Applying Nonparametric Methods to Analyses of Short-Term Fine Particulate Matter Exposure and Hospital Admissions for Cardiovascular Diseases among Older Adults
Abstract
:1. Introduction
2. Materials and Methods
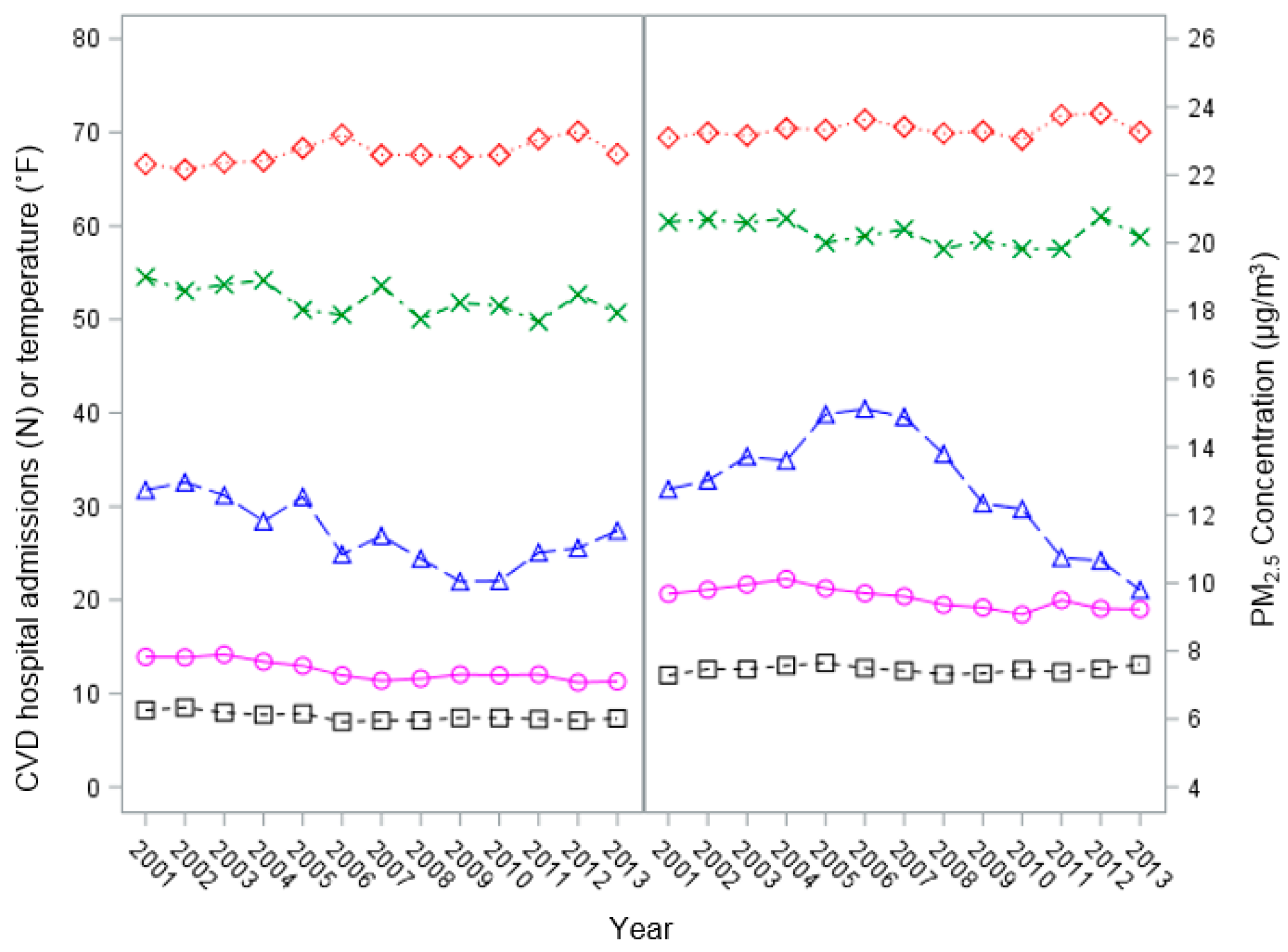
2.1. Hospital Admission Data
2.2. PM2.5 Data
2.3. Meteorological Data
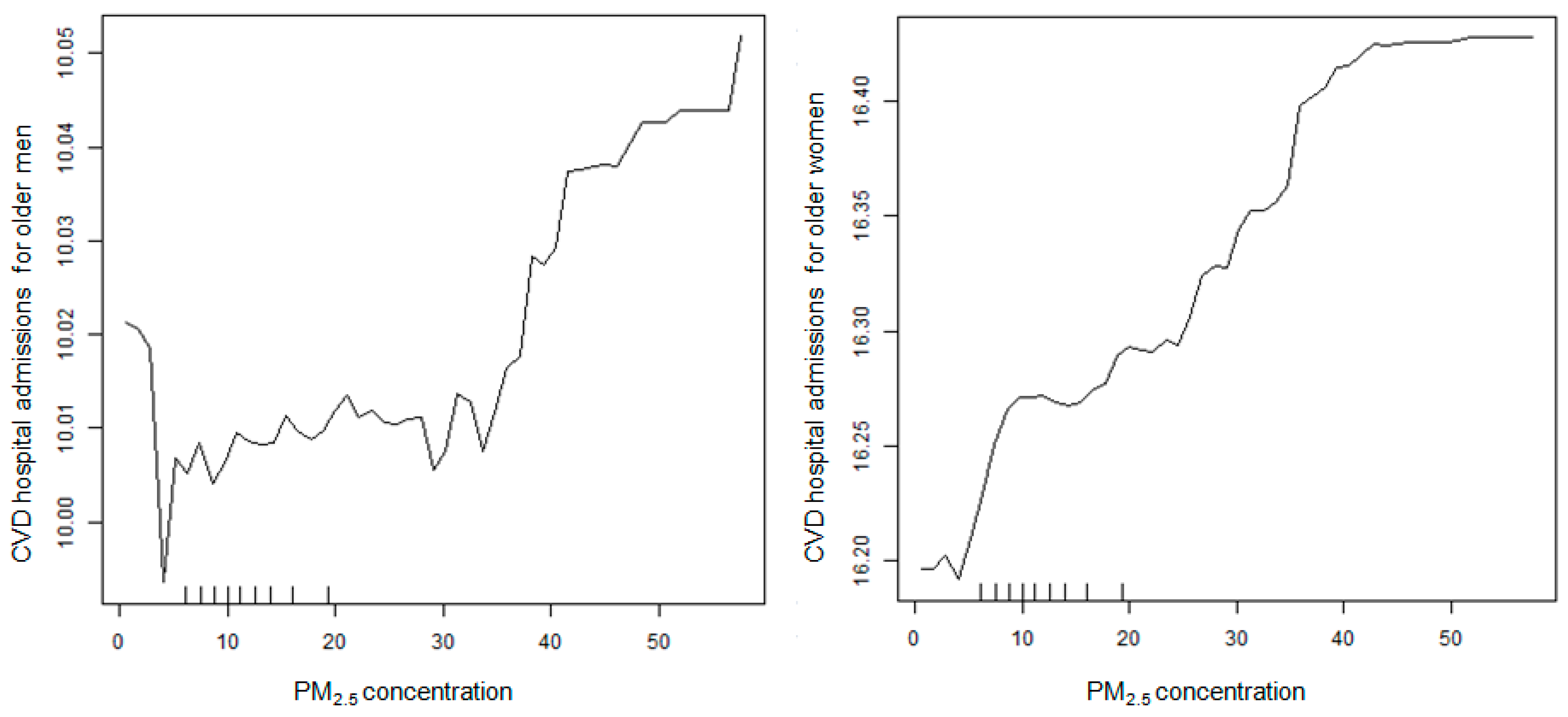
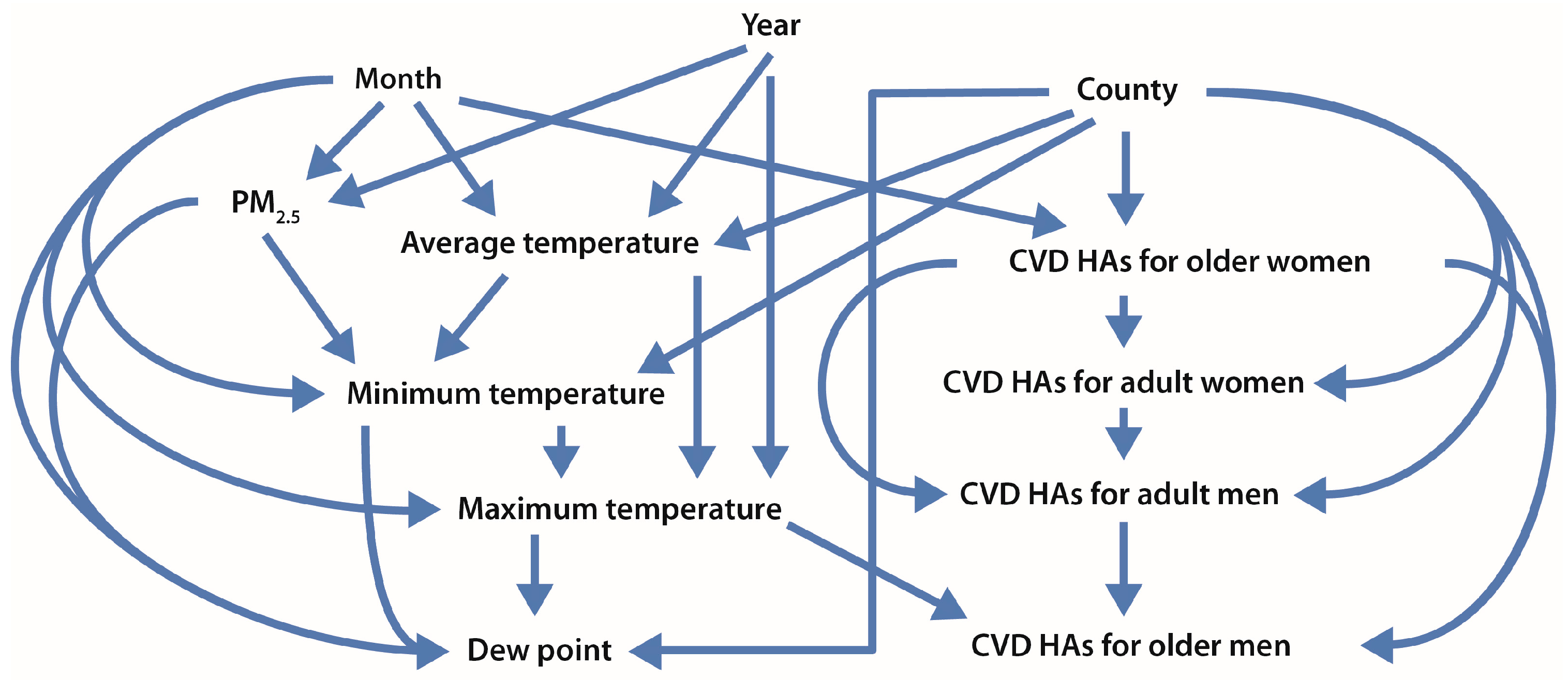
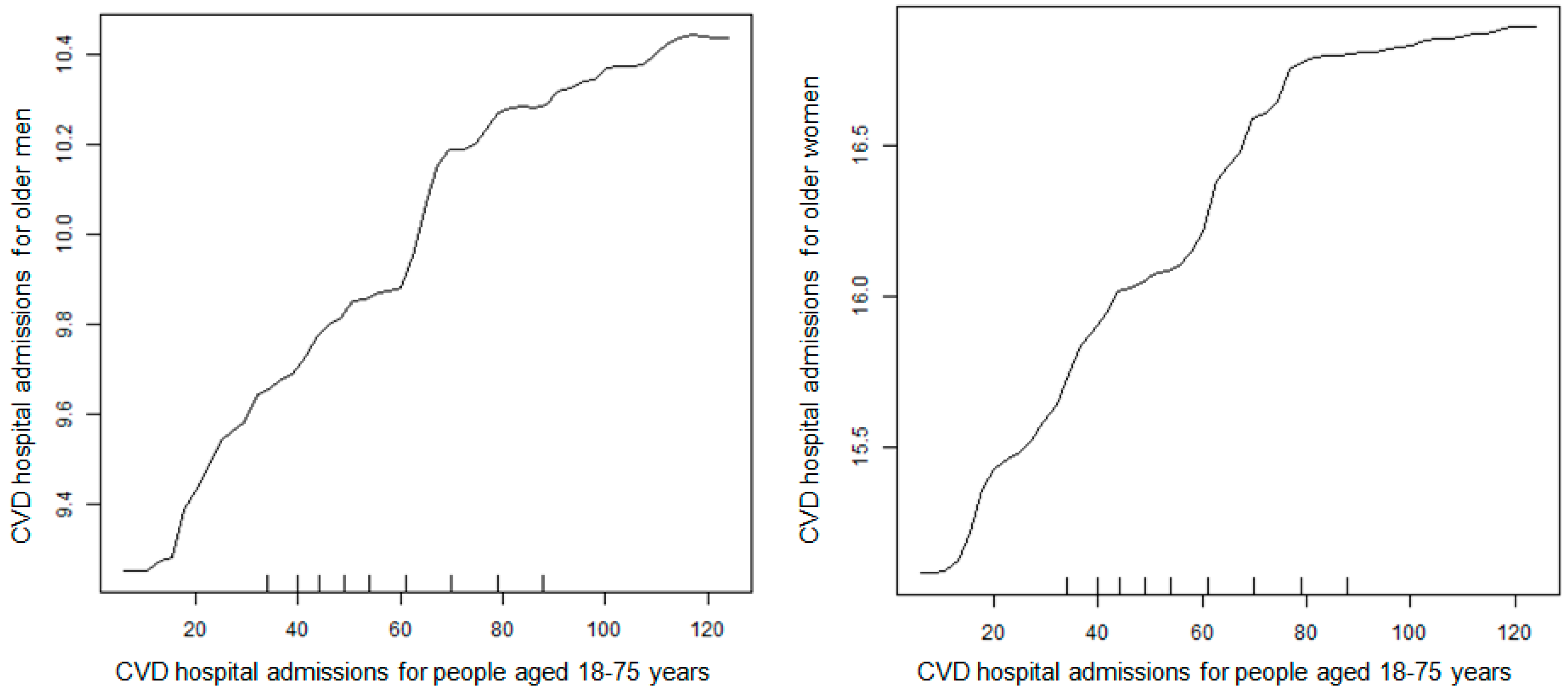
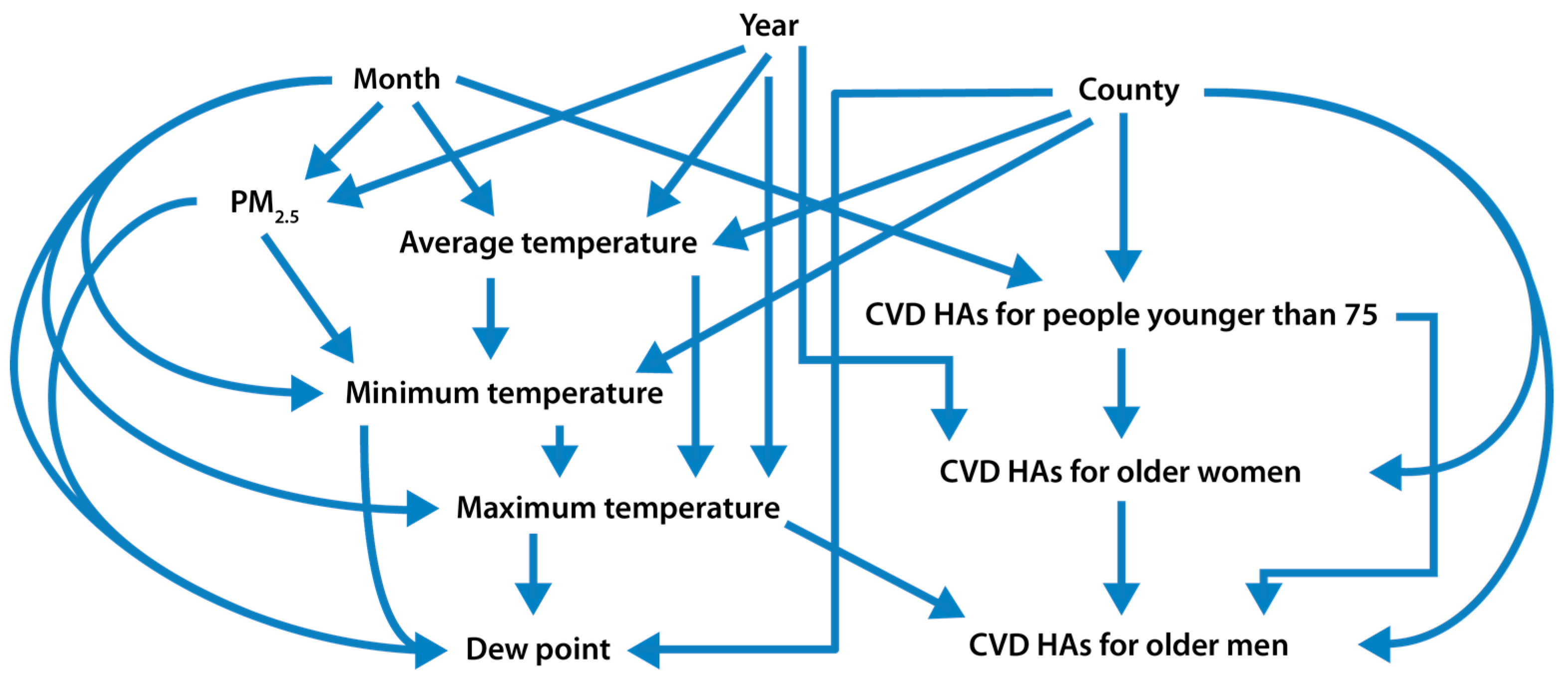
2.4. Statistical Analysis
- Information principle: Causes provide unique information that helps predict their effects and that cannot be obtained from other variables. (Conversely, if in some dataset the response R is conditionally independent of exposure concentration C, given the values of a covariate vector Z, then there is no evidence that C is a direct cause of R in that dataset.) This principle creates a bridge between well-developed statistical and machine learning methods for identifying informative variables that improve the prediction of dependent variables such as health effects, on the one hand, and the needs of causal inference, on the other. Only variables that help predict an effect by providing information that is not redundant with that from other variables (e.g., measured confounders) are candidates to be its causes. This constraint allows techniques of predictive analytics to be applied as a necessary condition for potential causation.
- Nonparametric analyses: Most of the top-performing causal inference algorithms use multivariate nonparametric methods (most commonly, classification and regression trees) to identify information dependency relations among variables and to help avoid biases due to model specification errors. If no significant change occurs in the conditional empirical cumulative distribution function of a dependent variable as the value of an explanatory variable varies for any combination of values of the remaining variables (i.e., the dependent variable and the explanatory variable are conditionally independent), then the dependent variable is conditionally independent of the explanatory variable. This lack of dependence does not provide evidence that the explanatory variable is a cause of the dependent variable, because effects are not conditionally independent of their direct causes.
- Model ensembles: Rather than relying on any single statistical model or nonparametric analysis, the top-performing causal analytics algorithms typically fit hundreds of nonparametric models (e.g., regression trees) to randomly generated bootstrap samples of the data and average the resulting predictions of how the dependent variable (e.g., income, education, smoking) depends on other variables. Such averaging over an ensemble of model results usually yields better estimates of how the dependent variable depends on other variables (e.g., pollutant concentrations) with lower bias and error variance than the estimates from any single predictive model.
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Miller, K.A.; Siscovick, D.S.; Sheppard, L.; Shepherd, K.; Sullivan, J.H.; Anderson, G.L.; Kaufman, J.D. Long-term exposure to air pollution and incidence of cardiovascular events in women. N. Engl. J. Med. 2007, 356, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Dehbi, H.M.; Blangiardo, M.; Gulliver, J.; Fecht, D.; de Hoogh, K.; Al-Kanaani, Z.; Tillin, T.; Hardy, R.; Chaturvedi, N.; Hansell, A.L. Air pollution and cardiovascular mortality with over 25 years follow-up: A combined analysis of two British cohorts. Environ. Health Perspect. 2016, 122, 138–144. [Google Scholar]
- Dominici, F.; Greenstone, M.; Sunstein, C.R. Science and regulation. Particulate matter matters. Science 2014, 344, 257–259. [Google Scholar] [CrossRef] [PubMed]
- Zigler, C.M.; Dominici, F. Point: Clarifying policy evidence with potential-outcomes thinking-beyond exposure-response estimation in air pollution epidemiology. Am. J. Epidemiol. 2014, 180, 1133–1140. [Google Scholar] [CrossRef] [PubMed]
- Cromar, K.R.; Gladson, L.A.; Perlmutt, L.D.; Ghazipura, M.; Ewart, G.W. American Thoracic Society and marron institute report. Estimated excess morbidity and mortality caused by air pollution above American Thoracic Society-recommended standards, 2011–2013. Ann. Am. Thorac. Soc. 2016, 13, 1195–1201. [Google Scholar] [CrossRef] [PubMed]
- Lo, W.C.; Shie, R.H.; Chan, C.C.; Lin, H.H. Burden of disease attributable to ambient fine particulate matter exposure in Taiwan. J. Formos. Med. Assoc. 2016, 116, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Allen, R.T.; Hales, N.M.; Baccarelli, A.; Jerrett, M.; Ezzati, M.; Dockery, D.W.; Pope, C.A., III. Countervailing effects of income, air pollution, smoking, and obesity on aging and life expectancy: Population-based study of U.S. Counties. Environ. Health 2016, 15, 86. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, J.; Austin, E.; Bind, M.A.; Zanobetti, A.; Koutrakis, P. Estimating causal associations of fine particles with daily deaths in Boston. Am. J. Epidemiol. 2015, 182, 644–650. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kloog, I.; Coull, B.A.; Kosheleva, A.; Zanobetti, A.; Schwartz, J.D. Estimating causal effects of long-term PM2.5 exposure on mortality in New Jersey. Environ. Health Perspect. 2016, 124, 1182–1188. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, G. Letter to the Editor re: Estimating causal associations of fine particles with daily deaths in Boston. Am. J. Epidemiol. 2016, 183, 594. [Google Scholar] [CrossRef] [PubMed]
- Dorie, V.; Hill, J.; Shalit, U.; Cervone, D.; Scott, M. Is your SATT where it’s at? A causal inference data analysis challenge. In Proceedings of the 2016 Atlantic Causal Inference Conference, New York, NY, USA, 26–27 May 2016. [Google Scholar]
- Pope, C.A., III. Epidemiology of fine particulate air pollution and human health: Biologic mechanisms and who’s at risk? Environ. Health Perspect. 2000, 108 (Suppl. 4), 713–723. [Google Scholar] [CrossRef] [PubMed]
- Haley, V.B.; Talbot, T.O.; Felton, H.D. Surveillance of the short-term impact of fine particle air pollution on cardiovascular disease hospitalizations in New York State. Environ. Health 2009, 8, 42. [Google Scholar] [CrossRef] [PubMed]
- Cox, L.A., Jr. Rethinking the meaning of concentration-response functions and the estimated burden of adverse health effects attributed to exposure concentrations. Risk Anal. 2016, 36, 1770–1779. [Google Scholar]
- Pearl, J. An introduction to causal inference. Int. J. Biostat. 2010. [Google Scholar] [CrossRef] [PubMed]
- Fleischer, N.L.; Diez Roux, A.V. Using directed acyclic graphs to guide analyses of neighbourhood health effects: An introduction. J. Epidemiol. Community Health 2008, 62, 842–846. [Google Scholar] [CrossRef] [PubMed]
- Cox Associates Consulting. Available online: http://cox-associates.com/ (accessed on 7 January 2017).
- Bell, M.L.; Ebisu, K.; Peng, R.D.; Walker, J.; Samet, J.M.; Zeger, S.L.; Dominici, F. Seasonal and regional short-term effects of fine particles on hospital admissions in 202 US counties, 1999–2005. Am. J. Epidemiol. 2008, 168, 1301–1310. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, R.W.; Kang, S.; Anderson, H.R.; Mills, I.C.; Walton, H.A. Epidemiological time series studies of PM2.5 and daily mortality and hospital admissions: A systematic review and meta-analysis. Thorax 2014, 69, 660–665. [Google Scholar] [CrossRef] [PubMed]
- Bell, M.L.; Ebisu, K.; Leaderer, B.P.; Gent, J.F.; Lee, H.J.; Koutrakis, P.; Wang, Y.; Dominici, F.; Peng, R.D. Associations of PM2.5 constituents and sources with hospital admissions: Analysis of four counties in Connecticut and Massachusetts (USA) for persons ≥ 65 years of age. Environ. Health Perspect. 2014, 122, 138–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saltelli, A.; Ratto, M.; Andres, T.; Campolongo, F.; Cariboni, J.; Gatelli, D.; Saisana, M.; Tarantola, S. Introduction to Sensitivity Analysis. In Global Sensitivity Analysis. The Primer; John Wiley & Sons, Ltd.: West Sussex, UK, 2008; pp. 1–51. [Google Scholar]
| Days with Data | Mean | SD | Minimum | 10th Percentile | 25th Percentile | Median | 75th Percentile | 90th Percentile | Maximum | Total | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| CVD HAs (N) | |||||||||||
| All, 18 to 75 Years | 9188 | 58.1 | 20.5 | 6.0 | 34.0 | 42.0 | 54.0 | 75.0 | 88.0 | 124.0 | 534,009 |
| All, 75+ Years | 9188 | 26.3 | 9.4 | 0.0 | 15.0 | 19.0 | 25.0 | 33.0 | 39.0 | 59.0 | 241,567 |
| Women, 18+ Years | 9188 | 42.3 | 14.7 | 2.0 | 25.0 | 31.0 | 40.0 | 54.0 | 63.0 | 93.0 | 389,072 |
| Men, 18+ Years | 9188 | 42.1 | 15.2 | 2.0 | 24.0 | 30.0 | 39.0 | 54.0 | 64.0 | 93.0 | 386,415 |
| Women, 75+ Years | 9188 | 16.3 | 6.3 | 0.0 | 9.0 | 12.0 | 16.0 | 20.0 | 25.0 | 43.0 | 149,557 |
| Men, 75+ Years | 9188 | 10.0 | 4.5 | 0.0 | 5.0 | 7.0 | 9.0 | 13.0 | 16.0 | 28.0 | 91,987 |
| PM2.5 and Meteorological factors | |||||||||||
| Daily Average PM2.5 Concentration (μg/m3) | 9188 | 12.2 | 5.5 | 0.6 | 6.2 | 8.2 | 11.2 | 15.0 | 19.3 | 57.5 | |
| Daily Average Temperature (°F) | 9188 | 69.1 | 14.7 | 18.0 | 47.7 | 58.0 | 71.3 | 82.0 | 86.0 | 100.0 | |
| Daily Minimum Temperature (°F) | 9188 | 58.9 | 15.2 | 12.0 | 36.7 | 46.5 | 61.5 | 72.5 | 76.3 | 88.0 | |
| Daily Maximum Temperature (°F) | 9188 | 78.8 | 14.9 | 21.5 | 57.3 | 69.3 | 81.0 | 91.0 | 96.0 | 111.0 | |
| Daily Dew Point Temperature (°F) | 9188 | 55.6 | 15.5 | 5.5 | 32.3 | 44.0 | 60.0 | 68.5 | 72.5 | 77.3 | |
| Daily Average PM2.5 Concentration | Daily Average Temperature | Daily Minimum Temperature | Daily Maximum Temperature | Daily Dew Point Temperature | |
|---|---|---|---|---|---|
| Daily Average PM2.5 Concentration | 1 | 0.26 | 0.23 | 0.27 | 0.25 |
| <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||
| Daily Average Temperature | 0.26 | 1 | 0.98 | 0.98 | 0.89 |
| <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||
| Daily Minimum Temperature | 0.23 | 0.98 | 1 | 0.92 | 0.92 |
| <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||
| Daily Maximum Temperature | 0.27 | 0.98 | 0.92 | 1 | 0.82 |
| <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||
| Daily Dew Point Temperature | 0.25 | 0.89 | 0.92 | 0.82 | 1 |
| <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Daily Counts of CVD HAs | Predictor | Linear Model 1 | Poisson Regression Model 1 | Quasi−Poisson Regression Model 2 | |||||
|---|---|---|---|---|---|---|---|---|---|
| Beta Coefficient 3 | p-Value | Beta Coefficient 3 | Percent Increase 4 | p-Value | Beta Coefficient 3 | Percent Increase 4 | p-Value | ||
| Women, 75+ | Daily PM2.5 Concentration | 0.035 (0.017, 0.053) | 0.0001 | 0.0021 (0.0012, 0.0031) | 2.16 (1.17, 3.16) | <0.0001 | 0.0021 (0.00010, 0.0032) | 2.16 (1.05, 3.29) | 0.0001 |
| CVD HAs for Men (75+) | 0.31 (0.29, 0.34) | <0.0001 | 0.018 (0.016, 0.019) | <0.0001 | 0.018 (0.016, 0.019) | <0.0001 | |||
| Men, 75+ | Daily PM2.5 Concentration | −0.000082 (−0.014, 0.014) | 0.999 | 0.00015 (−0.0011, 0.0014) | 0.15 (−1.1, 1.4) | 0.82 | 0.00015 (−0.0013, 0.0015) | 0.15 (−1.2, 1.56) | 0.83 |
| CVD HAs for Women (75+) | 0.19 (0.17, 0.20) | <0.0001 | 0.017 (0.016, 0.018) | <0.0001 | 0.017 (0.016, 0.019) | <0.0001 | |||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cox, L.A.; Liu, X.; Shi, L.; Zu, K.; Goodman, J. Applying Nonparametric Methods to Analyses of Short-Term Fine Particulate Matter Exposure and Hospital Admissions for Cardiovascular Diseases among Older Adults. Int. J. Environ. Res. Public Health 2017, 14, 1051. https://doi.org/10.3390/ijerph14091051
Cox LA, Liu X, Shi L, Zu K, Goodman J. Applying Nonparametric Methods to Analyses of Short-Term Fine Particulate Matter Exposure and Hospital Admissions for Cardiovascular Diseases among Older Adults. International Journal of Environmental Research and Public Health. 2017; 14(9):1051. https://doi.org/10.3390/ijerph14091051
Chicago/Turabian StyleCox, Louis Anthony (Tony), Xiaobin Liu, Liuhua Shi, Ke Zu, and Julie Goodman. 2017. "Applying Nonparametric Methods to Analyses of Short-Term Fine Particulate Matter Exposure and Hospital Admissions for Cardiovascular Diseases among Older Adults" International Journal of Environmental Research and Public Health 14, no. 9: 1051. https://doi.org/10.3390/ijerph14091051




