Comparison of Physiological and Psychological Relaxation Using Measurements of Heart Rate Variability, Prefrontal Cortex Activity, and Subjective Indexes after Completing Tasks with and without Foliage Plants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
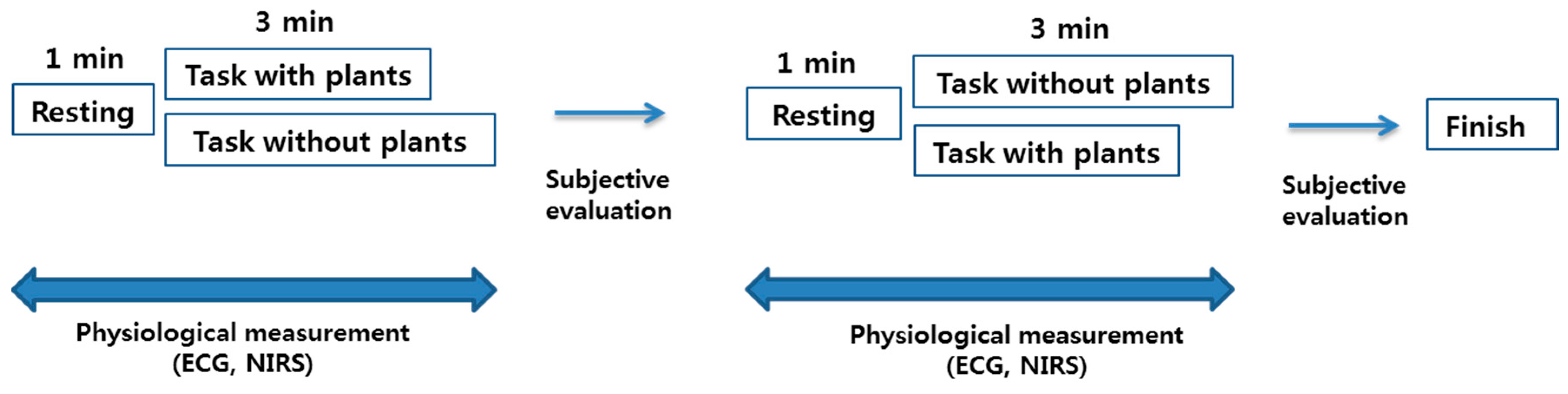
2.2. Experimental Protocol
2.3. Measurement of Heart Rate Variability and Prefrontal Cortex Activity
2.4. Subjective Evaluation
2.5. Data Analysis
3. Results and Discussion
3.1. Physiological Relaxation
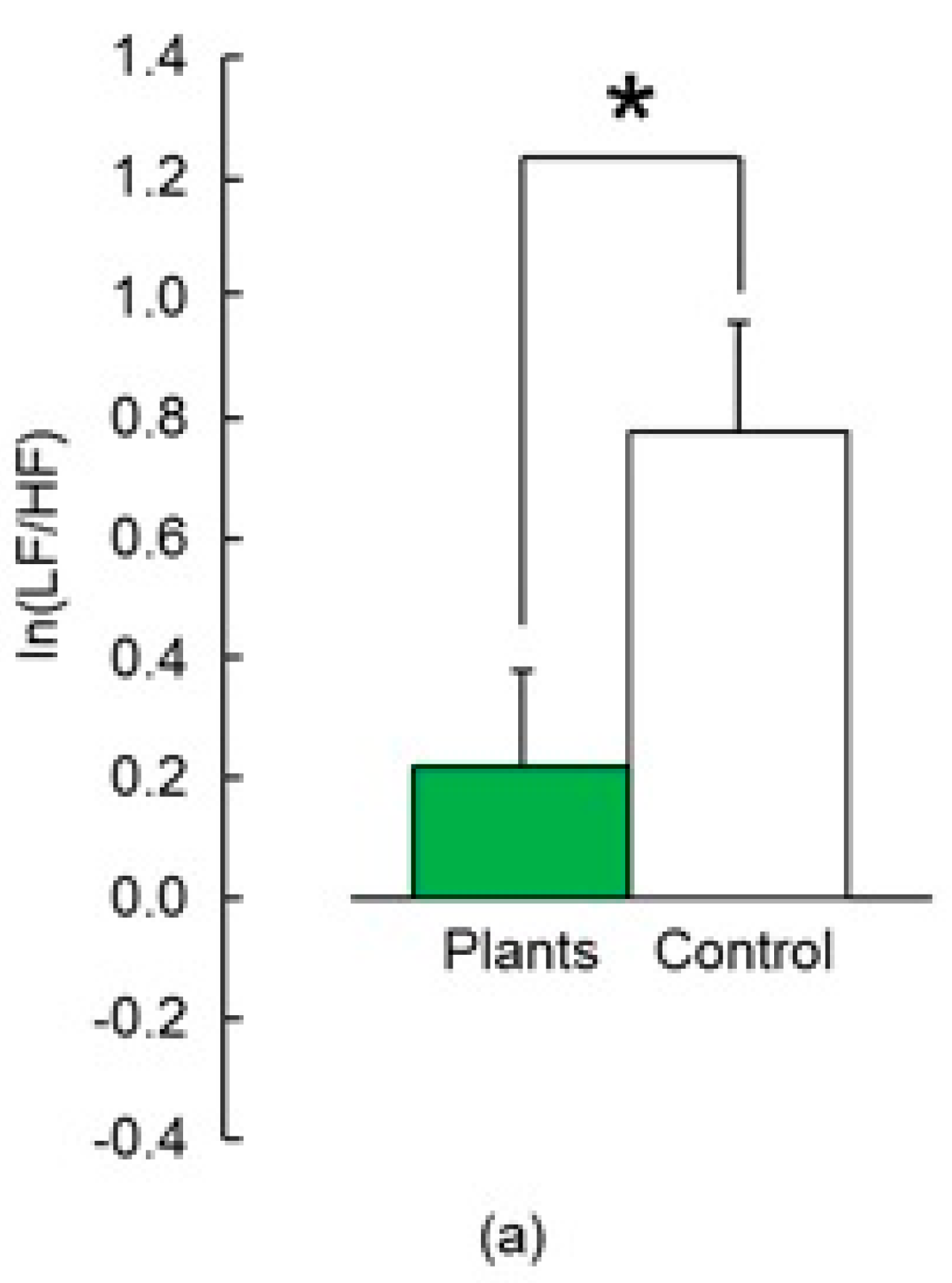
3.1.1. Heart Rate Variability
3.1.2. Prefrontal Cortex Activity
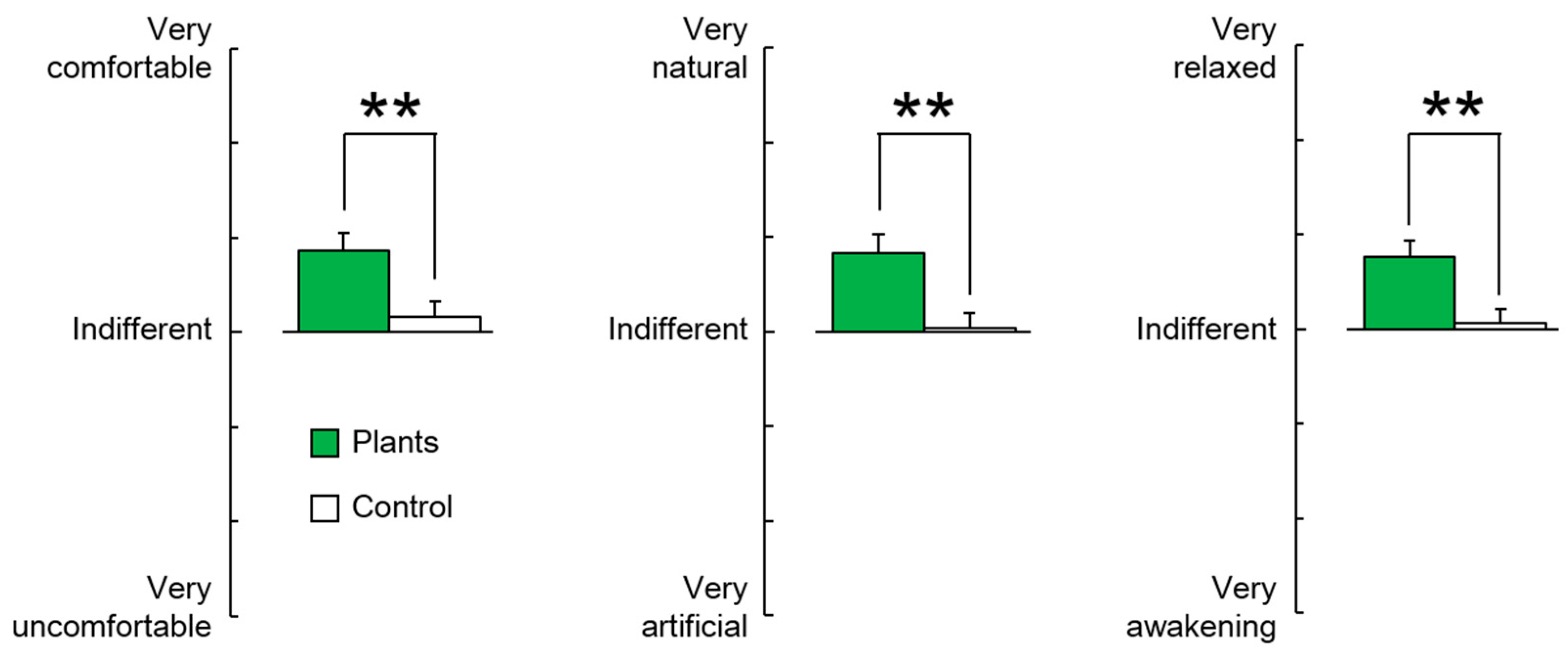
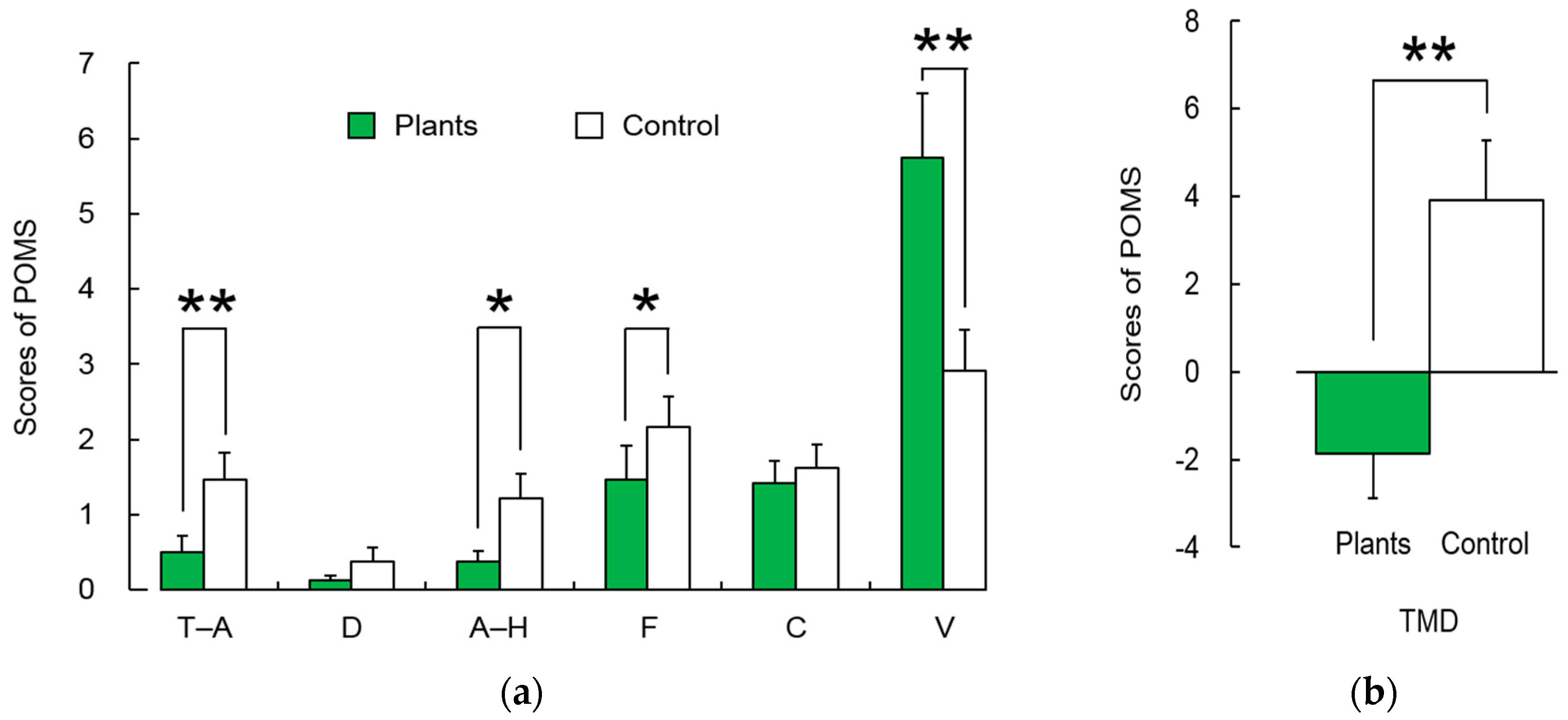
3.2. Subjective Evaluation
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dye, C. Health and urban living. Science 2008, 319, 766–769. [Google Scholar] [CrossRef] [PubMed]
- Peen, J.; Schoevers, R.A.; Beekman, A.T.; Dekker, J. The current status of urbanrural differences in psychiatric disorders. Acta Psychiatr. Scand. 2010, 121, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Krabbendam, L.; Van Os, J. Schizophrenia and Urbanicity: A Major Environmental influence—Conditional on Genetic Risk. Schizophr. Bull. 2005, 31, 795–799. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, C.B.; Mortensen, P.B. Evidence of a Dose-Response Relationship between Urbanicity during Upbringing and Schizophrenia Risk. Arch. Gen. Psychiatry 2001, 58, 1039–1046. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, C.; Stewart, I.D.; Ibrahim, N.; Facchini, A.; Mele, R. Developing a Multi-Layered Indicator Set for Urban Metabolism Studies in Megacities. Ecol. Ind. 2014, 47, 7–15. [Google Scholar] [CrossRef]
- Pezawas, L.; Meyer-Lindenberg, A.; Drabant, E.M.; Verchinski, B.A.; Munoz, K.E.; Kolachana, B.S.; Egan, M.F.; Mattay, V.S.; Hariri, A.R.; Weinberger, D.R. 5-HTTLPR Polymorphism Impacts Human Cingulate-Amygdala Interactions: A Genetic Susceptibility Mechanism for Depression. Nat. Neurosci. 2005, 8, 828. [Google Scholar] [CrossRef] [PubMed]
- Diorio, D.; Viau, V.; Meaney, M.J. The Role of the Medial Prefrontal Cortex (Cingulate Gyrus) in the Regulation of Hypothalamic-Pituitary-Adrenal Responses to Stress. J. Neurosci. 1993, 13, 3839–3847. [Google Scholar] [PubMed]
- Bowler, D.E.; Buyung-Ali, L.M.; Knight, T.M.; Pullin, A.S. A systematic review of evidence for the added benefits to health of exposure to natural environments. BMC Public Health 2010, 10, 456. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.M.; Jones, R.; Tocchini, K. Shinrin-Yoku (Forest Bathing) and Nature Therapy: A State-of-the-Art Review. Int. J. Environ. Res. Public Health 2017, 14, 851. [Google Scholar] [CrossRef] [PubMed]
- Franco, L.S.; Shanahan, D.F.; Fuller, R.A. A Review of the Benefits of Nature Experiences: More than Meets the Eye. Int. J. Environ. Res. Public Health 2017, 14, 864. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Ikei, H.; Miyazaki, Y. Physiological Effects of Nature Therapy: A Review of the Research in Japan. Int. J. Environ. Res. Public Health 2016, 13, 781. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, R.S.; Simons, R.F.; Losito, B.D.; Fiorito, E.; Miles, M.A.; Zelson, M. Stress recovery during exposure to natural and urban environments. J. Environ. Psychol. 1991, 11, 201–230. [Google Scholar] [CrossRef]
- Kaplan, S. The restorative benefits of nature: Toward an integrative framework. J. Environ. Psychol. 1995, 15, 169–182. [Google Scholar] [CrossRef]
- Wilson, E.O. Biophilia; Harvard University Press: Cambridge, MA, USA, 1984. [Google Scholar]
- Ikei, H.; Song, C.; Miyazaki, Y. Physiological Effects of Wood on Humans: A Review. J. Wood Sci. 2017, 63, 1–23. [Google Scholar] [CrossRef]
- Park, S.A.; Song, C.; Choi, J.Y.; Son, K.C.; Miyazaki, Y. Foliage plants cause physiological and psychological relaxation as evidenced by measurements of prefrontal cortex activity and profile of mood states. HortScience 2016, 51, 1308–1312. [Google Scholar] [CrossRef]
- Ikei, H.; Song, C.; Igarashi, M.; Namekawa, T.; Miyazaki, Y. Physiological and psychological relaxing effects of visual stimulation with foliage plants in high school students. Adv. Hortc. Sci. 2014, 28, 111–116. [Google Scholar]
- Ikei, H.; Komatsu, M.; Song, C.; Himoro, E.; Miyazaki, Y. The physiological and psychological relaxing effects of viewing rose flowers in office workers. J. Physiol. Anthropol. 2014, 33, 6. [Google Scholar] [CrossRef] [PubMed]
- Bringslimark, T.; Hartig, T.; Patil, G.G. The psychological benefits of indoor plants: A critical review of the experimental literature. J. Environ. Psychol. 2009, 29, 422–433. [Google Scholar] [CrossRef]
- Lee, J.; Park, B.J.; Tsunetsugu, Y.; Ohira, T.; Kagawa, T.; Miyazaki, Y. Effect of Forest Bathing on Physiological and Psychological Responses in Young Japanese Male Subjects. Public Health 2011, 125, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Park, B.J.; Tsunetsugu, Y.; Kasetani, T.; Kagawa, T.; Miyazaki, Y. The Physiological Effects of Shinrin-Yoku (Taking in the Forest Atmosphere or Forest Bathing): Evidence from Field Experiments in 24 Forests across Japan. Environ. Health Prev. Med. 2010, 15, 18. [Google Scholar] [CrossRef] [PubMed]
- Park, B.J.; Tsunetsugu, Y.; Kasetani, T.; Morikawa, T.; Kagawa, T.; Miyazaki, Y. Physiological Effects of Forest Recreation in a Young Conifer Forest in Hinokage Town, Japan. Silva Fenn. 2009, 43, 291–301. [Google Scholar] [CrossRef]
- Tsunetsugu, Y.; Lee, J.; Park, B.J.; Tyrväinen, L.; Kagawa, T.; Miyazaki, Y. Physiological and Psychological Effects of Viewing Urban Forest Landscapes Assessed by Multiple Measurements. Landsc. Urban Plan. 2013, 113, 90–93. [Google Scholar] [CrossRef]
- Tsunetsugu, Y.; Park, B.J.; Ishii, H.; Hirano, H.; Kagawa, T.; Miyazaki, Y. Physiological Effects of Shinrin-Yoku (Taking in the Atmosphere of the Forest) in an Old-Growth Broadleaf Forest in Yamagata Prefecture, Japan. J. Physiol. Anthropol. 2007, 26, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, R.S. View through a window may influence recovery from surgery. Science 1984, 224, 420–421. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, M.; Ikei, H.; Song, C.; Miyazaki, Y. Effects of olfactory stimulation with rose and orange oil on prefrontal cortex activity. Complement Ther. Med. 2014, 22, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, M.; Song, C.; Ikei, H.; Miyazaki, Y. Effects of olfactory stimulation with perilla essential oil on prefrontal cortex activity. J. Altern. Complement. Med. 2014, 20, 545–549. [Google Scholar] [CrossRef] [PubMed]
- Ikei, H.; Song, C.; Miyazaki, Y. Physiological effect of olfactory stimulation by Hinoki cypress (Chamaecyparis obtusa) leaf oil. J. Physiol. Anthropol. 2015, 34, 44. [Google Scholar] [CrossRef] [PubMed]
- Tsunetsugu, Y.; Park, B.J.; Miyazaki, Y. Physiological Effects of Visual, Olfactory, Auditory, and Tactile Factors in the Forest Environment. In Forest Medicine, 1st ed.; Li, Q., Ed.; Nova Science Publishers Inc.: New York, NY, USA, 2012; pp. 169–181. [Google Scholar]
- Kimura, A.; Sugiyama, H.; Sasaki, S.; Yatagai, M. Psychological and Physiological Effects in Humans Induced by the Visual and Olfactory Stimulations of an Interior Environment made of Hiba (Thujopsis Dolabrata) Wood. Mokuzai Gakkaishi 2011, 57, 150–159. [Google Scholar] [CrossRef]
- Ikei, H.; Song, C.; Miyazaki, Y. Physiological effect of contact to Japanese cypress wood with the palm. In Proceedings of the 67th Annual Meeting of Japan Wood Research Society, Fukuoka, Japan, 17–19 March 2017. (In Japanese). [Google Scholar]
- Lee, M.S.; Park, B.J.; Lee, J.; Park, K.T.; Ku, J.H.; Lee, J.W.; Oh, K.O.; Miyazaki, Y. Physiological relaxation induced by horticultural activity: Transplanting work using flowering plants. J. Physiol. Anthropol. 2013, 32, 15. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Lee, J.; Park, B.J.; Miyazaki, Y. Interaction with Indoor Plants may Reduce Psychological and Physiological Stress by Suppressing Autonomic Nervous System Activity in Young Adults: A Randomized Crossover Study. J. Physiol. Anthropol. 2015, 34, 21. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Apps. WHO. Available online: Int/bmi/index.jsp (accessed on 27 July 2012).
- Piantadosi, S. Crossover designs. In Clinical Trials: A Methodologic Perspective; Steven, P., Ed.; Wiley: New York, NY, USA, 2005; pp. 515–527. [Google Scholar]
- Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: Standards of measurement, physiological interpretation, and clinical use. Circulation 1996, 93, 1043–1065. [Google Scholar]
- Pagani, M.; Lombardi, F.; Guzzetti, S.; Rimoldi, O.; Furlan, R.; Pizzinelli, P.; Sandrone, G.; Malfatto, G.; Dell’Orto, S.; Piccaluga, E.; et al. Power spectral analysis of heart rate and arterial pressure variabilities as a marker of sympatho-vagal interaction in man and conscious dog. Circ. Res. 1986, 59, 178–193. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Park, B.J.; Miyazaki, Y. Normative references of heart rate variability and salivary alpha-amylase in a healthy young male population. J. Physiol. Anthropol. 2012, 31, 9. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Mizuno, T.; Shikayama, T.; Miwa, M. Development of a wireless near-infrared tissue oxygen monitor system with high sampling rate. In Digital Holography and Three-Dimensional Imaging; OSA Publishing: Washington, DC, USA, 2012. [Google Scholar]
- Perry, S. NIRS for measuring cerebral hemodynamic responses during exercise. In Functional Neuroimaging in Exercise and Sport Sciences; Boecker, H., Hillman, C.H., Scheef, L., Strüder, H.K., Eds.; Springer: New York, NY, USA, 2012; pp. 335–349. [Google Scholar]
- Jöbsis, F.F. Noninvasive, infrared monitoring of cerebral and myocardial oxygen sufficiency and circulatory parameters. Science 1977, 198, 1264–1267. [Google Scholar] [CrossRef] [PubMed]
- Perrey, S. Non-invasive NIR spectoroscopy of human brain function during exercise. Methods 2008, 45, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Osgood, C.E.; Suci, G.J.; Tannenbaum, P.H. The Measurement of Meaning; University of Illinois Press: Chicago, IL, USA, 1957. [Google Scholar]
- McNair, D.M.; Heuchert, J.P.; Shilony, E. Profile of Mood States Bibliography 1964–2002; Multi-Health Systems Inc.: North Tonawanda, NY, USA, 2003. [Google Scholar]
- Osgood, C.E. The nature and measurement of meaning. Psychol. Bull. 1952, 49, 197–237. [Google Scholar] [CrossRef] [PubMed]
- Yeun, E.J.; Shin-Park, K.K. Verification of the profile of mood states-brief: Cross-cultural analysis. J. Clin. Psychol. 2006, 62, 1173–1180. [Google Scholar] [CrossRef] [PubMed]
- Baker, F.; Denniston, M.; Zabora, J.; Polland, A.; Dudley, W.N. A POMS short form for cancer patients: Psychometric and structural evaluation. Psychooncology 2002, 11, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Taelman, J.; Vandeput, S.; Spaepen, A.; Huffel, S. Influence of mental stress on heart rate and heart rate variability. In Proceedings of the 4th European Conference of the International Federation for Medical and Biological Engineering, Antwerp, Belgium, 23–28 November 2008; Springer: Berlin/Heidelberg, Germany, 2009; pp. 1366–1369. [Google Scholar]
- Igarashi, M.; Aga, M.; Ikei, H.; Namekawa, T.; Miyazaki, Y. Physiological and psychological effects on high school students of viewing real and artificial pansies. Int. J. Environ. Res. Public Health 2015, 12, 2521–2531. [Google Scholar] [CrossRef] [PubMed]
- MacNeilage, P.F.; Rogers, L.J.; Vallortigara, G. Origins of the left & right brain. Sci. Am. 2009, 301, 60–67. [Google Scholar] [PubMed]
- Miyazaki, Y.; Park, B.J.; Lee, J. Nature therapy. In Designing Our Future: Local Perspectives on Bioproduction, Ecosystems and Humanity; Osaki, M., Braimoh, A., Nakagami, K., Eds.; United Nations University Press: New York, NY, USA, 2011; pp. 407–412. [Google Scholar]
- Rogers, R.L.; Meyer, J.S.; Mortel, K.F.; Mahurin, R.K.; Thornby, J. Age-related reductions in cerebral vasomotor reactivity and the law of initial value: A 4-year prospective longitudinal study. J. Cereb. Blood Flow Metab. 1985, 5, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Scher, H.; Furedy, J.J. Individual differences in phasic cardiac reactivity to psychological stress and the law of initial value. Psychophysiology 1985, 22, 345–348. [Google Scholar] [CrossRef] [PubMed]
- Park, S.A.; Lee, K.S.; Son, K.C. Determining exercise intensities of gardening tasks as a physical activity using metabolic equivalents in older adults. HortScience 2011, 46, 1706–1710. [Google Scholar]
- Park, S.A.; Lee, K.S.; Son, K.C.; Shoemaker, C. Metabolic cost of horticulture activities in older adults. J. Jpn. Soc. Hortc. Sci. 2012, 81, 295–299. [Google Scholar] [CrossRef]
- Park, S.A.; Lee, J.Y.; Lee, K.S.; Son, K.C. Metabolic costs of daily activities in community-dwelling older adults. Int. J. Gerontol. 2014, 8, 228–229. [Google Scholar] [CrossRef]
- Park, S.A.; Oh, S.R.; Lee, K.S.; Son, K.C. Electromyographic analysis of upper limb and hand muscles during horticultural activity motions. HortTechnology 2013, 23, 51–56. [Google Scholar]
- Park, S.A.; Lee, A.Y.; Kim, J.J.; Lee, K.S.; So, J.M.; Son, K.C. Electromyographic analysis of upper and lower limb muscles during gardening tasks. Korean J. Hortc. Sci. Technol. 2014, 32, 710–720. [Google Scholar] [CrossRef]
- Park, S.A.; Lee, A.Y.; Son, K.C.; Lee, W.L.; Kim, D.S. Gardening intervention for physical and psychological health benefits in elderly women at community centers. HortTechnology 2016, 26, 474–483. [Google Scholar]
- Park, S.A.; Shoemaker, C.; Haub, M. Can older gardeners meet the physical activity recommendation through gardening? HortTechnology 2008, 18, 639–643. [Google Scholar]
- Lee, A.Y.; Park, S.A.; Kim, J.J.; So, J.M.; Son, K.C. Kinematic and kinetic analysis of upper limb motions during horticultural activities. Korean J. Hortc. Sci. Technol. 2016, 34, 940–958. [Google Scholar]
- Park, S.A.; Lee, J.Y.; Lee, A.Y.; Park, S.W.; Son, K.C. Horticultural therapy program based on the stress immunization training for reducing depression symptom in the patients with stroke. J. Korean Soc. People Plant Environ. 2015, 18, 159–167. [Google Scholar] [CrossRef]
- Park, S.A.; Lee, A.Y.; Park, H.G.; Son, K.C.; Kim, D.S.; Lee, W.L. Gardening intervention as a low-to moderate-intensity physical activity for improving blood lipid profiles, blood pressure, inflammation, and oxidative stress in women over the age of 70: A pilot study. HortScience 2017, 52, 200–205. [Google Scholar] [CrossRef]
- Bird, W. Natural Fit: Can Green Space and Biodiversity Increase Levels of Physical Activity; Royal Society for the Protection of Birds: Sandy, UK, 2004. [Google Scholar]
- Lekies, K.S.; Sheavly, M.E. Fostering children’s interests in gardening. Appl. Environ. Educ. Commun. 2007, 6, 67–75. [Google Scholar] [CrossRef]



| Variable | Mean | Std. Dev. |
|---|---|---|
| Age (years) | 24.2 | 2.7 |
| Height (cm) | 171.1 | 6.3 |
| Body weight (kg) | 67.8 | 11.4 |
| Body mass index (kg∙m−2) 1 | 23.0 | 2.8 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.-A.; Song, C.; Oh, Y.-A.; Miyazaki, Y.; Son, K.-C. Comparison of Physiological and Psychological Relaxation Using Measurements of Heart Rate Variability, Prefrontal Cortex Activity, and Subjective Indexes after Completing Tasks with and without Foliage Plants. Int. J. Environ. Res. Public Health 2017, 14, 1087. https://doi.org/10.3390/ijerph14091087
Park S-A, Song C, Oh Y-A, Miyazaki Y, Son K-C. Comparison of Physiological and Psychological Relaxation Using Measurements of Heart Rate Variability, Prefrontal Cortex Activity, and Subjective Indexes after Completing Tasks with and without Foliage Plants. International Journal of Environmental Research and Public Health. 2017; 14(9):1087. https://doi.org/10.3390/ijerph14091087
Chicago/Turabian StylePark, Sin-Ae, Chorong Song, Yun-Ah Oh, Yoshifumi Miyazaki, and Ki-Cheol Son. 2017. "Comparison of Physiological and Psychological Relaxation Using Measurements of Heart Rate Variability, Prefrontal Cortex Activity, and Subjective Indexes after Completing Tasks with and without Foliage Plants" International Journal of Environmental Research and Public Health 14, no. 9: 1087. https://doi.org/10.3390/ijerph14091087







