Timeliness of Childhood Primary Immunization and Risk Factors Related with Delays: Evidence from the 2014 Zhejiang Provincial Vaccination Coverage Survey
Abstract
:1. Introduction
2. Methods
2.1. Target Children
2.2. Sample Size
2.3. Sampling Procedures in the Field
2.4. Data Collection
2.5. Measurements
2.6. Statistical Analysis
2.7. Ethical Considerations
3. Results
3.1. Demographic Characteristics of Surveyed Children
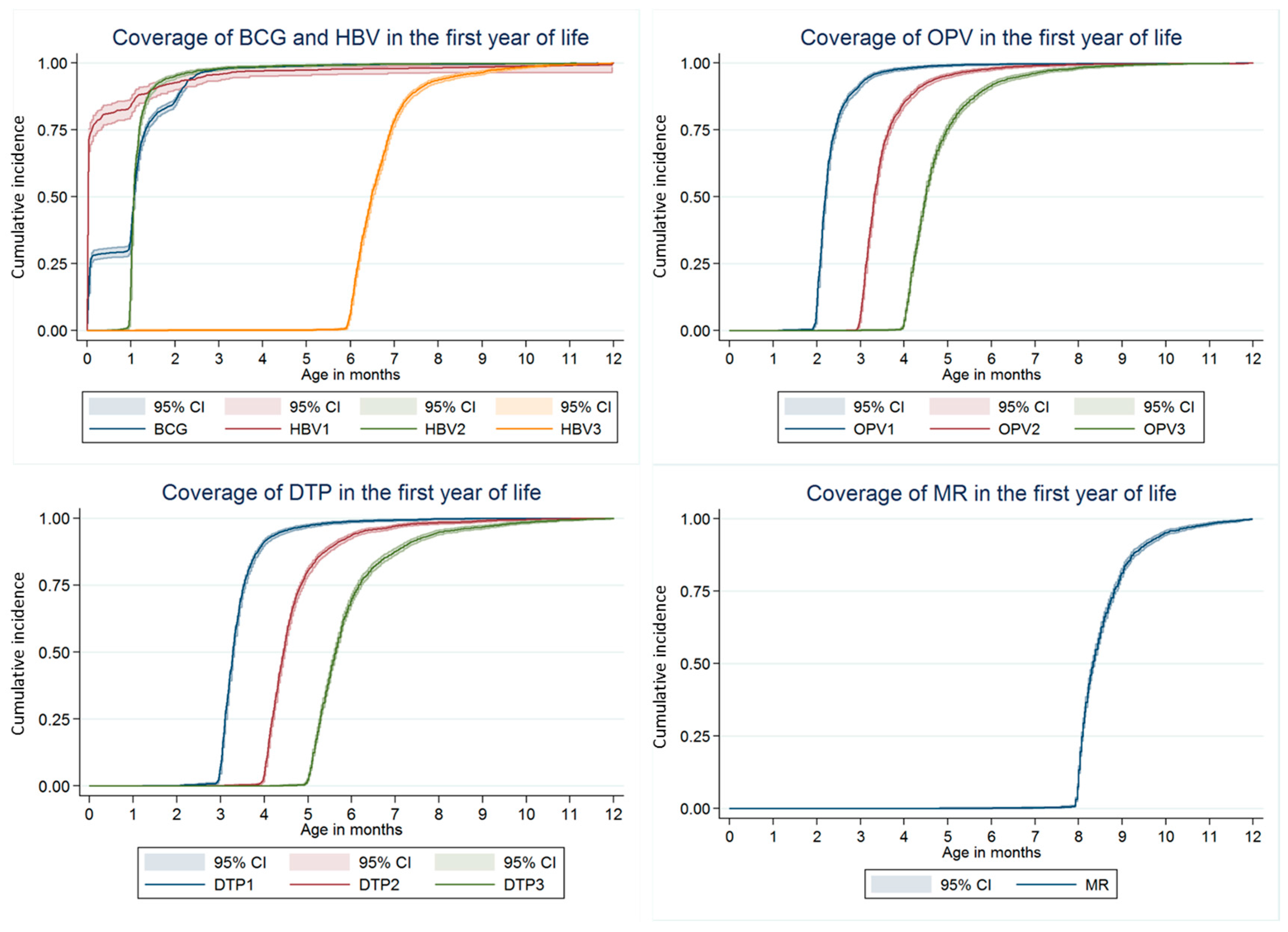
3.2. Vaccination Coverage
3.3. Reasons for Non-Vaccination
3.4. Risk Factors Related with Delayed Immunization
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bilous, J.; Eggers, R.; Gasse, F.; Jarrett, S.; Lydon, P.; Magan, A.; Okwo-Bele, J.M.; Salama, P.; Vandelaer, J.; Villeneuve, P.; et al. A new global immunisation vision and strategy. Lancet 2006, 367, 1464–1466. [Google Scholar] [CrossRef]
- Wang, Z.; Yan, R.; He, H.; Li, Q.; Chen, G.; Yang, S.; Chen, E. Difficulties in eliminating measles and controlling rubella and mumps: A cross-sectional study of a first measles and rubella vaccination and a second measles, mumps, and rubella vaccination. PLoS ONE 2014, 9, e89361. [Google Scholar] [CrossRef] [PubMed]
- Heininger, U.; Stehr, K.; Cherry, J.D. Serious pertussis overlooked in infants. Eur. J. Pediatr. 1992, 151, 342–343. [Google Scholar] [CrossRef] [PubMed]
- Hofstetter, A.M.; DuRivage, N.; Vargas, C.Y.; Camargo, S.; Vawdrey, D.K.; Fisher, A.; Stockwell, M.S. Text message reminders for timely routine mmr vaccination: A randomized controlled trial. Vaccine 2015, 33, 5741–5746. [Google Scholar] [CrossRef] [PubMed]
- Schoeps, A.; Ouedraogo, N.; Kagone, M.; Sie, A.; Muller, O.; Becher, H. Socio-demographic determinants of timely adherence to BCG, Penta3, measles, and complete vaccination schedule in Burkina Faso. Vaccine 2013, 32, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.J.; Eom, H.S.; Kim, E.S.; Choe, Y.J.; Bae, G.R.; Lee, D.H. Reemergence of measles in South Korea: Implications for immunization and surveillance programs. Jpn. J. Infect. Dis. 2013, 66, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Cutts, F.T.; Claquin, P.; Danovaro-Holliday, M.C.; Rhoda, D.A. Monitoring vaccination coverage: Defining the role of surveys. Vaccine 2016, 34, 4103–4109. [Google Scholar] [CrossRef] [PubMed]
- Fadnes, L.T.; Nankabirwa, V.; Sommerfelt, H.; Tylleskar, T.; Tumwine, J.K.; Engebretsen, I.M.; PROMISE-EBF Study Group. Is vaccination coverage a good indicator of age-appropriate vaccination? A prospective study from Uganda. Vaccine 2011, 29, 3564–3570. [Google Scholar] [CrossRef] [PubMed]
- Babirye, J.N.; Engebretsen, I.M.; Makumbi, F.; Fadnes, L.T.; Wamani, H.; Tylleskar, T.; Nuwaha, F. Timeliness of childhood vaccinations in Kampala Uganda: A community-based cross-sectional study. PLoS ONE 2012, 7, e35432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akmatov, M.K.; Mikolajczyk, R.T. Timeliness of childhood vaccinations in 31 low and middle-income countries. J. Epidemiol. Glob. Community Health 2012, 66, e14. [Google Scholar] [CrossRef] [PubMed]
- Luman, E.T.; Barker, L.E.; Shaw, K.M.; McCauley, M.M.; Buehler, J.W.; Pickering, L.K. Timeliness of childhood vaccinations in the United States: Days undervaccinated and number of vaccines delayed. JAMA 2005, 293, 1204–1211. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Chen, Y.; Guo, J.; Tang, X.; Shen, L. Completeness and timeliness of vaccination and determinants for low and late uptake among young children in eastern China. Hum. Vaccin. Immunother. 2014, 10, 1408–1415. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Li, Q.; Luo, S.; Lou, L.; Qi, X.; Xie, S. Timeliness vaccination of measles containing vaccine and barriers to vaccination among migrant children in east China. PLoS ONE 2013, 8, e73264. [Google Scholar] [CrossRef] [PubMed]
- Cutts, F.T. The use of the WHO cluster survey method for evaluating the impact of the expanded programme on immunization on target disease incidence. J. Trop. Med. Hyg. 1988, 91, 231–239. [Google Scholar] [PubMed]
- Gaumer, G.L.; Glazier, R.E., Jr.; Cowen, K.S. A review of pps research coverage. Health Aff. 1987, 6, 148–151. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, Y.; Zhang, B.; Li, Q. An evaluation of voluntary varicella vaccination coverage in Zhejiang province, east China. Int. J. Environ. Res. Public Health 2016, 13, 560. [Google Scholar] [CrossRef] [PubMed]
- Shrivastwa, N.; Gillespie, B.W.; Lepkowski, J.M.; Boulton, M.L. Vaccination timeliness in children under India’s universal immunization program. Pediatr. Infect. Dis J. 2016, 35, 955–960. [Google Scholar] [CrossRef] [PubMed]
- Lernout, T.; Theeten, H.; Hens, N.; Braeckman, T.; Roelants, M.; Hoppenbrouwers, K.; Van Damme, P. Timeliness of infant vaccination and factors related with delay in Flanders, Belgium. Vaccine 2014, 32, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Le Polain de Waroux, O.; Schellenberg, J.R.; Manzi, F.; Mrisho, M.; Shirima, K.; Mshinda, H.; Alonso, P.; Tanner, M.; Schellenberg, D.M. Timeliness and completeness of vaccination and risk factors for low and late vaccine uptake in young children living in rural southern Tanzania. Int. Health 2013, 5, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Valcarcel Salamanca, B.; Hagerup-Jenssen, M.E.; Flem, E. Uptake and timeliness of rotavirus vaccination in Norway: The first year post-introduction. Vaccine 2016, 34, 4684–4689. [Google Scholar] [CrossRef] [PubMed]
- Akmatov, M.K.; Kretzschmar, M.; Kramer, A.; Mikolajczyk, R.T. Timeliness of vaccination and its effects on fraction of vaccinated population. Vaccine 2008, 26, 3805–3811. [Google Scholar] [CrossRef] [PubMed]
- Poorolajal, J.; Khazaei, S.; Kousehlou, Z.; Bathaei, S.; Zahiri, A. Delayed vaccination and related predictors among infants. Iran. J. Public Health 2012, 41, 65–71. [Google Scholar] [PubMed]
- Domek, G.J.; Contreras-Roldan, I.L.; O’Leary, S.T.; Bull, S.; Furniss, A.; Kempe, A.; Asturias, E.J. SMS text message reminders to improve infant vaccination coverage in Guatemala: A pilot randomized controlled trial. Vaccine 2016, 34, 2437–2443. [Google Scholar] [CrossRef] [PubMed]
- Regan, A.K.; Blyth, C.C.; Mak, D.B.; Richmond, P.C.; Effler, P.V. Using sms to monitor adverse events following trivalent influenza vaccination in pregnant women. Aust. N. Z. J. Obstet. Gynaecol. 2014, 54, 522–528. [Google Scholar] [CrossRef] [PubMed]
- Bexelius, C.; Merk, H.; Sandin, S.; Ekman, A.; Nyren, O.; Kuhlmann-Berenzon, S.; Linde, A.; Litton, J.E. SMS versus telephone interviews for epidemiological data collection: Feasibility study estimating influenza vaccination coverage in the Swedish population. Eur. J. Epidemiol. 2009, 24, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Tran, V.; Leung, A.S.; Alexander, D.C.; Zhu, B. BCG vaccines: Their mechanisms of attenuation and impact on safety and protective efficacy. Hum. Vaccines 2009, 5, 70–78. [Google Scholar] [CrossRef]
- Verreck, F.A.W.; Tchilian, E.Z.; Vervenne, R.A.W.; Sombroek, C.C.; Kondova, I.; Eissen, O.A.; Sommandas, V.; van der Werff, N.M.; Verschoor, E.; Braskamp, G.; et al. Variable BCG efficacy in rhesus populations: Pulmonary BCG provides protection where standard intra-dermal vaccination fails. Tuberculosis 2017, 104, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Hawkridge, A.; Hatherill, M.; Little, F.; Goetz, M.A.; Barker, L.; Mahomed, H.; Sadoff, J.; Hanekom, W.; Gaiter, L.; South African BCG trial team. Efficacy of percutaneous versus intradermal BCG in the prevention of tuberculosis in South Africa infants: Randomised trial. Rev. Port Pneumol. 2009, 15, 747–749. [Google Scholar] [CrossRef]
- Romano, M.; Huygen, K. An update on vaccines for tuberculosis—There is more to it than just waning of BCG efficacy with time. Expert Opin. Biol. Ther. 2012, 12, 1601–1610. [Google Scholar] [CrossRef] [PubMed]
- Bielicki, J.A.; Achermann, R.; Berger, C. Timing of measles immunization and effective population vaccine coverage. Pediatrics 2012, 130, e600–e606. [Google Scholar] [CrossRef] [PubMed]
- Gay, N.J. Eliminating measles—No quick fix. Bull. World Health Organ. 2000, 78, 949. [Google Scholar] [PubMed]
- World Health Organization. The Children’s Vaccine Initiative (CVI) and WHO’S Global Programme for Vaccines and Immunization (GPV): Recommendations from the Scientific Advisory Group of Experts (SAGE). Available online: http://apps.who.int/iris/handle/10665/230575 (accessed on 19 September 2017).
- Romanelli, F.; Freeman, T. Immunization training: Right or privilege? Am. J. Pharm. Educ. 2012, 76, 57. [Google Scholar] [CrossRef] [PubMed]
- Marcum, Z.A.; Maffeo, C.M.; Kalsekar, I. The impact of an immunization training certificate program on the perceived knowledge, skills and attitudes of pharmacy students toward pharmacy-based immunizations. Pharm. Pract. 2010, 8, 103–108. [Google Scholar] [CrossRef]
- Uskun, E.; Uskun, S.B.; Uysalgenc, M.; Yagiz, M. Effectiveness of a training intervention on immunization to increase knowledge of primary healthcare workers and vaccination coverage rates. Public Health 2008, 122, 949–958. [Google Scholar] [CrossRef] [PubMed]
- Owais, A.; Hanif, B.; Siddiqui, A.R.; Agha, A.; Zaidi, A.K. Does improving maternal knowledge of vaccines impact infant immunization rates? A community-based randomized-controlled trial in Karachi, Pakistan. BMC Public Health 2011, 11, 239. [Google Scholar] [CrossRef] [PubMed]
- Rammohan, A.; Awofeso, N.; Fernandez, R.C. Paternal education status significantly influences infants’ measles vaccination uptake, independent of maternal education status. BMC Public Health 2012, 12, 336. [Google Scholar] [CrossRef] [PubMed]
- Nankabirwa, V.; Tylleskar, T.; Tumwine, J.K.; Sommerfelt, H.; Promise-ebf Study Group. Maternal education is associated with vaccination status of infants less than 6 months in eastern Uganda: A cohort study. BMC Pediatrics 2010, 10, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takum, T.; Padung, D.; Joshua, V.; Manickam, P.; Murhekar, M.V. Programmatic and beneficiary-related factors for low vaccination coverage in Papum Pare district, Arunachal Pradesh, India. J. Trop. Pediatr. 2011, 57, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Mutua, M.K.; Kimani-Murage, E.; Ettarh, R.R. Childhood vaccination in informal urban settlements in Nairobi, Kenya: Who gets vaccinated? BMC Public Health 2011, 11, 6. [Google Scholar] [CrossRef] [PubMed]
- Ganczak, M.; Dmytrzyk-Danilow, G.; Karakiewicz, B.; Korzen, M.; Szych, Z. Determinants influencing self-paid vaccination coverage, in 0–5 years old Polish children. Vaccine 2013, 31, 5687–5692. [Google Scholar] [CrossRef] [PubMed]
- Burgess, D.C.; Burgess, M.A.; Leask, J. The mmr vaccination and autism controversy in United Kingdom 1998–2005: Inevitable community outrage or a failure of risk communication? Vaccine 2006, 24, 3921–3928. [Google Scholar] [CrossRef] [PubMed]
- Babirye, J.N.; Rutebemberwa, E.; Kiguli, J.; Wamani, H.; Nuwaha, F.; Engebretsen, I.M. More support for mothers: A qualitative study on factors affecting immunisation behaviour in Kampala, Uganda. BMC Public Health 2011, 11, 723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ettarh, R.R.; Mutua, M.K.; Kyobutungi, C. Ethnicity and delay in measles vaccination in a Nairobi slum. Trop. Med. Health 2012, 40, 59–62. [Google Scholar] [CrossRef] [PubMed]
- Enkhtuya, B.; Badamusuren, T.; Dondog, N.; Khandsuren, L.; Elbegtuya, N.; Jargal, G.; Surenchimeg, V.; Grundy, J. Reaching every district—Development and testing of a health micro-planning strategy for reaching difficult to reach populations in Mongolia. Rural Remote Health 2009, 9, 1045. [Google Scholar] [PubMed]
| Variables | No. of Children (%) N = 2772 |
|---|---|
| Sex of child | |
| Male | 1395 (50.3) |
| Female | 1377 (49.7) |
| Number of children in the surveyed household | |
| 1 | 1952 (70.4) |
| 2 | 651 (23.5) |
| ≥3 | 169 (6.1) |
| Place of delivery | |
| Hospital | 2525 (91.1) |
| Home | 247 (8.9) |
| Age of mother (years) | |
| <30 | 1826 (65.9) |
| ≥30 | 946 (34.1) |
| Maternal education level | |
| <senior middle school a | 583 (21.0) |
| ≥senior middle school | 2189 (79.0) |
| Maternal employment status | |
| Home fulltime | 367 (13.2) |
| Employed | 2405 (86.8) |
| Residence | |
| Urban | 1362 (49.1) |
| Rural | 1410 (50.9) |
| Immigration status | |
| Resident | 1661 (59.9) |
| Migrant | 1111 (40.1) |
| Family income per month (US dollars) b | 1038.9 ± 15.8 |
| Vaccine Dose | General Coverage | Acceptable Timely Coverage | Age-Appropriate Coverage | |||
|---|---|---|---|---|---|---|
| n | % (95% CI) | n | % (95% CI) | n | % (95% CI) | |
| BCG | 2759 | 99.5 (99.3–99.8) | 2746 | 99.1 (98.7–99.4) | 703 | 25.4 (23.7–27.0) |
| HBV1 | 2763 | 99.7 (99.5–99.9) | 2760 | 99.6 (99.3–99.8) | 2635 | 95.1 (94.3–95.9) |
| HBV2 | 2762 | 99.6 (99.4–99.9) | 2755 | 99.4 (99.1–99.7) | 2619 | 94.5 (93.7–95.3) |
| HBV3 | 2760 | 99.6 (99.3–99.8) | 2751 | 99.2 (98.9–99.6) | 2118 | 76.4 (74.8–77.9) |
| OPV1 | 2765 | 99.8 (99.6–99.9) | 2754 | 99.4 (99.1–99.7) | 2534 | 91.3 (90.3–92.4) |
| OPV2 | 2764 | 99.7 (99.5–99.9) | 2746 | 99.1 (98.7–99.4) | 2327 | 83.9 (82.5–85.4) |
| OPV3 | 2762 | 99.6 (99.4–99.9) | 2732 | 98.6 (98.1–99.0) | 2062 | 74.4 (72.7–76.0) |
| DTP1 | 2765 | 99.8 (99.6–99.9) | 2746 | 99.1 (98.7–99.4) | 2487 | 89.7 (88.6–90.9) |
| DTP2 | 2762 | 99.6 (99.4–99.9) | 2732 | 98.6 (98.1–99.0) | 2182 | 78.7 (77.2–80.3) |
| DTP3 | 2755 | 99.4 (99.1–99.7) | 2701 | 97.4 (96.9–98.0) | 1865 | 67.3 (65.5–69.0) |
| MR | 2756 | 99.4 (99.1–99.7) | 2665 | 96.1 (95.4–96.9) | 2172 | 78.4 (76.8–79.9) |
| FI a | 2566 | 92.6 (91.8–93.3) | 2406 | 86.8 (85.4–88.3) | - | - |
| Reasons | BCG (n = 12) | HBV1 (n = 9) | HBV2 (n = 10) | HBV3 (n = 12) | OPV1 (n = 7) | OPV2 (n = 8) | OPV3 (n = 10) | DTP1 (n = 7) | DTP2 (n = 10) | DTP3 (n = 17) | MR (n = 16) | All (n = 119) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | |
| Was not aware that the child needed this vaccination | 1 | 7.7 | 3 | 25.0 | 1 | 14.3 | 1 | 10.0 | 2 | 28.6 | 1 | 10.0 | 3 | 17.6 | 12 | 10.1 | ||||||||
| The immunization clinic was overcrowded | 2 | 20.0 | 2 | 16.7 | 1 | 14.3 | 1 | 12.5 | 1 | 10.0 | 1 | 14.3 | 1 | 10.0 | 2 | 11.8 | 1 | 6.25 | 12 | 10.1 | ||||
| The schedule of the immunization clinic was incompatible with working hours | 6 | 60.0 | 4 | 33.3 | 1 | 14.3 | 2 | 25 | 2 | 20.0 | 2 | 20.0 | 3 | 17.6 | 4 | 25 | 24 | 20.2 | ||||||
| Fear of adverse events | 9 | 69.2 | 2 | 28.6 | 1 | 12.5 | 3 | 30.0 | 3 | 42.9 | 3 | 30.0 | 3 | 17.6 | 2 | 12.5 | 26 | 21.8 | ||||||
| The child was sick when the vaccine was due | 1 | 10.0 | 2 | 16.7 | 2 | 25 | 1 | 14.3 | 1 | 10.0 | 3 | 17.6 | 7 | 43.8 | 17 | 14.3 | ||||||||
| The physicians had contraindicated the vaccine | 9 | 100.0 | 1 | 10.0 | 1 | 8.3 | 2 | 28.6 | 2 | 25 | 2 | 20.0 | 2 | 20.0 | 2 | 11.8 | 2 | 12.5 | 23 | 19.3 | ||||
| Considered vaccination not important | 3 | 23.1 | 1 | 10.0 | 1 | 5.88 | 5 | 4.2 | ||||||||||||||||
| Demographic and Socio-Economic Variables | BCG | HBV1 | HBV3 | OPV1 | OPV3 | DTP1 | DTP3 | MR | |
|---|---|---|---|---|---|---|---|---|---|
| Mother’s education level | <senior middle school | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| ≥senior middle school | NS | 0.91 (0.87–0.99) * | 0.85 (0.80–0.92) * | NS | NS | NS | 0.84 (0.75–0.92) * | 0.79 (0.72–0.87) ** | |
| Mother’s occupation | Home fulltime | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| Employed | NS | NS | 1.57 (1.38–1.70) ** | NS | 1.25 (1.17–1.40) * | 1.05 (1.00–1.09) * | 1.42 (1.35–1.55) ** | 1.61 (1.40–1.74) ** | |
| Number of children in the surveyed household | 1 | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| 2 | 1.06 (0.86–1.14) | NS | 1.05 (0.91–1.17) | NS | 1.07 (0.92–1.25) | NS | NS | NS | |
| ≥3 | 1.18 (1.12–1.31) * | NS | 1.12 (1.03–1.19) * | NS | 1.09 (1.03–1.15) * | NS | NS | NS | |
| Place of delivery | Hospital | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| Home | NS | 1.76 (1.43–2.21) ** | NS | NS | NS | 1.17 (1.10–1.27) * | NS | 1.38 (1.20–1.51) ** | |
| Family income per month a | NS | NS | 0.79 (0.72–0.90) ** | NS | 0.88 (0.82–0.96) * | NS | 0.85 (0.80–0.93) * | 0.75 (0.69–0.88) ** | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Y.; Li, Q.; Chen, Y. Timeliness of Childhood Primary Immunization and Risk Factors Related with Delays: Evidence from the 2014 Zhejiang Provincial Vaccination Coverage Survey. Int. J. Environ. Res. Public Health 2017, 14, 1086. https://doi.org/10.3390/ijerph14091086
Hu Y, Li Q, Chen Y. Timeliness of Childhood Primary Immunization and Risk Factors Related with Delays: Evidence from the 2014 Zhejiang Provincial Vaccination Coverage Survey. International Journal of Environmental Research and Public Health. 2017; 14(9):1086. https://doi.org/10.3390/ijerph14091086
Chicago/Turabian StyleHu, Yu, Qian Li, and Yaping Chen. 2017. "Timeliness of Childhood Primary Immunization and Risk Factors Related with Delays: Evidence from the 2014 Zhejiang Provincial Vaccination Coverage Survey" International Journal of Environmental Research and Public Health 14, no. 9: 1086. https://doi.org/10.3390/ijerph14091086




