J.; Lotufo, P.A. Diabetes mellitus and sensorineural hearing loss: Is there an association? Baseline of the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil). Clinics (Sao Paulo) 2017, 72, 5–10. [Google Scholar] [CrossRef]
- Kim, M.B. Diabetes mellitus and the incidence of hearing loss: A cohort study. Int. J. Epidemiol. 2017, 46, 727. [Google Scholar] [CrossRef] [PubMed]
- Diaz de Leon-Morales, L.V.; Jauregui-Renaud, K.; Garay-Sevilla, M.E.; Hernandez-Prado, J.; Malacara-Hernandez, J.M. Auditory impairment in patients with type 2 diabetes mellitus. Arch. Med. Res. 2005, 36, 507–510. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Johnson, M.A.; Shea Miller, K.; De Chicchis, A.R. Hearing loss and cardiovascular disease risk factors in older adults. J. Nutr. Health Aging 2007, 11, 515–518. [Google Scholar] [PubMed]
- Stansfeld, S.A.; Matheson, M.P. Noise pollution: Non-auditory effects on health. Br. Med. Bull. 2003, 68, 243–257. [Google Scholar] [CrossRef] [PubMed]
- Nageris, B.I.; Raveh, E.; Zilberberg, M.; Attias, J. Asymmetry in noise-induced hearing loss: Relevance of acoustic reflex and left or right handedness. Otol. Neurotol. 2007, 28, 434–437. [Google Scholar] [CrossRef] [PubMed]
- Dobie, R.A. Does occupational noise cause asymmetric hearing loss? Ear Hear. 2014, 35, 577–579. [Google Scholar] [CrossRef] [PubMed]
- Hiller, W.; Goebel, G. Factors influencing tinnitus loudness and annoyance. Arch. Otolaryngol. Head Neck. Surg. 2006, 132, 1323–1330. [Google Scholar] [CrossRef] [PubMed]
- Schecklmann, M.; Landgrebe, M.; Langguth, B. the TRI Database Study Group. Phenotypic characteristics of hyperacusis in tinnitus. PLoS ONE 2014, 9, e86944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilles, A.; Goelen, S.; Van de Heyning, P. Tinnitus: A cross-sectional study on the audiologic characteristics. Otol. Neurotol. 2014, 35, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, M.D.; Katon, W.; Dobie, R.; Sakai, C.; Russo, J.; Harrop-Griffiths, J. Disabling tinnitus. Association with affective disorder. Gen. Hosp. Psychiat. 1988, 10, 285–291. [Google Scholar] [CrossRef]
- Kehrle, H.M.; Sampaio, A.L.; Granjeiro, R.C.; de Oliveira, T.S.; Oliveira, C.A. Tinnitus Annoyance in Normal-Hearing Individuals: Correlation With Depression and Anxiety. Ann. Otol. Rhinol. Laryngol. 2016, 125, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Ottoni, A.O.; Barbosa-Branco, A.; Boger, M.E.; Garavelli, S.L. Study of the noise spectrum on high frequency thresholds in workers exposed to noise. Braz. J. Otorhinolaryngol. 2012, 78, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Mehrparvar, A.H.; Mirmohammadi, S.J.; Ghoreyshi, A.; Mollasadeghi, A.; Loukzadeh, Z. High-frequency audiometry: A means for early diagnosis of noise-induced hearing loss. Noise Health 2011, 13, 402–406. [Google Scholar] [CrossRef] [PubMed]
- Antonioli, C.A.; Momensohn-Santos, T.M.; Benaglia, T.A. High-frequency Audiometry Hearing on Monitoring of Individuals Exposed to Occupational Noise: A Systematic Review. Int. Arch. Otorhinolaryngol. 2016, 20, 281–289. [Google Scholar] [PubMed]

| Demographic Characteristics | LOW-RISK | HIGH-RISK | p-Value |
|---|---|---|---|
| Age (mean (SD)) | 53.5 (13.5) | 56.6 (12.4) | 0.08 |
| Male (freq. (%)) | 37 (54.4) | 55 (80.9) | 0.001 |
| Female (freq. (%)) | 31 (45.6) | 13 (19.2) | 0.001 |
| Family history (freq. (%)) | |||
| No hearing loss | 59 (86.8) | 54 (79.4) | |
| Hearing loss | 9 (13.2) | 14 (20.6) | 0.253 |
| Time of noise exposure in years (mean (SD)) | 18.4 (8.1) | 19.3 (6.7) | 0.72 |
| Comorbidity (freq. (%)) | |||
| No comorbidity | 44 (64.7) | 41 (60.3) | |
| At least one comorbidity | 24 (35.3) | 27 (39.7) | |
| Heart disease | 7 (29.2) | 5 (18.5) | |
| Diabetes | 4 (16.7) | 3 (11.1) | |
| Hypertension | 18 (75) | 21 (77.8) | |
| Vascular diseases | 4 (16.7) | 6 (22.2) | 0.60 |
| PTA | LOW-RISK | HIGH-RISK | p-Value |
|---|---|---|---|
| Average Right/Left Ear (mean (SD)) | |||
| 500 Hz | 16.8 (7.2) | 22.0 (12.6) | 0.002 |
| 1000 Hz | 17.0 (7.2) | 24.3 (15.3) | <0.001 |
| 2000 Hz | 21.5 (16.8) | 28.8 (18.4) | 0.008 |
| 4000 Hz | 28.4 (16.3) | 46.1 (21.4) | <0.001 |
| 8000 Hz | 37.1 (20.9) | 58.8 (23.2) | <0.001 |
| Right Ear (mean (SD)) | |||
| 500 Hz | 16.8 (7.2) | 22.0 (12.6) | 0.004 |
| 1000 Hz | 17.2 (7.2) | 24.3 (15.3) | <0.001 |
| 2000 Hz | 19.2 (9.2) | 28.8 (18.4) | <0.001 |
| 4000 Hz | 29.0 (15.1) | 46.1 (21.4) | <0.001 |
| 8000 Hz | 37.6 (20.3) | 58.8 (23.1) | <0.001 |
| Left Ear (mean (SD)) | |||
| 500 Hz | 16.8 (7.2) | 23.3 (11.8) | <0.001 |
| 1000 Hz | 17.0 (7.2) | 25.1 (14.9) | <0.001 |
| 2000 Hz | 18.8 (9.5) | 31.5 (18.4) | <0.001 |
| 4000 Hz | 29.0 (15.9) | 52.4 (19.9) | <0.001 |
| 8000 Hz | 38.2 (20.3) | 60.7 (22.3) | <0.001 |
| Tinnitus Characteristics and Questionnaire Scores | LOW-RISK | HIGH-RISK | p-Value |
|---|---|---|---|
| Tinnitus side (freq. (%)) | |||
| Left | 19 (27.9) | 18 (26.5) | 0.05 |
| Right | 13 (19.1) | 4 (5.9) | |
| Bilateral | 36 (52.9) | 46 (67.6) | |
| Tinnitus Sound (freq. (%)) | |||
| Buzzing | 11 (16.2) | 19 (27.9) | 0.06 |
| High-pitched | 9 (13.2) | 17 (25.0) | |
| Low-pitched | 7 (10.3) | 8 (11.8) | |
| Other | 12 (17.6) | 7 (10.3) | |
| Whistle | 29 (42.6) | 17 (25.0) | |
| Questionnaire scores (mean (SD)) | |||
| THI | 30.6 (18.1) | 33.1 (18.8) | 0.22 |
| HHI | 9.4 (13.4) | 18.8 (20.3) | <0.001 |
| HQ | 11.8 (7.9) | 13.4 (8.3) | 0.12 |
| Occupation | Male (%) | Age (y) | Work (y) | Bilateral Tin (%) | Tin onset (y) | THI | HHI | HQ | PTA (0.5–2 kHz) | PTA (4–8 kHz) |
|---|---|---|---|---|---|---|---|---|---|---|
| HIGH-RISK | ||||||||||
| Armed Forces (n = 11) | 100 | 54.8 | 19.9 | 72.7 | 9.8 | 24.1 | 11 | 9.7 | 16.8 | 44.5 |
| Carpenters (n = 8) | 100 | 54.2 | 14.7 | 62.5 | 9.7 | 29.7 | 21.7 | 15.1 | 24.7 | 52 |
| Manufacturing Workers (n = 4) | 0 | 44.5 | 11.2 | 50 | 8 | 50.5 | 23.5 | 16.2 | 25.4 | 46.2 |
| Drivers (n = 9) | 100 | 61.1 | 16.5 | 55.5 | 12.6 | 29.1 | 17.1 | 10.6 | 31.2 | 60 |
| Miners (n = 4) | 100 | 55 | 20.7 | 75 | 8.5 | 38 | 47 | 14.2 | 47.5 | 78.1 |
| Musicians (n = 2) | 100 | 47.5 | 13 | 0 | 6.5 | 21 | 30 | 22 | 11.6 | 33.7 |
| Railroaders (n = 3) | 100 | 61.3 | 21 | 33.3 | 15.3 | 42 | 2.6 | 8 | 31.6 | 65.8 |
| School Teachers (n = 12) | 33.3 | 63.7 | 21 | 91.6 | 16.6 | 33.6 | 15.1 | 17.4 | 22.3 | 45.6 |
| Construction Workers (n = 15) | 93.3 | 54.8 | 23 | 73.3 | 8.8 | 37 | 23.4 | 12.6 | 23.8 | 54.5 |
| LOW-RISK | ||||||||||
| Entrepreneurs (n = 11) | 81.8 | 48.7 | 18.5 | 63.6 | 11.6 | 28 | 13.1 | 13.8 | 16.1 | 31.8 |
| Hospital Workers (n = 4) | 50 | 38.7 | 16.7 | 25 | 6.2 | 21 | 1.5 | 10 | 13.3 | 15 |
| Office Workers (n = 35) | 51.4 | 53.7 | 19.2 | 54.2 | 6.6 | 33.7 | 8.9 | 11.1 | 17.2 | 31.4 |
| Professionals (n = 18) | 44.4 | 59.5 | 21.8 | 33.7 | 9.5 | 27.8 | 11.9 | 9.6 | 23.2 | 40.9 |
| Variable | Odds Ratio | Confidence Interval | p-Value |
|---|---|---|---|
| Age | 1.02 | 0.99–1.05 | 0.16 |
| Male | 3.54 | 1.64–7.66 | 0.001 |
| Family history | 1.70 | 0.68–4.24 | 0.26 |
| Comorbidity | 1.20 | 0.6–2.42 | 0.60 |
| HHI | 1.03 | 1.01–1.06 | 0.003 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ralli, M.; Balla, M.P.; Greco, A.; Altissimi, G.; Ricci, P.; Turchetta, R.; De Virgilio, A.; De Vincentiis, M.; Ricci, S.; Cianfrone, G. Work-Related Noise Exposure in a Cohort of Patients with Chronic Tinnitus: Analysis of Demographic and Audiological Characteristics. Int. J. Environ. Res. Public Health 2017, 14, 1035. https://doi.org/10.3390/ijerph14091035
Ralli M, Balla MP, Greco A, Altissimi G, Ricci P, Turchetta R, De Virgilio A, De Vincentiis M, Ricci S, Cianfrone G. Work-Related Noise Exposure in a Cohort of Patients with Chronic Tinnitus: Analysis of Demographic and Audiological Characteristics. International Journal of Environmental Research and Public Health. 2017; 14(9):1035. https://doi.org/10.3390/ijerph14091035
Chicago/Turabian StyleRalli, Massimo, Maria Paola Balla, Antonio Greco, Giancarlo Altissimi, Pasquale Ricci, Rosaria Turchetta, Armando De Virgilio, Marco De Vincentiis, Serafino Ricci, and Giancarlo Cianfrone. 2017. "Work-Related Noise Exposure in a Cohort of Patients with Chronic Tinnitus: Analysis of Demographic and Audiological Characteristics" International Journal of Environmental Research and Public Health 14, no. 9: 1035. https://doi.org/10.3390/ijerph14091035






