Responses of Biogeochemical Characteristics and Enzyme Activities in Sediment to Climate Warming under a Simulation Experiment in Geographically Isolated Wetlands of the Hulunbuir Grassland, China
Abstract
:1. Introduction
2. Materials and Methods
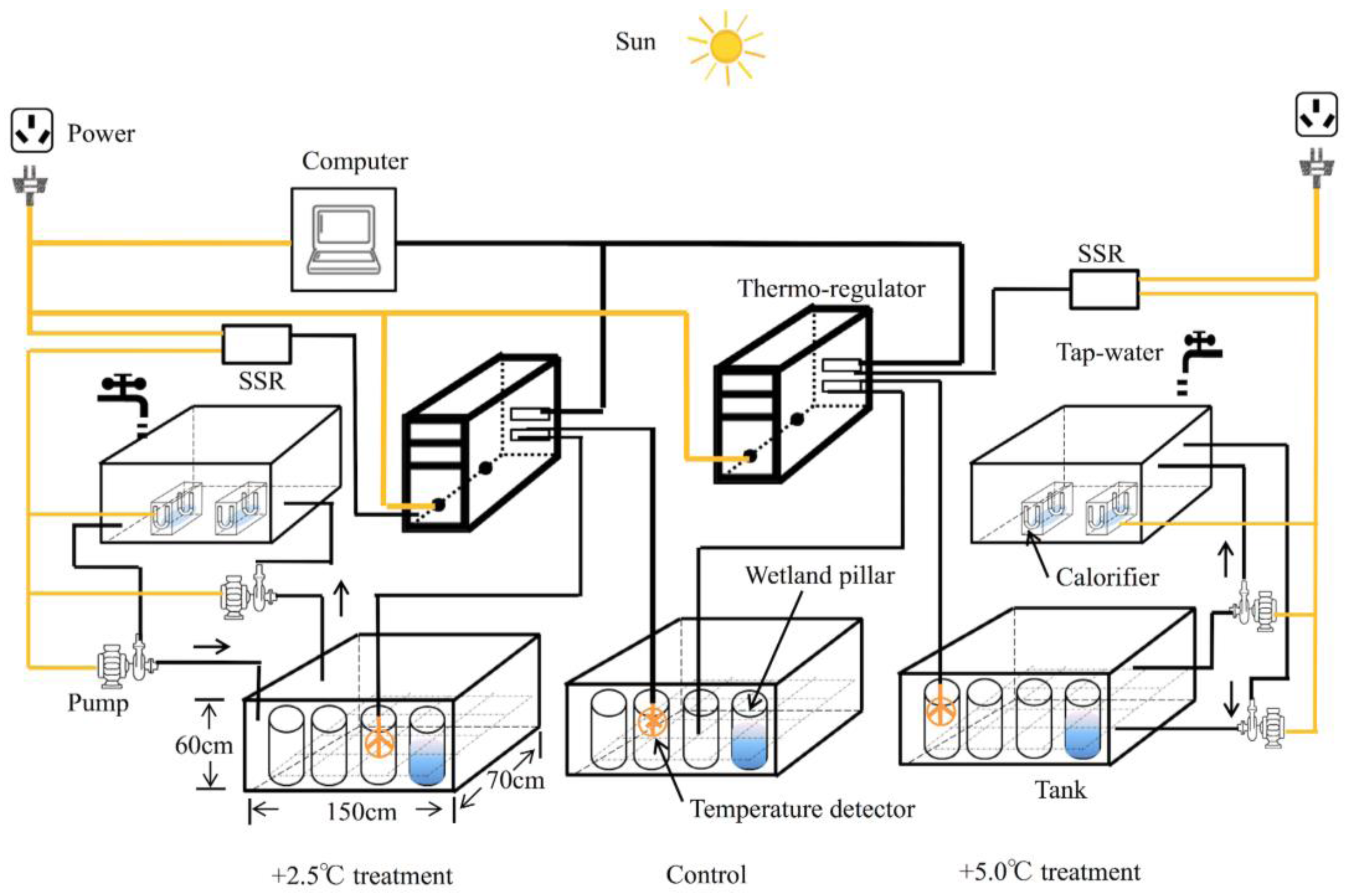
2.1. Micro-Environment Simulating Disposition
2.2. Study Sites
2.3. Sampling Methods
2.4. Sediment Biogeochemical Characteristics Analysis
2.5. Enzyme Activity Assays
2.6. Statistical Analyses
3. Results
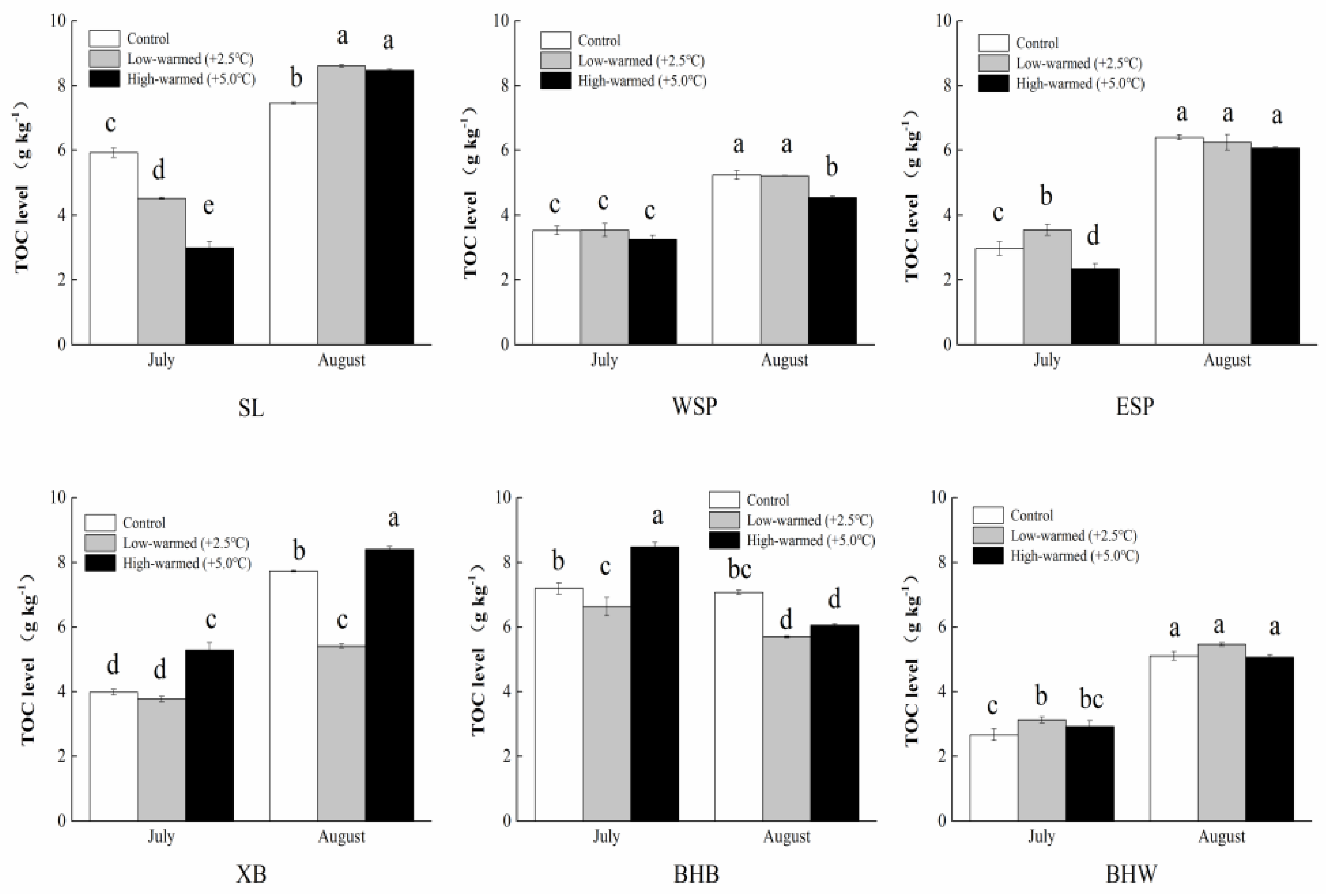
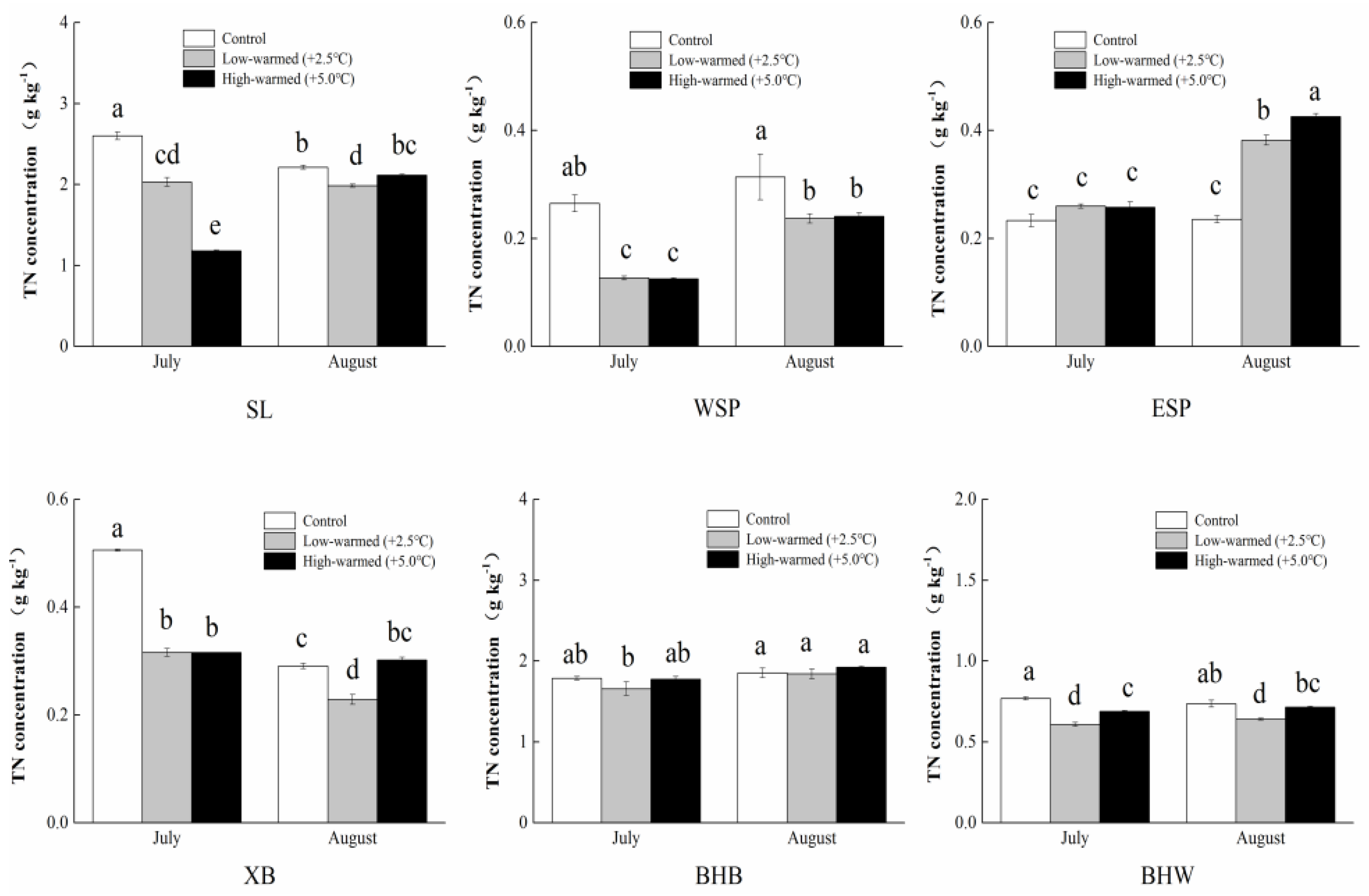
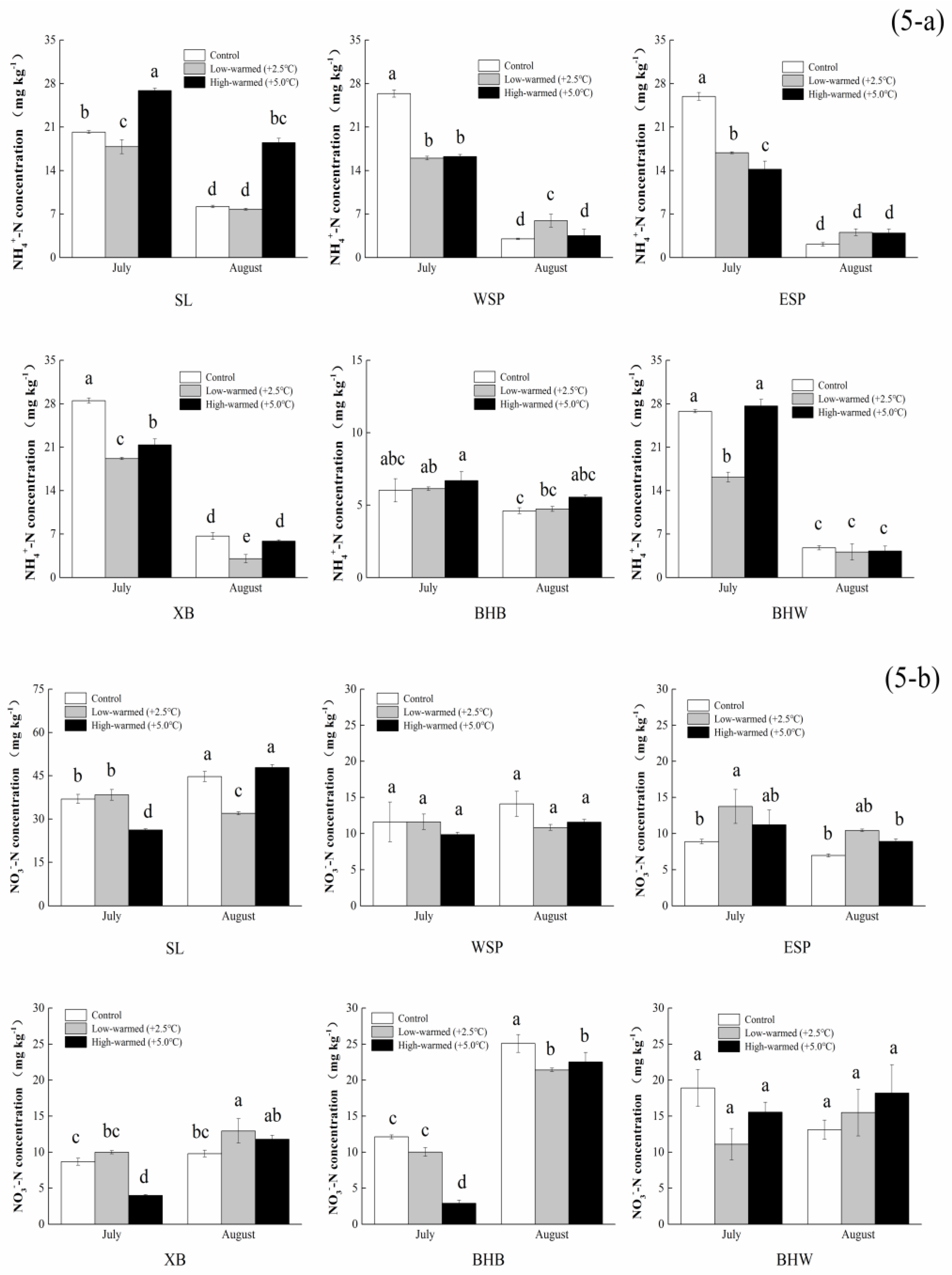
3.1. Sediment Biogeochemical Properties
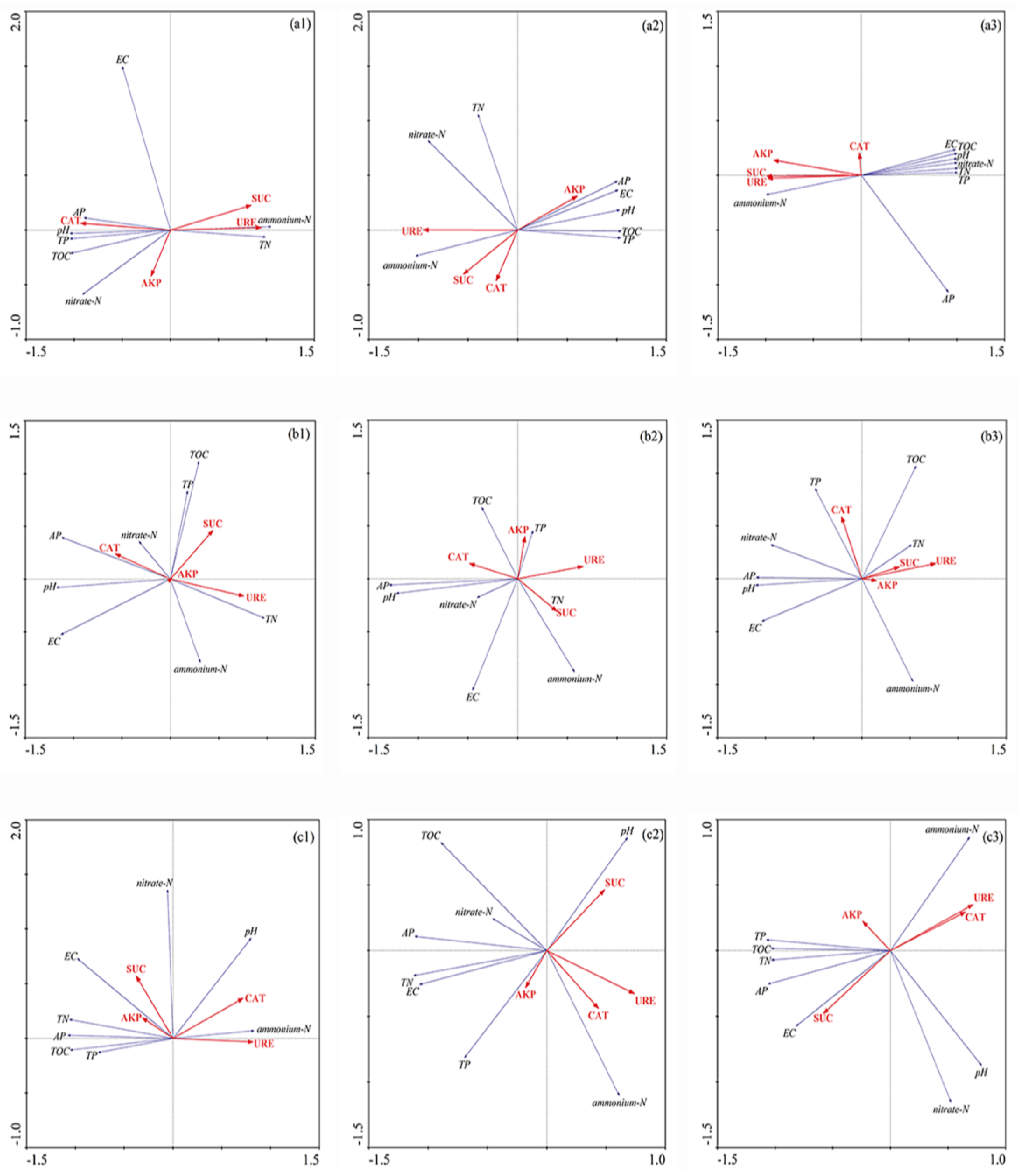
3.2. Redundancy Analysis and Pearson Correlation of Biogeochemical Properties and Enzyme Activity in Sediment of Different Flooding Periods in GIW
4. Discussion
4.1. Effects of Sediment Biogeochemical Characteristics Responding to Ambient Temperature Warming
4.2. Effects of Sediment Enzyme Activities Responding to Ambient Temperature Warming
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tiner, R.W. Geographically isolated wetlands of the United States. Wetlands 2003, 23, 494–516. [Google Scholar] [CrossRef]
- McLaughlin, D.L.; Kaplan, D.A.; Cohen, M.J. A Significant nexus: Geographically Isolated wetlands influence landscape hydrology. Water Resour. Res. 2014, 50, 7153–7166. [Google Scholar] [CrossRef]
- Leibowitz, S.G. Isolated wetlands and their functions: An ecological perspective. Wetlands 2003, 23, 517–531. [Google Scholar] [CrossRef]
- Cable Rains, M.; Fogg, G.E.; Harter, T.; Dahlgren, R.A.; Williamson, R.J. The Role of perched aquifers in hydrological connectivity and biogeochemical processes in Vernal Pool Landscapes, Central Valley, California. Hydrol. Process. 2006, 20, 1157–1175. [Google Scholar] [CrossRef]
- Creed, I.F.; Sanford, S.E.; Beall, F.D.; Molot, L.A.; Dillon, P.J. Cryptic Wetlands: Integrating hidden wetlands in regression models of the export of dissolved organic carbon from forested landscapes. Hydrol. Process. 2003, 17, 3629–3648. [Google Scholar] [CrossRef]
- Euliss, N.H.; Gleason, R.A.; Olness, A.; McDougal, R.L.; Murkin, H.R.; Robarts, R.D.; Bourbonniere, R.A.; Warner, B.G. North American Prairie wetlands are important nonforested land-based carbon storage sites. Sci. Total Environ. 2006, 361, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Gleason, R.A.; Tangen, B.A.; Browne, B.A.; Euliss, N.H. Greenhouse Gas Flux from cropland and restored wetlands in the Prairie Pothole Region. Soil Biol. Biochem. 2009, 41, 2501–2507. [Google Scholar] [CrossRef]
- Houghton, J.T.; Ding, Y.; Griggs, D.J.; Noguer, M.; van der Linden, P.J.; Dai, X.; Maskell, K.; Johnson, C.A. Climate Change 2001: The Scientific Basis; The Press Syndicate of the University of Cambridge: Cambridge, UK, 2001. [Google Scholar]
- Verhoeven, J.T.; Arheimer, B.; Yin, C.; Hefting, M.M. Regional and global concerns over wetlands and water quality. Trends Ecol. Evol. 2006, 21, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, Z.; Joseph, H.; Xu, X.; Wang, H.; Ruan, J.; Xu, X. The Release of phosphorus from sediment into water in subtropical wetlands: A warming microcosm experiment. Hydrol. Process. 2012, 26, 15–26. [Google Scholar]
- Lane, C.R.; Autrey, B.C.; Jicha, T.; Lehto, L.; Elonen, C.; Seifert-Monson, L. Denitrification potential in geographically isolated wetlands of North Carolina and Florida, USA. Wetlands 2015, 35, 459–471. [Google Scholar] [CrossRef]
- Baldrian, P.; Šnajdr, J.; Merhautová, V.; Dobiášová, P.; Cajthaml, T.; Valášková, V. Responses of the extracellular enzyme activities in hardwood forest to soil temperature and seasonality and the potential effects of climate change. Soil Biol. Biochem. 2013, 56, 60–68. [Google Scholar] [CrossRef]
- Kong, L.; Wang, Y.-B.; Zhao, L.-N.; Chen, Z.-H. Enzyme and root activities in surface-flow constructed wetlands. Chemosphere 2009, 76, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Keeler, B.L.; Hobbie, S.E.; Kellogg, L.E. Effects of long-term nitrogen addition on microbial enzyme activity in eight forested and grassland sites: Implications for litter and soil organic matter decomposition. Ecosystems 2009, 12, 1–15. [Google Scholar] [CrossRef]
- Yang, K.; Zhu, J.; Yan, Q.; Zhang, J. Soil enzyme activities as potential indicators of soluble organic nitrogen pools in forest ecosystems of Northeast China. Ann. For. Sci. 2012, 69, 795–803. [Google Scholar] [CrossRef]
- Wigley, T.M.; Raper, S.C. Interpretation of high projections for global-mean warming. Science 2001, 293, 451–454. [Google Scholar] [CrossRef] [PubMed]
- Elser, J.J.; Bracken, M.E.; Cleland, E.E.; Gruner, D.S.; Harpole, W.S.; Hillebrand, H.; Ngai, J.T.; Seabloom, E.W.; Shurin, J.B.; Smith, J.E. Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol. Lett. 2007, 10, 1135–1142. [Google Scholar] [CrossRef] [PubMed]
- Cole, L.; Bardgett, R.D.; Ineson, P.; Hobbs, P.J. Enchytraeid worm (Oligochaeta) influences on microbial community structure, nutrient dynamics and plant growth in blanket peat subjected to warming. Soil Biol. Biochem. 2002, 34, 83–92. [Google Scholar] [CrossRef]
- Xiang, W.; Freeman, C. Annual variation of temperature sensitivity of soil organic carbon decomposition in North Peatlands: Implications for thermal responses of carbon cycling to global warming. Environ. Geol. 2009, 58, 499–508. [Google Scholar] [CrossRef]
- Frey, S.D.; Drijber, R.; Smith, H.; Melillo, J. Microbial biomass, functional capacity, and community structure after 12 years of soil warming. Soil Biol. Biochem. 2008, 40, 2904–2907. [Google Scholar] [CrossRef]
- Wang, X.; Song, C.; Sun, X.; Wang, J.; Zhang, X.; Mao, R. Soil carbon and nitrogen across wetland types in discontinuous permafrost zone of the Xiao Xing’an mountains, Northeastern China. CATENA 2013, 101, 31–37. [Google Scholar] [CrossRef]
- Wang, H.; Holden, J.; Spera, K.; Xu, X.; Wang, Z.; Luan, J.; Xu, X.; Zhang, Z. Phosphorus fluxes at the sediment-water interface in subtropical wetlands subjected to experimental warming: A microcosm study. Chemosphere 2013, 90, 1794–1804. [Google Scholar] [CrossRef] [PubMed]
- Kalembasa, S.J.; Jenkinson, D.S. A comparative study of titrimetric and gravimetric methods for the determination of organic carbon in soil. J. Sci. Food Agric. 1973, 24, 1085–1090. [Google Scholar] [CrossRef]
- Parkinson, J.A.; Allen, S.E. A wet oxidation procedure suitable for the determination of nitrogen and mineral nutrients in biological material. Commun. Soil Sci. Plant Anal. 1975, 6, 1–11. [Google Scholar] [CrossRef]
- Olsen, S.R. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; United States Department of Agriculture: Washington, DC, USA, 1954.
- Song, Y.; Song, C.; Tao, B.; Wang, J.; Zhu, X.; Wang, X. Short-Term responses of soil enzyme activities and carbon mineralization to added nitrogen and litter in a freshwater marsh of Northeast China. Eur. J. Soil Biol. 2014, 61, 72–79. [Google Scholar] [CrossRef]
- Bergmeyer, H.U. Methoden Der Enzymatischen Analyse, 2nd ed.; Verlag Chemie: Weinheim, Germany, 1970. [Google Scholar]
- Guan, S.Y. Soil Enzyme and Study Method; Agricultural Press: Beijing, China, 1986. [Google Scholar]
- Tabatabai, M.A.; Fu, M. Extraction of enzymes from soils. Soil Biochem. 1992, 7, 197–227. [Google Scholar]
- Tabatabai, M.A.; Garcı́a-Manzanedo, A.M.; Acosta-Martınez, V. Substrate specificity of arylamidase in soils. Soil Biol. Biochem. 2002, 34, 103–110. [Google Scholar] [CrossRef]
- Dunne, E.J.; Reddy, R.; Clark, M.W. Biogeochemical indices of phosphorus retention and release by wetland soils and adjacent stream sediments. Wetlands 2006, 26, 1026–1041. [Google Scholar] [CrossRef]
- Alster, C.J.; German, D.P.; Lu, Y.; Allison, S.D. Microbial enzymatic responses to drought and to nitrogen addition in a Southern California grassland. Soil Biol. Biochem. 2013, 64, 68–79. [Google Scholar] [CrossRef]
- Bengtson, P.; Bengtsson, G. Rapid Turnover of DOC in Temperate forests accounts for increased CO2 production at elevated temperatures. Ecol. Lett. 2007, 10, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Simpson, M.J. Temperature and substrate controls on microbial phospholipid fatty acid composition during incubation of grassland soils contrasting in organic matter quality. Soil Biol. Biochem. 2009, 41, 804–812. [Google Scholar] [CrossRef]
- Liikanen, A.N.U.; Murtoniemi, T.; Tanskanen, H.; Väisänen, T.; Martikainen, P.J. Effects of temperature and oxygenavailability on greenhouse gas and nutrient dynamics in sediment of a Eutrophic Mid-Boreal Lake. Biogeochemistry 2002, 59, 269–286. [Google Scholar] [CrossRef]
- Serpa, D.; Falcao, M.; Duarte, P.; Da Fonseca, L.C.; Vale, C. Evaluation of ammonium and phosphate release from intertidal and subtidal sediments of a shallow coastal lagoon (Ria Formos-Portugal): A modelling approach. Biogeochemistry 2007, 82, 291–304. [Google Scholar] [CrossRef]
- Zhang, Z.-S.; Song, X.-L.; Lu, X.-G.; Xue, Z.-S. Ecological Stoichiometry of carbon, nitrogen, and phosphorus in Estuarine Wetland soils: Influences of vegetation coverage, plant communities, geomorphology, and seawalls. J. Soils Sediments 2013, 13, 1043–1051. [Google Scholar] [CrossRef]
- Stutter, M.I.; Langan, S.J.; Lumsdon, D.G. Vegetated buffer strips can lead to increased release of phosphorus to waters: A biogeochemical assessment of the mechanisms. Environ. Sci. Technol. 2009, 43, 1858–1863. [Google Scholar] [CrossRef] [PubMed]
- Newman, S.; McCormick, P.V.; Backus, J.G. Phosphatase activity as an early warning indicator of wetland eutrophication: Problems and prospects. J. Appl. Phycol. 2003, 15, 45–59. [Google Scholar] [CrossRef]
- Allison, S.D.; Treseder, K.K. Warming and drying suppress microbial activity and carbon cycling in boreal forest soils. Glob. Change Biol. 2008, 14, 2898–2909. [Google Scholar] [CrossRef]
- Chen, A.; Liang, H.; Chen, T.; Yang, W.; Ding, C. Influence of long-term irrigation with treated papermaking wastewater on soil ecosystem of a full-scale managed reed wetland. J. Soils Sediments 2016, 16, 1352–1359. [Google Scholar] [CrossRef]
- Mingorance, M.D.; Oliva, S.R.; Valdés, B.; Gata, F.P.; Leidi, E.O.; Guzmán, I.; Peña, A. Stabilized municipal sewage sludge addition to improve properties of an acid mine soil for plant growth. J. Soils Sediments 2014, 14, 703–712. [Google Scholar] [CrossRef]
- Hidri, Y.; Fourti, O.; Eturki, S.; Jedidi, N.; Charef, A.; Hassen, A. Effects of 15-Year application of municipal wastewater on microbial biomass, fecal pollution indicators, and heavy metals in a Tunisian Calcareous Soil. J. Soils Sediments 2014, 14, 155–163. [Google Scholar] [CrossRef]
- Sardans, J.; Peñuelas, J.; Estiarte, M. Changes in soil enzymes related to C and N cycle and in soil C and N content under prolonged warming and drought in a Mediterranean Shrubland. Appl. Soil Ecol. 2008, 39, 223–235. [Google Scholar] [CrossRef]
- Lehmeier, C.A.; Min, K.; Niehues, N.D.; Ballantyne, F.; Billings, S.A. Temperature-mediated Changes of exoenzyme-substrate reaction rates and their consequences for the carbon to nitrogen flow ratio of liberated resources. Soil Biol. Biochem. 2013, 57, 374–382. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Lauber, C.L.; Weintraub, M.N.; Ahmed, B.; Allison, S.D.; Crenshaw, C.; Contosta, A.R.; Cusack, D.; Frey, S.; Gallo, M.E.; et al. Stoichiometry of soil enzyme activity at global scale. Ecol. Lett. 2008, 11, 1252–1264. [Google Scholar] [CrossRef] [PubMed]
- Rustad, L.; Campbell, J.; Marion, G.; Norby, R.; Mitchell, M.; Hartley, A.; Cornelissen, J.; Gurevitch, J. A meta-analysis of the response of soil respiration, net nitrogen mineralization, and aboveground plant growth to experimental ecosystem warming. Oecologia 2001, 126, 543–562. [Google Scholar] [CrossRef] [PubMed]
- Sardans, J.; Peñuelas, J.; Estiarte, M. Warming and drought alter soil phosphatase activity and soil P availability in a Mediterranean Shrubland. Plant Soil 2006, 289, 227–238. [Google Scholar] [CrossRef]
- Haase, S.; Philippot, L.; Neumann, G.; Marhan, S.; Kandeler, E. Local response of bacterial densities and enzyme activities to elevated atmospheric CO2 and different N supply in the Rhizosphere of Phaseolus Vulgaris L. Soil Biol. Biochem. 2008, 40, 1225–1234. [Google Scholar] [CrossRef]
- Kang, H.; Freeman, C. Phosphatase and arylsulphatase activities in wetland soils: Annual Variation and controlling factors. Soil Biol. Biochem. 1999, 31, 449–454. [Google Scholar] [CrossRef]
- Selig, U.; Hübener, T.; Michalik, M. Dissolved and particulate phosphorus forms in a Eutrophic Shallow Lake. Aquat. Sci. 2002, 64, 97–105. [Google Scholar] [CrossRef]


| Parameters | SL | WSP | ESP | XB | BHW | BHB |
|---|---|---|---|---|---|---|
| pH | 8.67 ± 0.01 e | 10.27 ± 0.04 c | 10.44 ± 0.01 b | 9.71 ± 0.03 d | 10.61 ± 0.02 a | 10.31 ± 0.01 c |
| EC (μS·cm−1) | 328.33 ± 3.75 e | 2320.00 ± 26.45 b | 3053.33 ± 114.64 a | 453.33 ± 8.83 e | 1507.00 ± 4.61 d | 2110.00 ± 11.54 c |
| TOC (g·kg−1) | 5.925 ± 0.141 b | 3.523 ± 0.131 c | 2.971 ± 0.215 d | 3.976 ± 0.083 c | 2.663 ± 0.18 d | 7.194 ± 0.172 a |
| TN (g·kg−1) | 2.602 ± 0.049 a | 0.265 ± 0.016 e | 0.233 ± 0.011 e | 0.506 ± 0.002 d | 0.768 ± 0.013 c | 1.786 ± 0.022 b |
| NH4+-N (mg kg−1) | 20.194 ± 0.216 c | 26.398 ± 0.59 b | 25.938 ± 0.612 b | 28.52 ± 0.423 a | 26.802 ± 0.248 b | 6.02 ± 0.804 d |
| NO3−-N (mg·kg−1) | 36.96 ± 1.572 a | 11.606 ± 2.748 c | 8.879 ± 0.306 c | 8.67 ± 0.499 c | 18.922 ± 2.555 b | 12.122 ± 0.258 c |
| TP (g·kg−1) | 0.095 ± 0.003 c | 0.029 ± 0.013 c | 0.091 ± 0.008 c | 0.079 ± 0.008 c | 0.879 ± 0.024 b | 1.337 ± 0.075 a |
| AP (mg·kg−1) | 39.767 ± 0.873 c | 47.163 ± 1.221 c | 61.761 ± 0.413 b | 12.978 ± 0.464 d | 51.206 ± 6.172 b,c | 180.662 ± 6.513 a |
| SUC (mg glucose·g−1 24 h−1) | 10.371 ± 3.301 a | 0.138 ± 0.026 b | 0.168 ± 0.029 b | 3.478 ± 0.069 b | 1.596 ± 0.276 b | 0.451 ± 0.043 b |
| URE (mg NH4+-N·g−1·24 h−1) | 1.424 ± 0.002 a | 0.087 ± 0.001 d | 0.098 ± 0.011 d | 0.567 ± 0.052 c | 0.649 ± 0.002 b | 0.155 ± 0.021 d |
| AKP (mg phenol·g−1 24 h−1) | 0.041 ± 0.005 a | 0.036 ± 0.002 a | 0.061 ± 0.017 a | 0.035 ± 0.006 a | 0.065 ± 0.006 a | 0.066 ± 0.013 a |
| CAT (mg H2O2·g−1·20 min−1) | 0.117 ± 0.001 c | 0.305 ± 0.022 a | 0.299 ± 0.012 a | 0.242 ± 0.024 b | 0.229 ± 0.002 b | 0.101 ± 0.004 c |
| Sample Site | SUC (mg Glucose g−1·24 h−1) | URE (mg NH4+-N g−1 24 h−1) | AKP (mg Phenol·g−1 24 h−1) | CAT (mg H2O2 g−1 20 min−1) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | Low Warmed | High Warmed | Control | Low Warmed | High Warmed | Control | Low Warmed | High Warmed | Control | Low Warmed | High Warmed | |
| SL | 9.371 ± 1.266 | 18.687 ± 1.779 | 7.219 ± 0.063 | 1.424 ± 0.002 | 1.238 ± 0.008 | 1.073 ± 0.032 | 0.041 ± 0.005 | 0.026 ± 0.007 | 0.118 ± 0.013 | 0.117 ± 0.012 | 0.161 ± 0.006 | 0.218 ± 0.002 |
| WSP | 0.158 ± 0.014 | 0.128 ± 0.027 | 0.337 ± 0.005 | 0.087 ± 0.001 | 0.083 ± 0.015 | 0.039 ± 0.002 | 0.036 ± 0.002 | 0.047 ± 0.013 | 0.036 ± 0.003 | 0.305 ± 0.022 | 0.347 ± 0.019 | 0.288 ± 0.021 |
| ESP | 0.141 ± 0.009 | 2.511 ± 0.252 | 1.024 ± 0.067 | 0.098 ± 0.011 | 0.062 ± 0.004 | 0.056 ± 0.005 | 0.061 ± 0.017 | 0.065 ± 0.004 | 0.061 ± 0.025 | 0.299 ± 0.012 | 0.244 ± 0.011 | 0.259 ± 0.005 |
| XB | 3.478 ± 0.069 | 1.511 ± 0.077 | 3.491 ± 0.114 | 0.567 ± 0.052 | 0.422 ± 0.012 | 0.317 ± 0.015 | 0.035 ± 0.006 | 0.058 ± 0.005 | 0.055 ± 0.026 | 0.242 ± 0.024 | 0.065 ± 0.032 | 0.286 ± 0.019 |
| BHB | 0.484 ± 0.021 | 0.736 ± 0.026 | 3.225 ± 0.145 | 0.155 ± 0.021 | 0.166 ± 0.008 | 0.215 ± 0.021 | 0.066 ± 0.013 | 0.049 ± 0.005 | 0.072 ± 0.013 | 0.101 ± 0.004 | 0.164 ± 0.023 | 0.155 ± 0.007 |
| BHW | 1.596 ± 0.276 | 0.602 ± 0.028 | 0.503 ± 0.022 | 0.649 ± 0.002 | 0.811 ± 0.049 | 0.648 ± 0.029 | 0.065 ± 0.006 | 0.043 ± 0.008 | 0.083 ± 0.012 | 0.229 ± 0.002 | 0.217 ± 0.011 | 0.893 ± 0.003 |
| Sample Site | SUC (mg Glucose·g−1 24 h−1) | URE (mg NH4+-N·g−1 24 h−1) | AKP (mg Phenol·g−1 24 h−1) | CAT (mg H2O2·g−1 20 min−1) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | Low Warmed | High Warmed | Control | Low Warmed | High Warmed | Control | Low Warmed | High Warmed | Control | Low Warmed | High Warmed | |
| SW | 4.079 ± 0.103 | 16.112 ± 0.131 | 0.515 ± 0.036 | 0.411 ± 0.016 | 0.332 ± 0.005 | 0.606 ± 0.009 | 0.045 ± 0.007 | 0.041 ± 0.005 | 0.038 ± 0.010 | 0.202 ± 0.011 | 0.158 ± 0.004 | 0.215 ± 0.051 |
| WSP | 0.145 ± 0.011 | 1.995 ± 0.171 | 3.142 ± 0.134 | 0.107 ± 0.021 | 0.172 ± 0.007 | 0.109 ± 0.005 | 0.029 ± 0.008 | 0.108 ± 0.021 | 0.036 ± 0.002 | 0.363 ± 0.008 | 0.342 ± 0.008 | 0.361 ± 0.017 |
| ESP | 0.125 ± 0.016 | 0.694 ± 0.029 | 1.291 ± 0.048 | 0.163 ± 0.005 | 0.084 ± 0.005 | 0.104 ± 0.002 | 0.032 ± 0.005 | 0.049 ± 0.005 | 0.065 ± 0.003 | 0.314 ± 0.005 | 0.255 ± 0.022 | 0.307 ± 0.024 |
| XB | 18.472 ± 0.245 | 0.138 ± 0.009 | 1.455 ± 0.117 | 0.241 ± 0.016 | 0.116 ± 0.002 | 0.185 ± 0.008 | 0.059 ± 0.012 | 0.112 ± 0.017 | 0.046 ± 0.006 | 0.282 ± 0.012 | 0.368 ± 0.002 | 0.342 ± 0.023 |
| BHB | 6.115 ± 0.484 | 0.127 ± 0.007 | 7.542 ± 0.176 | 0.089 ± 0.003 | 0.188 ± 0.001 | 0.072 ± 0.001 | 0.072 ± 0.013 | 0.049 ± 0.005 | 0.086 ± 0.008 | 0.168 ± 0.006 | 0.169 ± 0.006 | 0.161 ± 0.015 |
| BHW | 0.405 ± 0.054 | 1.323 ± 0.024 | 4.006 ± 0.078 | 0.311 ± 0.018 | 0.329 ± 0.002 | 0.208 ± 0.001 | 0.038 ± 0.008 | 0.034 ± 0.007 | 0.025 ± 0.006 | 0.172 ± 0.005 | 0.155 ± 0.004 | 0.112 ± 0.008 |
| NO3−N (mg·kg−1) | TN (g·kg−1) | TP (g·kg−1) | SUC (mg·g−1·24 h−1) | URE (mg·g−1·24 h−1) | CAT (mg·g−1·20 min−1) | TOC (g·kg−1) | AKP (mg·g−1·24 h−1) | AP (mg·kg−1) | pH | EC (μS·cm−1) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| NH4+-N (mg·kg−1) | –0.112 | –0.638 ** | –0.717 ** | 0.002 | 0.069 | 0.765 ** | –0.886 ** | –0.360 | –0.919 ** | 0.046 | –0.109 |
| NO3−-N (mg·kg−1) | 0.790 ** | –0.076 | 0.735 ** | 0.878 ** | –0.554 * | 0.316 | –0.076 | –0.156 | –0.743 ** | –0.564 * | |
| TN (g·kg−1) | 0.302 | 0.649 ** | 0.706 ** | –0.903 ** | 0.804 ** | 0.037 | 0.334 | –0.700 ** | –0.519 * | ||
| TP (g·kg−1) | –0.281 | –0.194 | –0.545 * | 0.441 | 0.527 * | 0.814 ** | 0.420 | 0.156 | |||
| SUC (mg glucose·g−1·24 h−1) | 0.847 ** | –0.454 | 0.311 | –0.309 | –0.326 | –0.839 ** | –0.708 ** | ||||
| URE (mg NH4+-N g−1 24 h−1) | –0.500 * | 0.232 | –0.194 | –0.393 | –0.834 ** | –0.829 ** | |||||
| CAT (mg H2O2·g−1·20 min−1) | –0.857 ** | –0.192 | –0.529 * | 0.480 * | 0.448 | ||||||
| TOC (g kg−1) | 0.085 | 0.656 ** | –0.452 | –0.257 | |||||||
| AKP (mg phenol·g−1 24 h−1) | 0.490 * | 0.410 | 0.376 | ||||||||
| AP (mg·kg−1) | 0.327 | 0.421 | |||||||||
| pH | 0.759 ** |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, L.; Su, D.; Lv, S.; Luo, Y.; Li, X.; Jiao, J.; Diao, Z.; Bu, H. Responses of Biogeochemical Characteristics and Enzyme Activities in Sediment to Climate Warming under a Simulation Experiment in Geographically Isolated Wetlands of the Hulunbuir Grassland, China. Int. J. Environ. Res. Public Health 2017, 14, 968. https://doi.org/10.3390/ijerph14090968
Han L, Su D, Lv S, Luo Y, Li X, Jiao J, Diao Z, Bu H. Responses of Biogeochemical Characteristics and Enzyme Activities in Sediment to Climate Warming under a Simulation Experiment in Geographically Isolated Wetlands of the Hulunbuir Grassland, China. International Journal of Environmental Research and Public Health. 2017; 14(9):968. https://doi.org/10.3390/ijerph14090968
Chicago/Turabian StyleHan, Liliang, Derong Su, Shihai Lv, Yan Luo, Xingfu Li, Jian Jiao, Zhaoyan Diao, and He Bu. 2017. "Responses of Biogeochemical Characteristics and Enzyme Activities in Sediment to Climate Warming under a Simulation Experiment in Geographically Isolated Wetlands of the Hulunbuir Grassland, China" International Journal of Environmental Research and Public Health 14, no. 9: 968. https://doi.org/10.3390/ijerph14090968





