Identification of Potential High-Risk Habitats within the Transmission Reach of Oncomelania hupensis after Floods Based on SAR Techniques in a Plane Region in China
Abstract
:1. Introduction
2. Materials and Methods
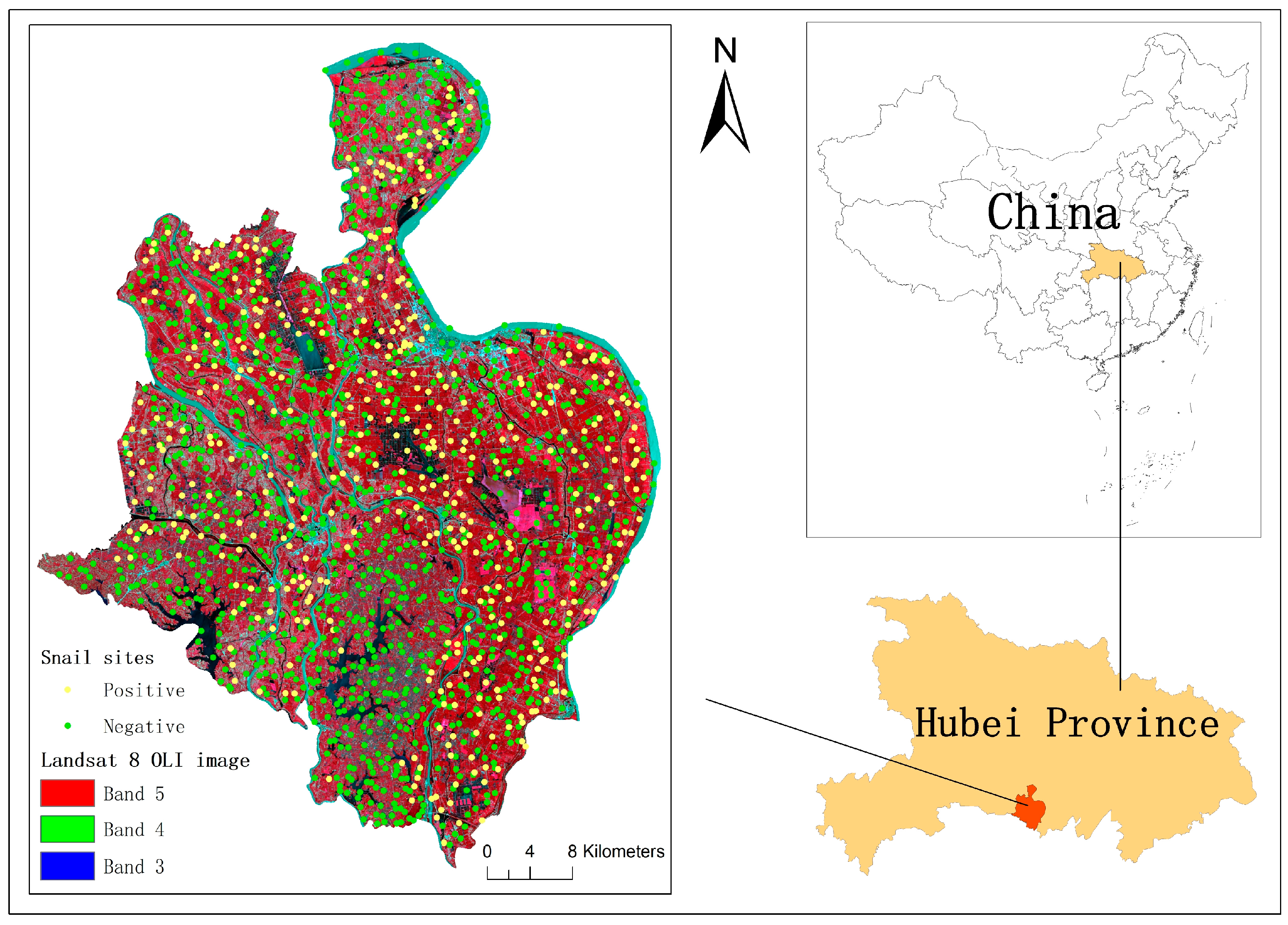
2.1. Study Area
2.2. Data Sources
2.3. Data Processing
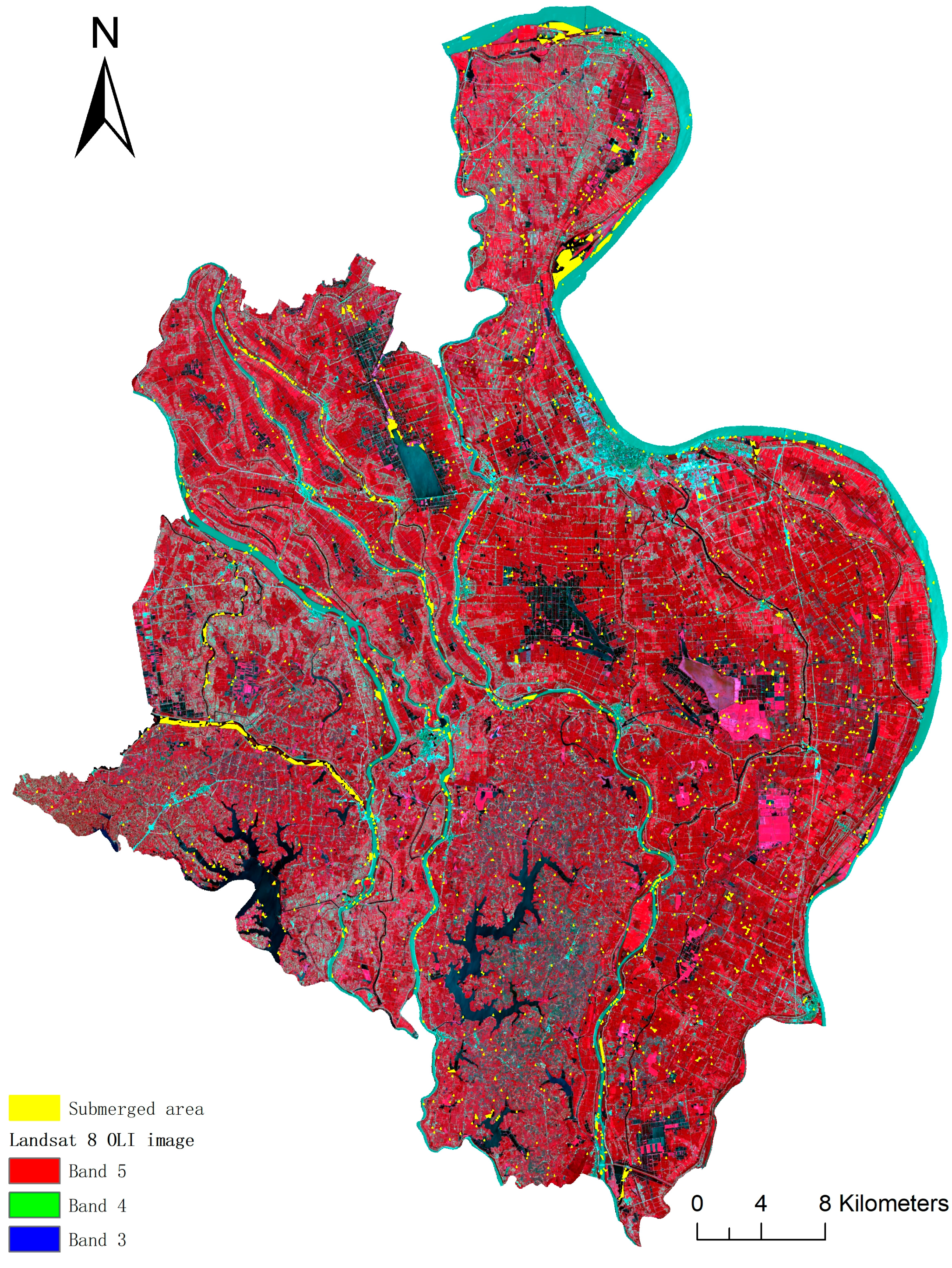
2.3.1. Submerged Area Extraction
2.3.2. Environmental Factor Data Extraction
2.3.3. Snail Site Selection
2.3.4. Land Use Data Processing
2.3.5. Landscape Pattern Index Calculation
2.3.6. Significant Factor and Probability Equation Acquisition
2.3.7. Potential Habitat Simulation
3. Results
3.1. Submerged Area after a Flood
3.2. Snail Survival and Natural Factors
3.3. Predicted Potential Snail Habitats within the Snail Transmission Reach after Flooding
4. Discussion
4.1. The Factors Influencing Snail Habitats after a Flood
4.2. Potential Habitats of Oncomelania Hupensis in Dispersal Ranges
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ross, A.G.P.; Sleigh, A.C.; Li, Y.S.; Davis, G.M.; Williams, G.M.; Jiang, Z.; Feng, Z.; McManus, D.P. Schistosomiasis in the People’s Republic of China: Prospects and challenges for the 21st century. Clin. Microbiol. Rev. 2001, 14, 270–295. [Google Scholar] [CrossRef] [PubMed]
- Steinmann, P.; Keiser, J.; Bos, R.; Tanner, M.; Utzinger, J. Schistosomiasis and water resources development: Systematic review, meta-analysis, and estimates of people at risk. Lancet Infect. Dis. 2006, 6, 411–425. [Google Scholar] [CrossRef]
- Zhou, Y.B.; Yang, M.X.; Zhao, G.M.; Wei, J.G.; Jiang, Q.W. Oncomelania hupensis (gastropoda: Rissooidea), intermediate host of schistosoma japonicum in China: Genetics and molecular phylogeny based on amplified fragment length polymorphisms. Malacologia 2007, 49, 367–382. [Google Scholar] [CrossRef]
- Wu, J.Y.; Zhou, Y.B.; Li, L.H.; Zheng, S.B.; Liang, S.; Coatsworth, A.; Ren, G.H.; Song, X.X.; He, Z.; Cai, B.; et al. Identification of optimum scopes of environmental factors for snails using spatial analysis techniques in Dongting Lake Region, China. Parasit. Vectors 2014, 7, 216. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.J.; Utzinger, J.; Sun, L.P.; Hong, Q.B.; Vounatsou, P.; Tanner, M.; Zhou, X.N. Effect of temperature on the development of schistosoma japonicum within Oncomelania hupensis, and hibernation of O. hupensis. Parasitol. Res. 2007, 100, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhu, X.; Zhou, G.; Cai, B. The compound effect of disastrous floods in Dongting Lake on concurrence of ecological disasters. Acta. Ecol. Sin. 2002, 22, 334–340. [Google Scholar]
- Utzinger, J.; Zhou, X.N.; Chen, M.G.; Bergquist, R. Conquering schistosomiasis in China: The long march. Acta. Trop. 2005, 96, 69–96. [Google Scholar] [PubMed]
- Zhou, X.N.; Wang, L.Y.; Chen, M.G.; Wu, X.H.; Jiang, Q.W.; Chen, X.Y.; Zheng, J.; Utzinger, J. The public health significance and control of schistosomiasis in China—Then and now. Acta Trop. 2005, 96, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.S.; Raso, G.; Zhao, Z.Y.; He, Y.K.; Ellis, M.K.; McManus, D.P. Large water management projects and schistosomiasis control, Dongting Lake Region, China. Emerg. Infect. Dis. 2007, 13, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.D.; Utzinger, J.; Zhou, X.N. Schistosomiasis control: Experiences and lessons from China. Lancet 2008, 372, 1793–1795. [Google Scholar] [CrossRef]
- Wu, X.H.; Zhang, S.Q.; Xu, X.J.; Huang, Y.X.; Steinmann, P.; Utzinger, J.; Wang, T.P.; Xu, J.; Zheng, J.; Zhou, X.N. Effect of floods on the transmission of schistosomiasis in the Yangtze River Valley, People’s Republic of China. Parasitol. Int. 2008, 57, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Ong, S.H.; Peng, W.X.; Zhou, Y.B.; Zhuang, J.L.; Zhao, G.M.; Jiang, Q.W. A model for the prediction of Oncomelania hupensis in the lake and Marshland regions, China. Parasitol. Int. 2008, 57, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Zhou, X.N.; Wu, X.H.; Steinmann, P.; Wang, X.H.; Yang, G.J.; Utzinger, J.; Li, H.J. Landscape pattern analysis and bayesian modeling for predicting Oncomelania hupensis distribution in Eryuan County, People’s Republic of China. Am. J. Trop. Med. Hyg. 2009, 81, 416–423. [Google Scholar] [PubMed]
- Kristensen, T.K.; Malone, J.B.; McCarroll, J.C. Use of satellite remote sensing and geographic information systems to model the distribution and abundance of snail intermediate hosts in Africa: A preliminary model for biomphalaria pfeifferi in ethiopia. Acta Trop. 2001, 79, 73–78. [Google Scholar] [CrossRef]
- Zhou, X.N.; Lin, D.D.; Yang, H.M.; Chen, H.G.; Sun, L.P.; Yang, G.J.; Hong, Q.B.; Brown, L.; Malone, J.B. Use of Landsat TM satellite surveillance data to measure the impact of the 1998 flood on snail intermediate host dispersal in the lower Yangtze River basin. Acta Trop. 2002, 82, 199–205. [Google Scholar] [CrossRef]
- Guo, J.G.; Vounatsou, P.; Cao, C.L.; Utzinger, J.; Zhu, H.Q.; Anderegg, D.; Zhu, R.; He, Z.Y.; Li, D.; Hu, F.; et al. A geographic information and remote sensing based model for prediction of Oncomelania hupensis habitats in the Poyang Lake area, China. Acta Trop. 2005, 96, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Y.; Xu, D.Z.; Zhou, X.N.; Zhou, Y.; Liu, S.J. Remote sensing and spatial statistical analysis to predict the distribution of Oncomelania hupensis in the marshlands of China. Acta Trop. 2005, 96, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Bates, P.D.; De Roo, A.P.J. A simple raster-based model for flood inundation simulation. J. Hydrol. 2000, 236, 54–77. [Google Scholar] [CrossRef]
- Martinez, J.M.; Le Toan, T. Mapping of flood dynamics and spatial distribution of vegetation in the Amazon floodplain using multitemporal sar data. Remote Sens. Environ. 2007, 108, 209–223. [Google Scholar] [CrossRef]
- Barreto, T.L.M.; Almeida, J.; Cappabianco, F.A.M. Estimating accurate water levels for rivers and reservoirs by using sar products: A multitemporal analysis. Pattern Recogn. Lett. 2016, 83, 224–233. [Google Scholar] [CrossRef]
- Ardhuin, F.; Collard, F.; Chapron, B.; Girard-Ardhuin, F.; Guitton, G.; Mouche, A.; Stopa, J.E. Estimates of ocean wave heights and attenuation in sea ice using the sar wave mode on sentinel-1a. Geophys. Res. Lett. 2015, 42, 2317–2325. [Google Scholar] [CrossRef]
- Grant, K.; Siegmund, R.; Wagner, M.; Hartmann, S. Satellite-based assessment of grassland yields. Int. Arch. Photogramm. Remote Sens. Spat. Inf. Sci. 2015, 7, 15–18. [Google Scholar] [CrossRef]
- Wiehle, S.; Lehner, S.; Pleskachevsky, A. Waterline detection and monitoring in the German Wadden sea using high resolution satellite-based radar measurements. Int. Arch. Photogramm. Remote Sens. Spat. Inf. Sci. 2015, 40, 1029. [Google Scholar] [CrossRef]
- Yang, G.J.; Vounatsou, P.; Zhou, X.N.; Utzinger, J.; Tanner, M. A review of geographic information system and remote sensing with applications to the epidemiology and control of schistosomiasis in China. Acta Trop. 2005, 96, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Li, W.; Sun, L.P.; Huang, Y.X.; Zhang, J.F.; Wu, F.; Hang, D.R.; Steinmann, P.; Liang, Y.S. Spatio-temporal analysis to identify determinants of Oncomelania hupensis infection with schistosoma japonicum in Jiangsu province, China. Parasit. Vectors 2013, 6, 138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitron, U. Landscape ecology and epidemiology of vector-borne diseases: Tools for spatial analysis. J. Med. Entomol. 1998, 35, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Turner, S.J. Landscape ecology concepts, methods and applications. Landsc. Ecol. 2005, 20, 1031–1033. [Google Scholar] [CrossRef]
- Brooker, S.; Hay, S.I.; Bundy, D.A.P. Tools from ecology: Useful for evaluating infection risk models? Trends Parasitol. 2002, 18, 70–74. [Google Scholar] [CrossRef]
- Van Benthem, B.H.B.; Vanwambeke, S.O.; Khantikul, N.; Burghoorn-Maas, C.; Panart, K.; Oskam, L.; Lambin, E.F.; Somboon, P. Spatial patterns of and risk factors for seropositivity for dengue infection. Am. J. Trop. Med. Hyg. 2005, 72, 201–208. [Google Scholar] [PubMed]
- Linard, C.; Lamarque, P.; Heyman, P.; Ducoffre, G.; Luyasu, V.; Tersago, K.; Vanwambeke, S.O.; Lambin, E.F. Determinants of the geographic distribution of puumala virus and lyme borreliosis infections in Belgium. Int. J. Health Geogr. 2007, 6, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ross, Z.; Jerrett, M.; Ito, K.; Tempalski, B.; Thurston, G.D. A land use regression for predicting fine particulate matter concentrations in the New York city region. Atmos. Environ. 2007, 41, 2255–2269. [Google Scholar] [CrossRef]
- Beelen, R.; Hoek, G.; Vienneau, D. Development of NO2 and NOx land use regression models for estimating air pollution exposure in 36 study areas in Europe—The escape project. Atmos. Environ. 2013, 72, 10–23. [Google Scholar] [CrossRef]
- Long, S.; Fatoyinbo, T.E.; Policelli, F. Flood extent mapping for Namibia using change detection and thresholding with sar. Environ. Res. Lett. 2014, 9, 035002. [Google Scholar] [CrossRef]
- Asrar, G.; Kanemasu, E.T.; Yoshida, M. Estimates of leaf-area index from spectral reflectance of wheat under different cultural-practices and solar angle. Remote Sens. Environ. 1985, 17, 1–11. [Google Scholar] [CrossRef]
- Baret, F.; Guyot, G. Potentials and limits of vegetation indexes for lai and apar assessment. Remote Sens. Environ. 1991, 35, 161–173. [Google Scholar] [CrossRef]
- Richardson, A.J.; Wiegand, C.L.; Wanjura, D.F.; Dusek, D.; Steiner, J.L. Multisite analyses of spectral-biophysical data for sorghum. Remote Sens. Environ. 1992, 41, 71–82. [Google Scholar] [CrossRef]
- Gilabert, M.A.; Gandia, S.; Melia, J. Analyses of spectral biophysical relationships for a corn canopy. Remote Sens. Environ. 1996, 55, 11–20. [Google Scholar] [CrossRef]
- Leprieur, C.; Verstraete, M.M.; Pinty, B. Evaluation of the performance of various vegetation indices to retrieve vegetation cover from avhrr data. Remote Sens. Rev. 1994, 10, 265–284. [Google Scholar] [CrossRef]
- Bannari, A.; Morin, D.; Bonn, F.; Huete, A.R. A review of vegetation indices. Remote Sens. Rev. 1995, 13, 95–120. [Google Scholar] [CrossRef]
- Elvidge, C.D.; Chen, Z. Comparison of broad-band and narrow-band red and near-infrared vegetation indices. Remote Sens. Environ. 1995, 54, 38–48. [Google Scholar] [CrossRef]
- Danson, F.M.; Plummer, S.E. Advances in environmental remote sensing. Oceanogr. Lit. Rev. 1996, 8, 838. [Google Scholar]
- Amiri, R.; Weng, Q.H.; Alimohammadi, A.; Alavipanah, S.K. Spatial-temporal dynamics of land surface temperature in relation to fractional vegetation cover and land use/cover in the Tabriz urban area, Iran. Remote Sens. Environ. 2009, 113, 2606–2617. [Google Scholar] [CrossRef]
- Chen, Y.H.; Shi, P.J.; Li, X.B.; Chen, J.; Li, J. A combined approach for estimating vegetation cover in urban/suburban environments from remotely sensed data. Comput. Geosci. 2006, 32, 1299–1309. [Google Scholar]
- Liu, K.; Su, H.B.; Li, X.K. Comparative assessment of two vegetation fractional cover estimating methods and their impacts on modeling urban latent heat flux using landsat imagery. Remote Sens. 2017, 9, 455. [Google Scholar] [CrossRef]
- Avdan, U.; Jovanovska, G. Algorithm for automated mapping of land surface temperature using Landsat 8 satellite data. J. Sens. 2016. [Google Scholar] [CrossRef]
- Kauth, R.J.; Thomas, G.S. The Tasselet Cap: A Graphic Description of the Spectral-Temporal Development of Agricultural Crops as Seen by Landsat. Available online: http://docs.lib.purdue.edu/cgi/viewcontent.cgi?article=1160&context=lars_symp (accessed on 29 November 2016).
- Crist, E.P.; Cicone, R.C. A physically-based transformation of thematic mapper data—The tm tasseled cap. IEEE Trans. Geosci. Remote Sens. 1984, 22, 256–263. [Google Scholar] [CrossRef]
- Qiu, J.; Li, R.D.; Xu, X.J.; Yu, C.H.; Xia, X.; Hong, X.C.; Chang, B.R.; Yi, F.J.; Shi, Y.Y. Identifying determinants of oncomelania hupensis habitats and assessing the effects of environmental control strategies in the plain regions with the waterway network of China at the microscale. Int. J. Environ. Res. Pub. Health 2014, 11, 6571–6585. [Google Scholar] [CrossRef]
- McGarigal, K.; Marks, B.J. Spatial Analysis Program for Quantifying Landscape Structure. Available online: http://www.umass.edu/landeco/pubs/mcgarigal.marks.1995.pdf (accessed on 30 November 2016).
- Swets, J.A. Measuring the accuracy of diagnostic systems. Science 1988, 240, 1285–1293. [Google Scholar] [CrossRef] [PubMed]
- Cross, E.R.; Bailey, R.C. Prediction of areas endemic for schistosomiasis through use of discriminant analysis of environmental data. Mil. Med. 1984, 149, 28–30. [Google Scholar] [PubMed]
- Zhu, H.M.; Xiang, S.; Yang, K.; Wu, X.H.; Zhou, X.N. Three gorges dam and its impact on the potential transmission of schistosomiasis in regions along the Yangtze River. EcoHealth 2008, 5, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.Y.; Zhou, Y.B.; Chen, Y.; Liang, S.; Li, L.H.; Zheng, S.B.; Zhu, S.P.; Ren, G.H.; Song, X.X.; Jiang, Q.W. Three gorges dam: Impact of water level changes on the density of schistosome-transmitting snail Oncomelania hupensis in Dongting Lake area, China. PLoS Negl. Trop. Dis. 2015, 9, e0003882. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Li, D.; Zhuang, D.F.; Wang, Y. The influence of natural factors on the spatio-temporal distribution of Oncomelania hupensis. Acta Trop 2016, 164, 194–207. [Google Scholar] [CrossRef] [PubMed]
- He, L.C.; Yuan, M.Z.; Peng, X.W.; Dong, J.; Zhou, H.R. Further observation on snail distribution in ditch water in lake regions. Chin. J. Schist. Control 2006, 18, 98. [Google Scholar]
- Wang, J.S.; Lu, J.Y.; Wei, G.Y.; Yao, S.M. Impact of Environment Changes on Oncomelania Spread. J. Yangtze River Sci. Res. Inst. 2007, 24, 16–19. [Google Scholar]
- Li, S.S.; Hong, H.W.; Yu, B.X.; Liu, J.; Qin, Z.H.; Li, P. Research on the molluscacidal effect by concreting ditches for schistosomiasis control in lake regions. J. Public Health Prev. Med. 2007, 18, 7–9. [Google Scholar]
- Remais, J.; Zhong, B.; Carlton, E.J.; Spear, R.C. Model approaches for estimating the influence of time-varying socio-environmental factors on macroparasite transmission in two endemic regions. Epidemics 2009, 1, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Li, D.M.; Zhan, C.H.; Hu, Z.M. Studies on Moving of Oncomelania in Water. Adv. Water Sci. 1997, 8, 270–274. [Google Scholar]
- Huang, Y.X.; Sun, L.P.; Hong, Q.B.; Gao, Y.; Zhang, L.H.; Gao, Y.; Chen, H.; Guo, J.H.; Liang, Y.S.; Zhu, Y.C. Longitudinal observation on fluctuation trend of distribution and spread of oncomelania snails after water in marshiland of lower reaches of Yangtze River. Chin. J. Schist. Control 2004, 16, 253–256. [Google Scholar]
- Wu, S.W.; Liu, X.S.; Peng, X.P.; Xiao, J.W.; Yao, X.M.; Zhao, Z.Y.; Wu, Q.Q.; Wu, M.Q.; Pi, H.; Chen, Y. Study on formative factors attributable to a newly endemic area of schitosomiasis control strategeis within the range of irragation system from Huangshi reseroir. Chin. J. Schist. Control 2001, 13, 137–140. [Google Scholar]
- Spear, R.C.; Seto, E.; Liang, S.; Birkner, M.; Hubbard, A.; Qiu, D.; Yang, C.; Zhong, B.; Xu, F.; Gu, X. Factors influencing the transmission of schistosoma japonicum in the mountains of Sichuan province of China. Am. J. Trop. Med. Hyg. 2017, 70, 48–56. [Google Scholar]


| Data | Classification |
|---|---|
| Sentinel-1A data | RS data |
| Landsat 8 OLI image | RS data |
| Elevation data | RS data |
| Snail survey data | Snail data |
| Village-scale vector map | vector data |
| Land-use data | vector data |
| Soil texture data | Raster data |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, Y.; Qiu, J.; Li, R.; Shen, Q.; Huang, D. Identification of Potential High-Risk Habitats within the Transmission Reach of Oncomelania hupensis after Floods Based on SAR Techniques in a Plane Region in China. Int. J. Environ. Res. Public Health 2017, 14, 986. https://doi.org/10.3390/ijerph14090986
Shi Y, Qiu J, Li R, Shen Q, Huang D. Identification of Potential High-Risk Habitats within the Transmission Reach of Oncomelania hupensis after Floods Based on SAR Techniques in a Plane Region in China. International Journal of Environmental Research and Public Health. 2017; 14(9):986. https://doi.org/10.3390/ijerph14090986
Chicago/Turabian StyleShi, Yuanyuan, Juan Qiu, Rendong Li, Qiang Shen, and Duan Huang. 2017. "Identification of Potential High-Risk Habitats within the Transmission Reach of Oncomelania hupensis after Floods Based on SAR Techniques in a Plane Region in China" International Journal of Environmental Research and Public Health 14, no. 9: 986. https://doi.org/10.3390/ijerph14090986




