Selective Removal of the Genotoxic Compound 2-Aminopyridine in Water using Molecularly Imprinted Polymers Based on Magnetic Chitosan and β-Cyclodextrin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
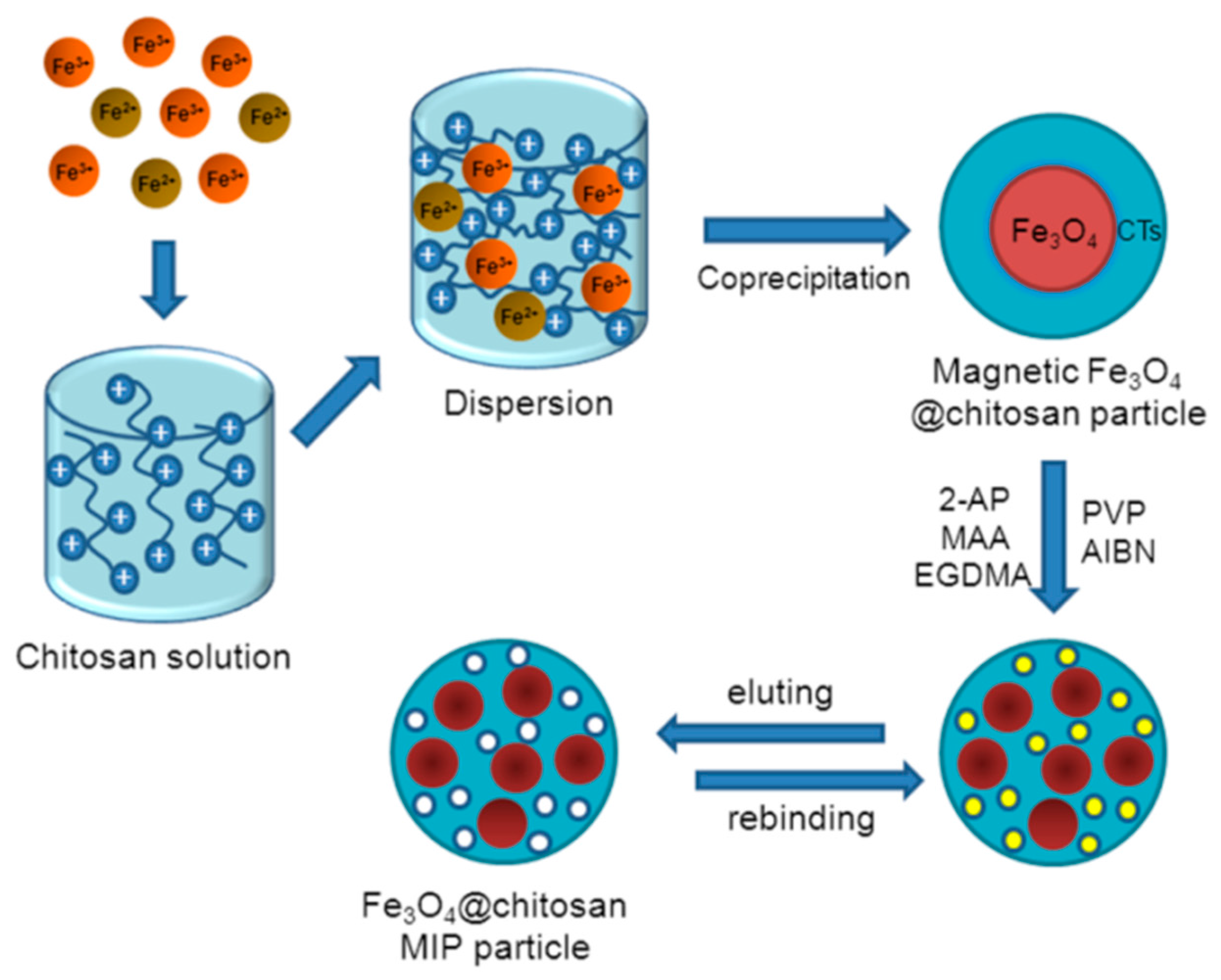
2.2. Synthesis of Magnetic Chitosan Imprinted Polymer
2.2.1. Synthesis of Fe3O4-Chitosan Particles
2.2.2. Synthesis of Fe3O4-CTs@MIP
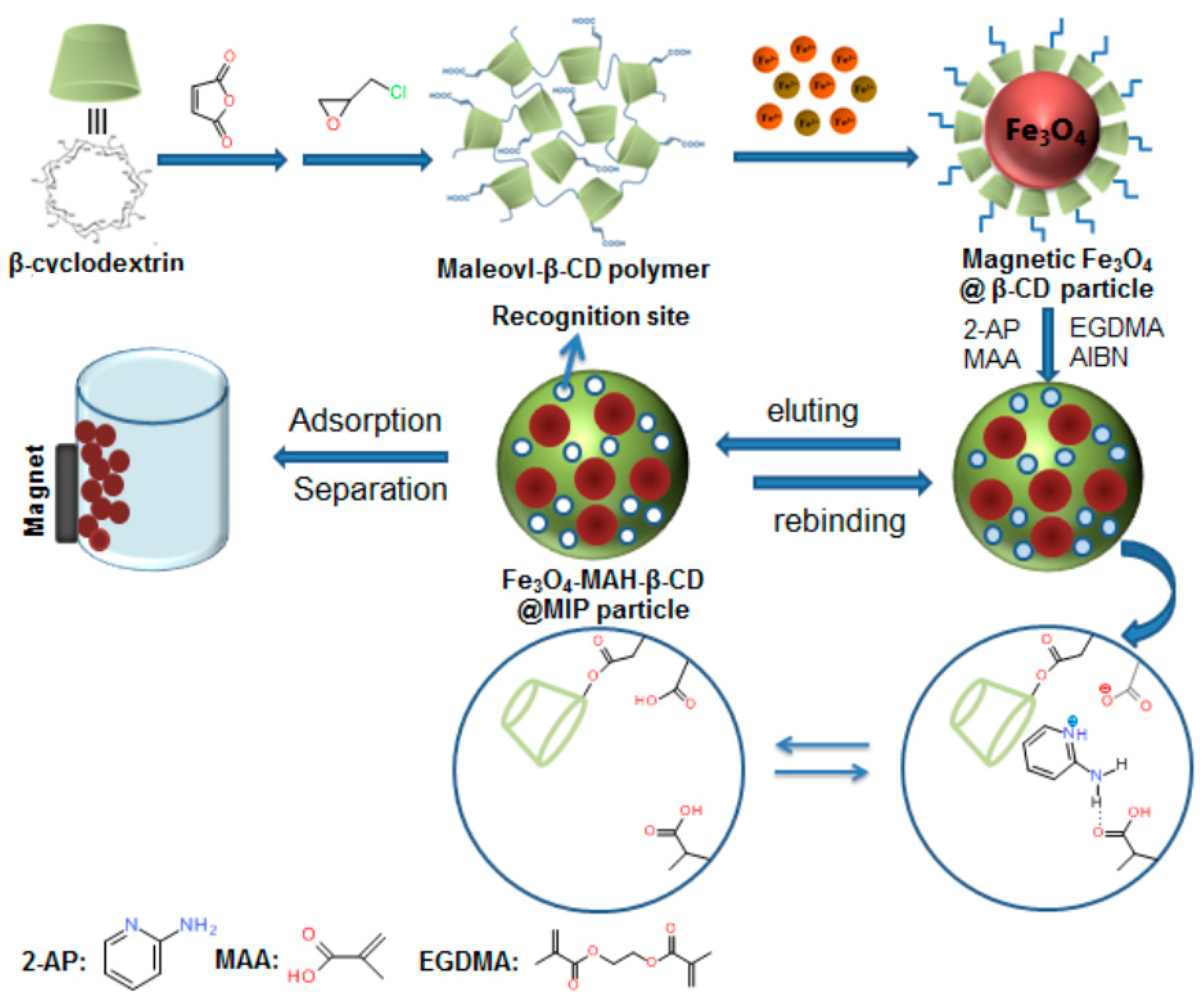
2.3. Synthesis of Magnetic β-Cyclodextrin Imprinted Polymer
2.3.1. Synthesis of MAH-β-CD
2.3.2. Synthesis of MAH-β-CD Polymer Coated Magnetic Particles
2.3.3. Synthesis of Fe3O4-MAH-β-CD@MIP
2.4. Characterization
2.5. Adsorption Experiments
2.6. Selective Adsorption
2.7. Influence of Coexistent Ions
2.8. The Contrast of MMIPs and Activated Carbon in Selective Adsorption Performance
2.9. Regeneration and Reusable Studies
2.10. Removal of 2-Aminopyridine from Different Water Samples
3. Results and Discussion
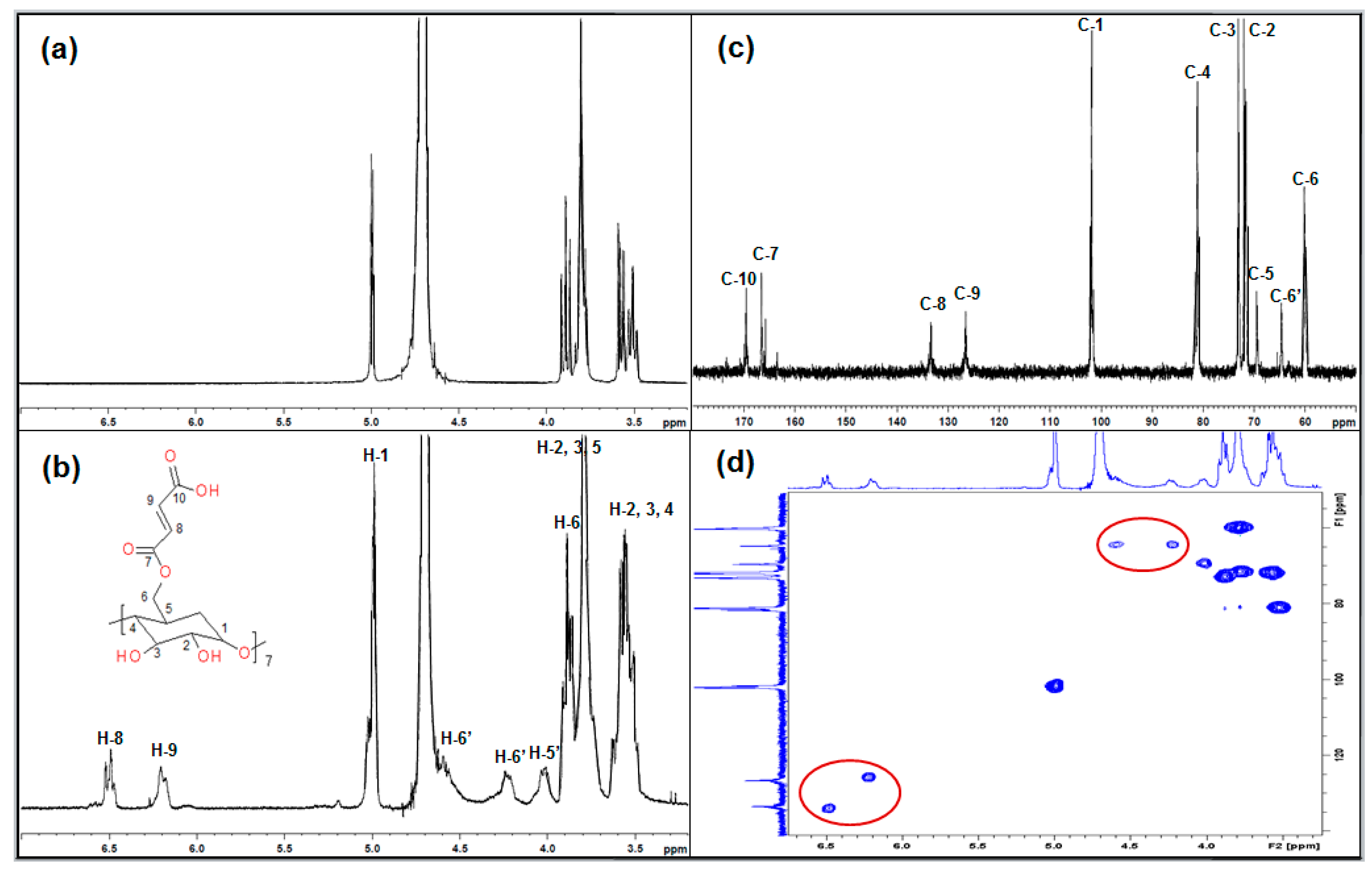
3.1. NMR Analysis of MAH-β-CD
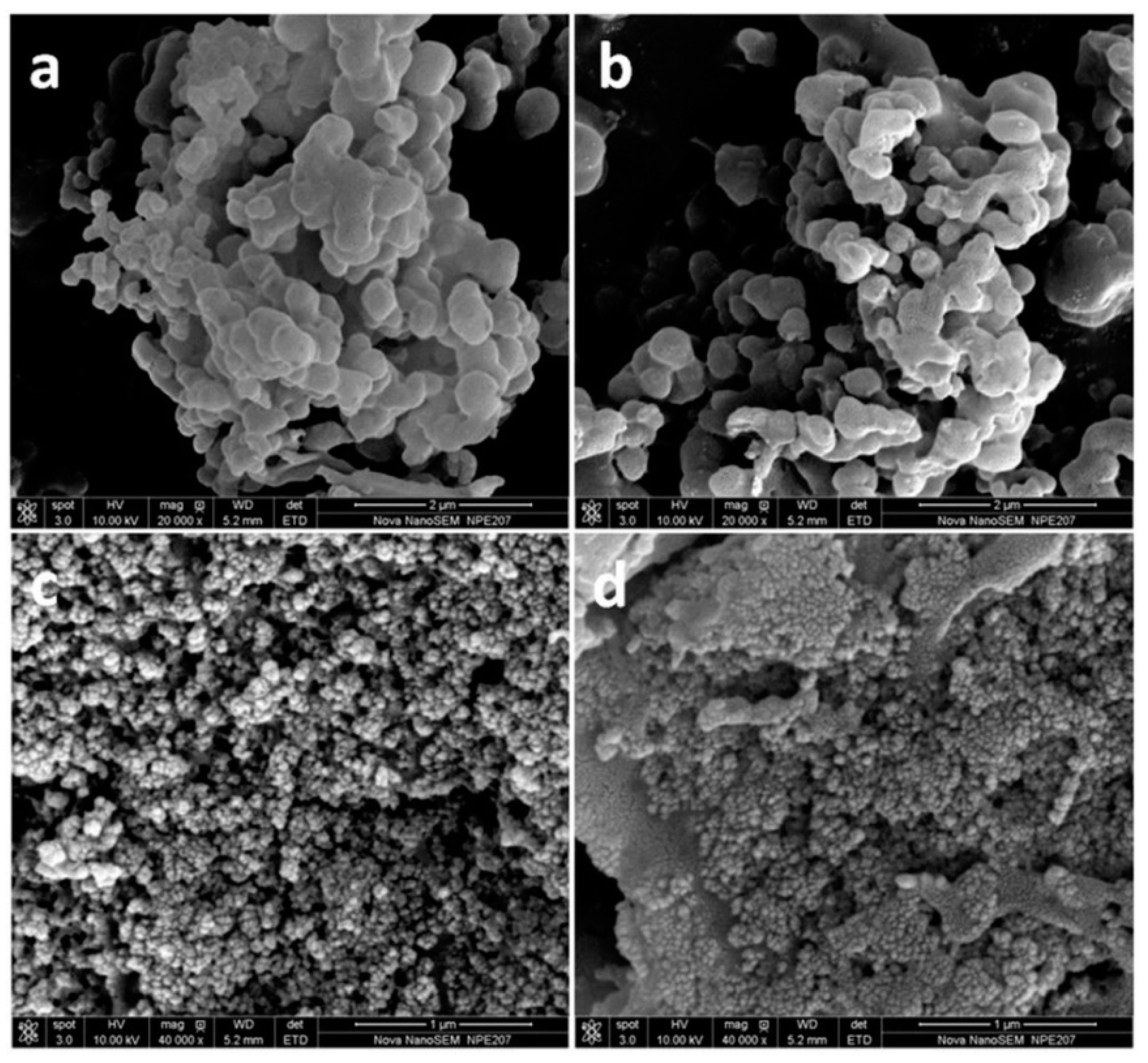
3.2. Characterization of the Magnetic Fe3O4-CTs@MIP and Fe3O4-MAH-β-CD@MIP
3.3. Adsorption Study
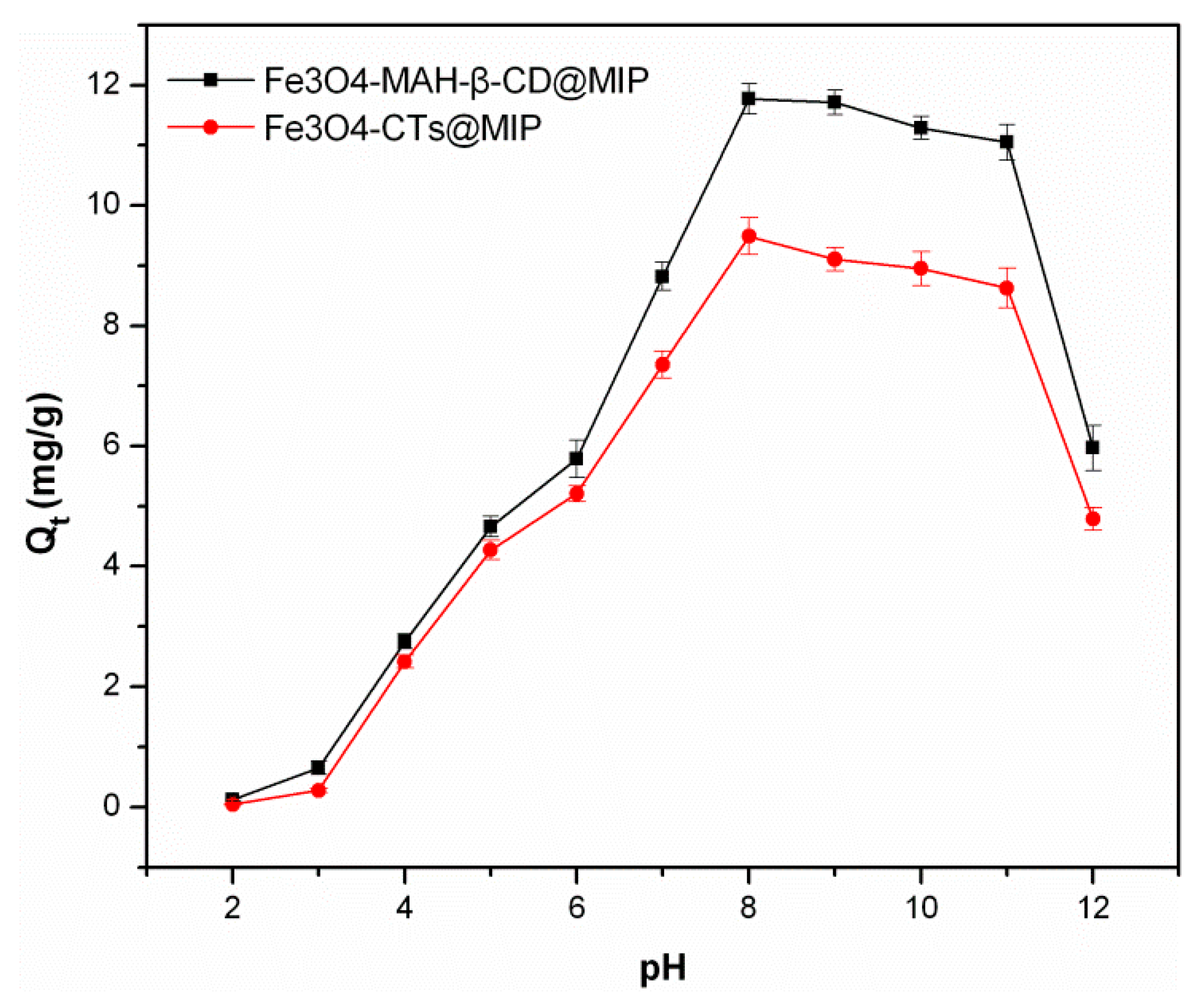
3.3.1. Effect of Solution pH on Adsorption
3.3.2. Adsorption Isotherm
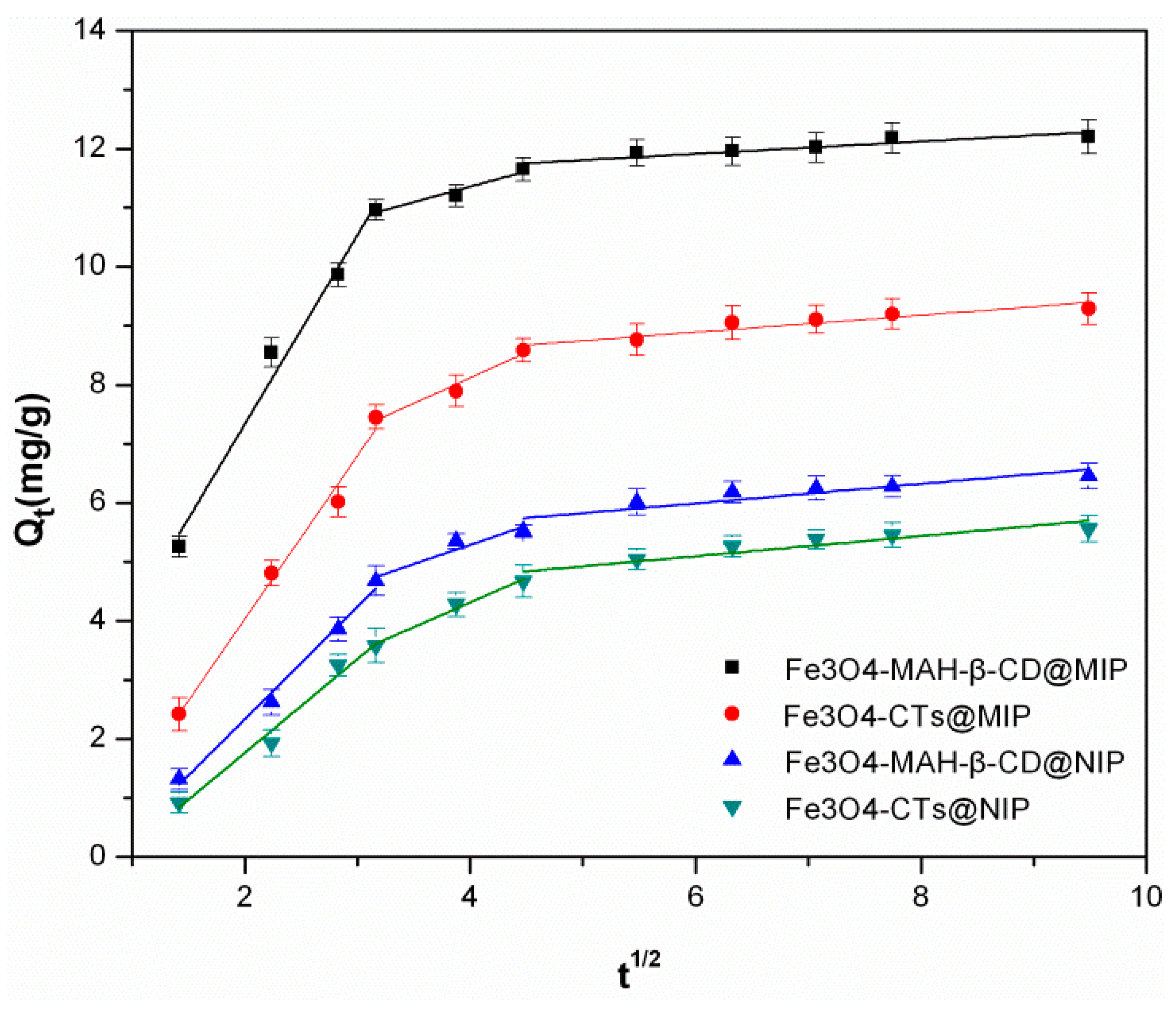
3.4. Adsorption Kinetics
3.5. Adsorption Thermodynamics
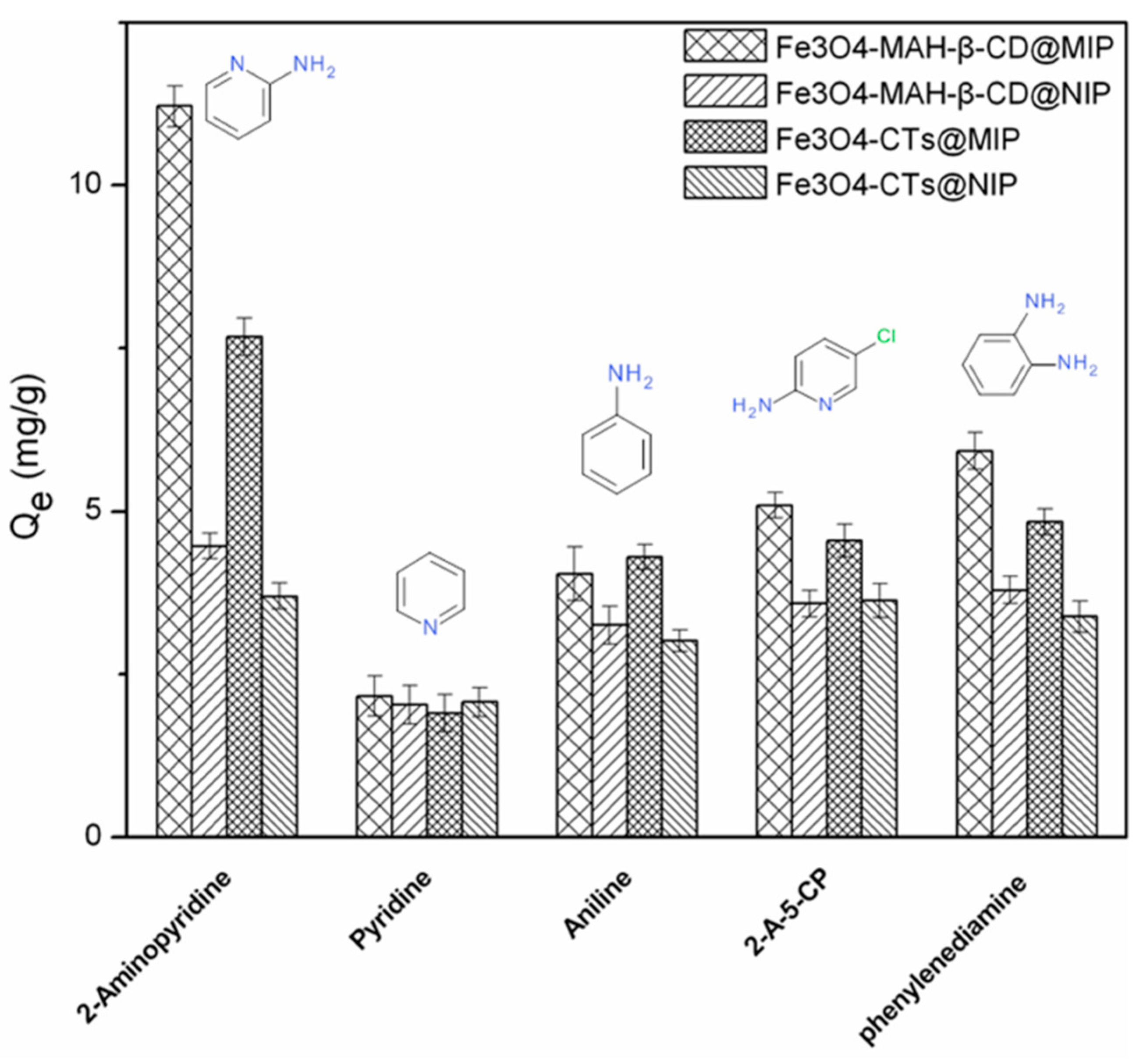
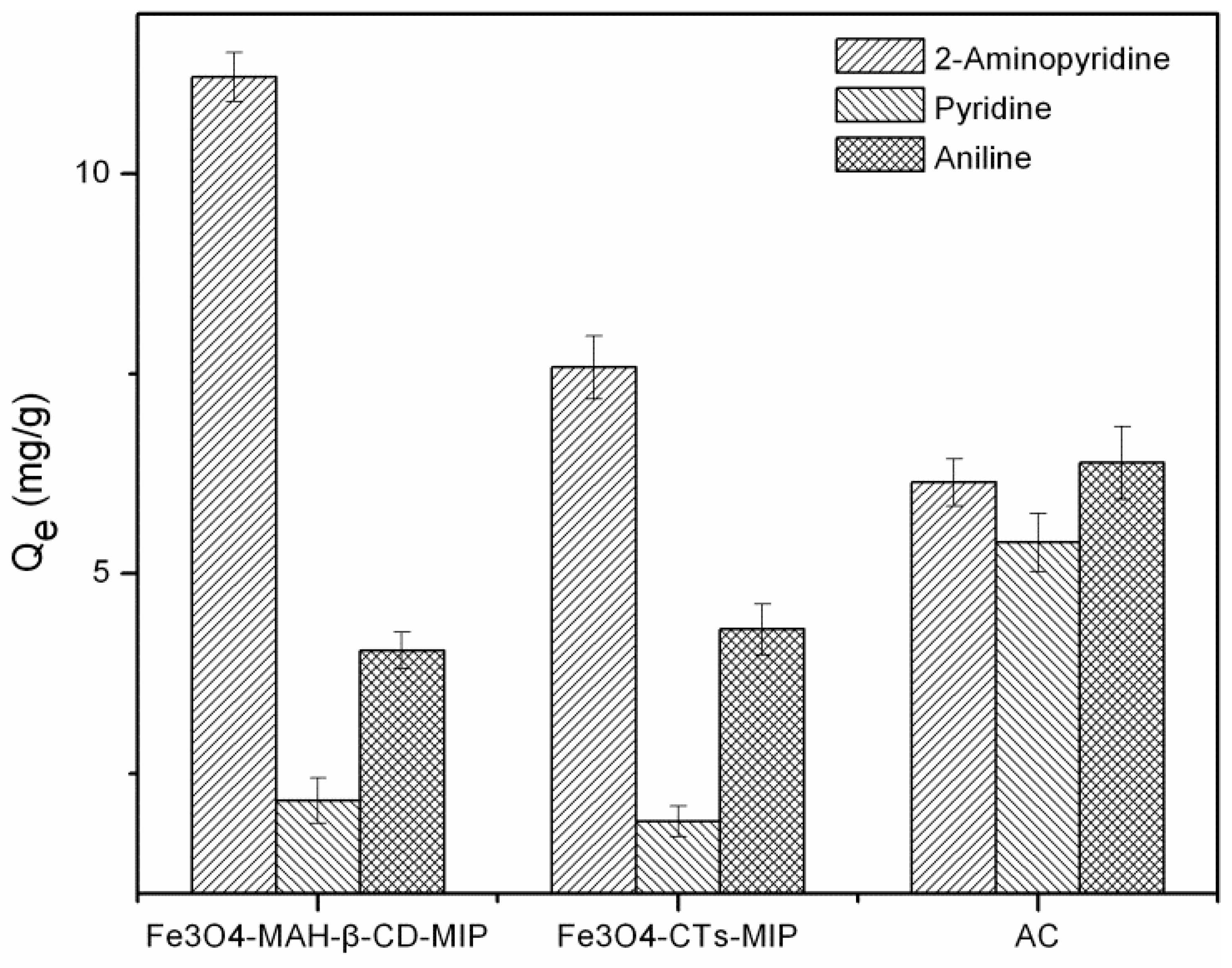
3.6. Selective Adsorption
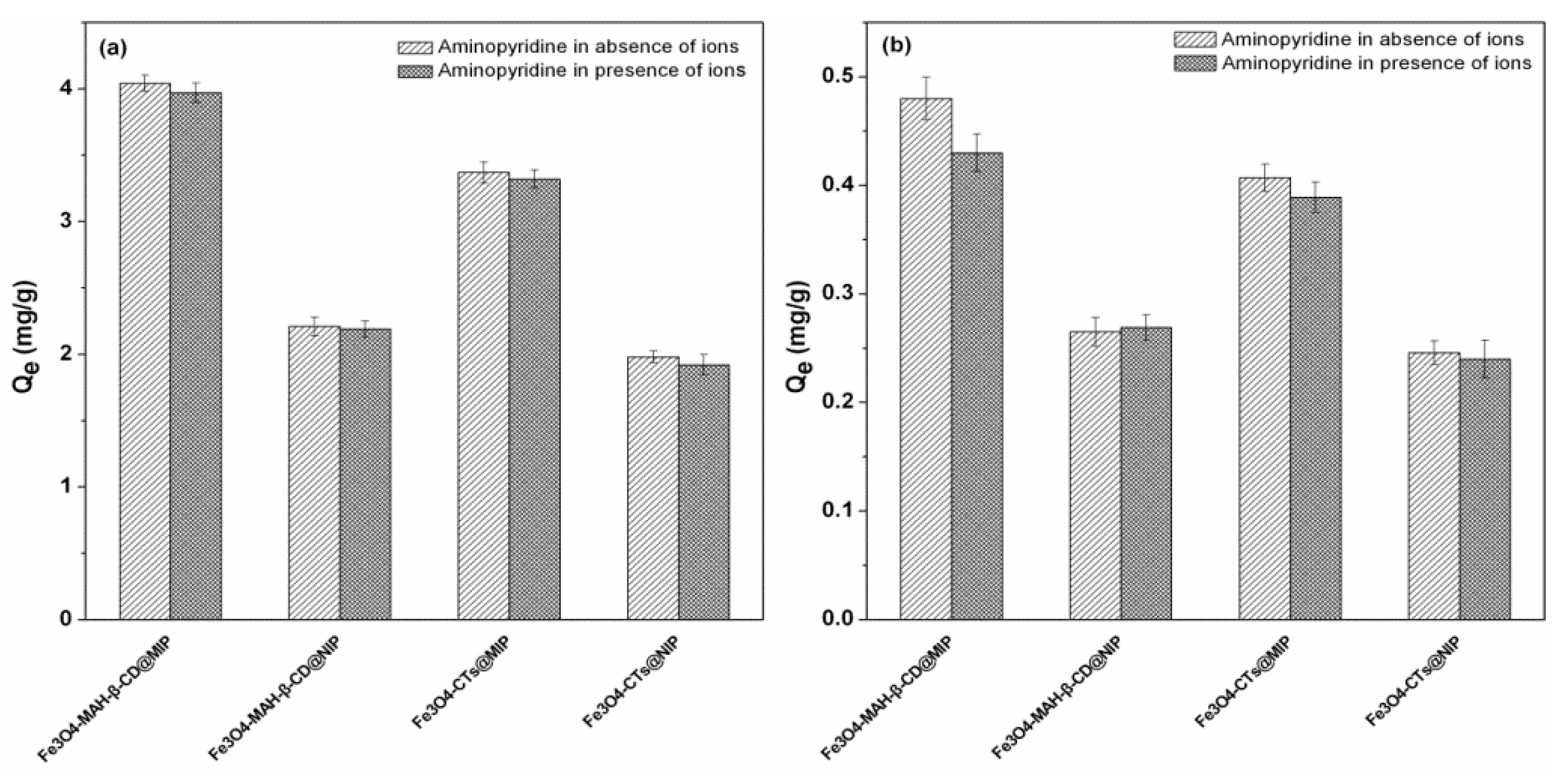
3.7. Influence of Coexistent Ions
3.8. Comparison of MIPs and Activated Carbon in Selective Adsorption Performance
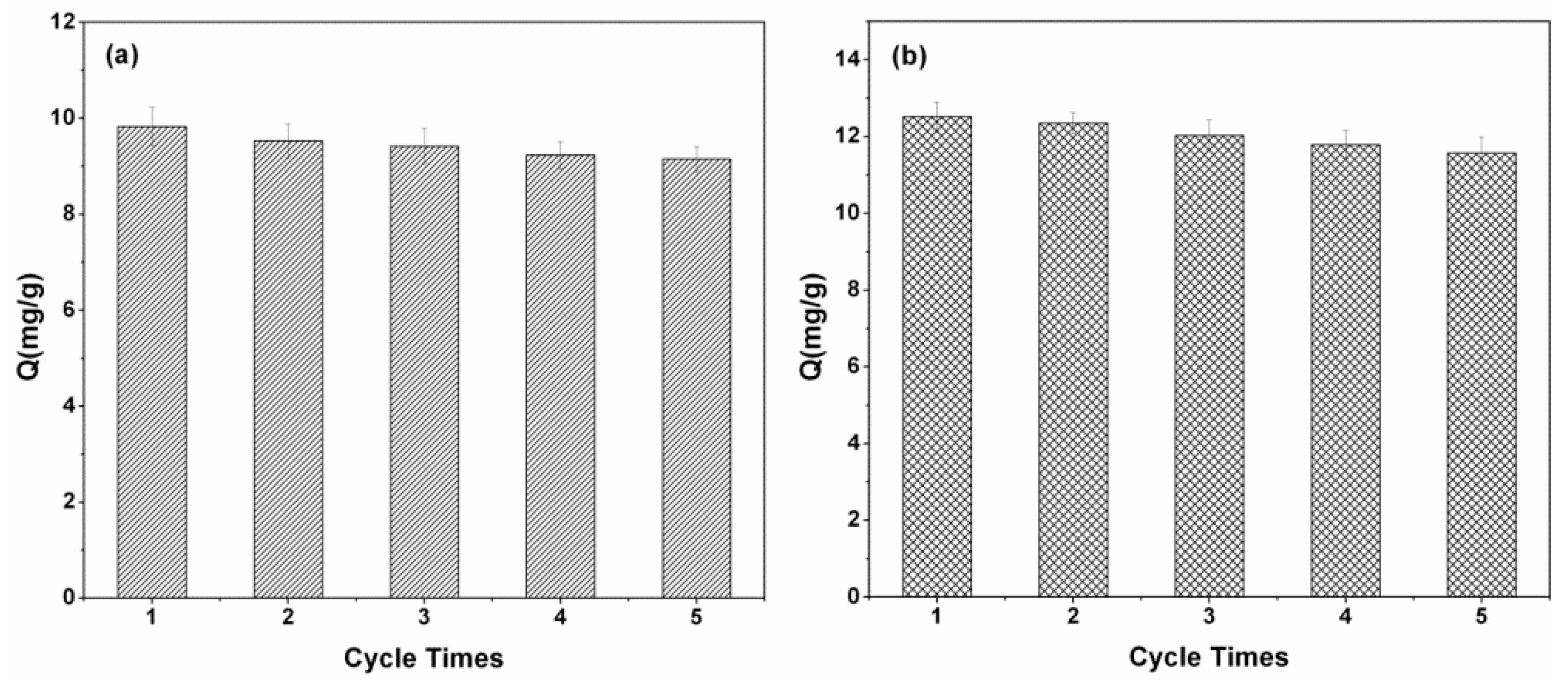
3.9. Regeneration and Reusable Studies
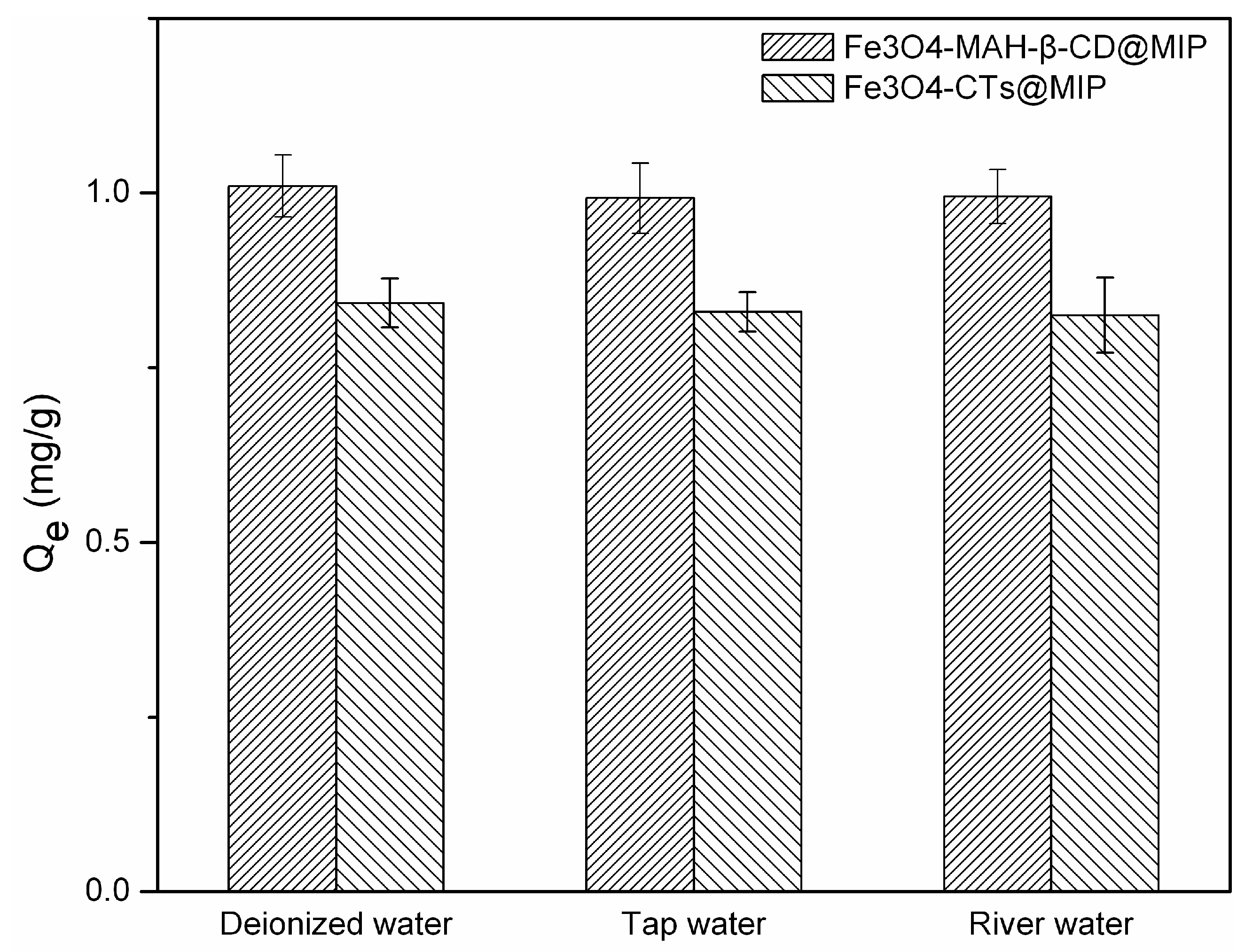
3.10. Removal of 2-Aminopyridine with MMIPs in Different Water Samples
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jassal, V.; Shanker, U.; Gahlot, S. Green synthesis of some iron oxide nanoparticles and their interaction with 2-Amino, 3-Amino and 4-Aminopyridines. Mater. Today Proc. 2016, 3, 1874–1882. [Google Scholar] [CrossRef]
- Padoley, K.V.; Mudliar, S.N.; Pandey, R.A. Heterocyclic nitrogenous pollutants in the environment and their treatment options—An overview. Bioresour. Technol. 2008, 99, 4029–4043. [Google Scholar] [CrossRef] [PubMed]
- Karale, R.; Manu, B.; Shrihari, S. Catelytic use of laterite iron for degradation of 2-aminopyridine using advanced oxidation process. Int. J. Sci. Eng. Res. 2013, 4, 207–210. [Google Scholar]
- Zhao, P.; Hao, J.C. 2,6-Diaminopyridine-imprinted polymer and its potency to hair-dye assay using graphene/ionic liquid electrochemical sensor. Biosens. Bioelectron. 2015, 64, 277–284. [Google Scholar] [CrossRef] [PubMed]
- De Fávere, V.T.; Hinze, W.L. Evaluation of the potential of chitosan hydrogels to extract polar organic species from nonpolar organic solvents: Application to the extraction of aminopyridines from hexane. J. Colloid Interface Sci. 2009, 330, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Speltini, A.; Scalabrini, A.; Maraschi, F.; Sturini, M.; Profumo, A. Newest applications of molecularly imprinted polymers for extraction of contaminants from environmental and food matrices: A review. Anal. Chim. Acta 2017, 974, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Ansaria, S.; Karimib, M. Recent configurations and progressive uses of magnetic molecularly imprinted polymers for drug analysis. Talanta 2017, 167, 470–485. [Google Scholar] [CrossRef] [PubMed]
- Kecili, R.; Billing, J.; Nivhede, D.; Sellergren, B.; Rees, A.; Yilmaz, E. Fast identification of selective resins for removal of genotoxic aminopyridine impurities via screening of molecularly imprinted polymer libraries. J. Chromatogr. A 2014, 1339, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Shakerian, F.; Kim, K.H.; Kwon, E.; Szulejko, J.E.; Kumar, P.; Dadfarnia, S.; Shabani, A.M.H. Advanced polymeric materials: Synthesis and analytical application of ion imprinted polymers as selective sorbents for solid phase extraction of metal ions. TrAC Trends Anal. Chem. 2016, 83, 55–69. [Google Scholar] [CrossRef]
- Vlatakis, G.; Andersson, L.I.; Müller, R.; Mosbach, K. Drug assay using antibody mimics made by molecular imprinting. Nature 1993, 361, 645–647. [Google Scholar] [CrossRef] [PubMed]
- Wulff, G. Molecular imprinting in cross-linked materials with the aid of molecular templates a way towards artificial antibodies. Angew. Chem. Int. Ed. 1995, 34, 1812–1832. [Google Scholar] [CrossRef]
- Chen, L.X.; Wang, X.Y.; Lu, W.H.; Wu, X.Q.; Li, J.H. Molecular imprinting: Perspectives and applications. Chem. Soc. Rev. 2016, 45, 2137–2211. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; He, X.W. Study of the nature of recognition in molecularly imprinted polymer selective for 2-aminopyridine. Anal. Chim. Acta 1999, 381, 85–91. [Google Scholar]
- Zheng, X.F.; Lian, Q.; Yang, H.; Wang, X.P. Surface molecularly imprinted polymer of chitosan grafted poly(methyl methacrylate) for 5-Fluorouracil and controlled release. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Rao, W.; Cai, R.; Yin, Y.L.; Long, F.; Zhang, Z.H. Magnetic dummy molecularly imprinted polymers based on multi-walled carbon nanotubes for rapid selective solid-phase extraction of 4-nonylphenol in aqueous samples. Talanta 2014, 128, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.L.; Dramou, P.; Xiong, N.Q.; He, H.; Yuan, D.H.; Dai, H.; Li, H.; He, X.M.; Peng, J.; Li, N. Preparation of molecularly imprinted polymers on the surface of magnetic carbon nanotubes with a pseudo template for rapid simultaneous extraction of four fluoroquinolones in egg samples. Analyst 2013, 138, 3287–3296. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.P.; Xu, X.K.; Xu, R.; Zhang, X.H.; Zhu, J.H. Preparation and characterization of molecular imprinted polymer functionalized with core/shell magnetic particles (Fe3O4@SiO2@MIP) for the simultaneous recognition and enrichment of four taxoids in taxus media. Chem. Eng. J. 2015, 279, 567–577. [Google Scholar] [CrossRef]
- Xu, W.Z.; Zhou, W.; Xu, P.P.; Pan, J.M.; Wu, X.Y.; Yan, Y.S. A molecularly imprinted polymer based on TiO2 as a sacrificial support for selective recognition of dibenzothiophene. Chem. Eng. J. 2011, 172, 191–198. [Google Scholar] [CrossRef]
- Wang, S.; Wang, B.; Si, H.T.; Shan, J.J.; Yang, X.L. Preparation of magnetic molecularly imprinted polymer beads and their recognition for baicalein. RSC Adv. 2015, 5, 8028–8036. [Google Scholar] [CrossRef]
- Chen, A.W.; Shang, C.; Shao, J.H.; Lin, Y.Q.; Luo, S.; Zhang, J.C.; Huang, H.L.; Lei, M.; Zeng, Q.G. Caron disulfide-modified magnetic ion-imprinted chitosan-Fe(III): A novel adsorbent for simultaneous removal of tetracycline and cadmium. Carbohyd. Polym. 2017, 155, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Huang, Y.A.; Zhu, Q.J.; Ye, C. Chitosan in Molecularly-Imprinted Polymers: Current and Future Prospects. Int. J. Mol. Sci. 2015, 16, 18328–18347. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.M.; Wang, X.J.; Wang, Y.H.; Sun, Y.L.; Li, J.B.; Luo, C.N. An ultrasensitive lysozyme chemiluminescence biosensor based on surface molecular imprinting using ionic liquid modified magnetic graphene oxide/β-cyclodextrin as supporting material. Anal. Chim. Acta 2016, 918, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Alsbaiee, A.; Smith, B.J.; Xiao, L.L.; Ling, Y.H.; Helbling, D.E.; Dichtel, W.R. Rapid removal of organic micropollutants from water by a porous β-cyclodextrin polymer. Nature 2016, 529, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Ou, H.X.; Chen, Q.H.; Pan, J.M.; Zhang, Y.L.; Yong, H.G.; Qi, X.Y. Selective removal of erythromycin by magnetic imprinted polymers synthesized from chitosan-stabilized Pickering emulsion. J. Hazard. Mater. 2015, 289, 28–37. [Google Scholar] [CrossRef] [PubMed]
- He, X.P.; Lian, Z.R.; Tan, L.J.; Wang, J.T. Preparation and characterization of magnetic molecularly imprinted polymers for selective trace extraction of dienestrol in seawater. J. Chromatogr. A 2016, 1469, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Y.; Zhou, T.; Du, Z.W.; Wei, Z.; Zhang, J.H. Recognition ability of temperature responsive molecularly imprinted polymer hydrogels. Soft Matter 2011, 7, 1986–1993. [Google Scholar] [CrossRef]
- Liu, L.; Xiao, L.; Zhu, H.; Shi, X. Preparation of magnetic and fluorescentbifunctional chitosan nanoparticles for optical determination of copper ion. Microchim. Acta 2012, 178, 413–419. [Google Scholar] [CrossRef]
- Badruddoza, A.Z.M.; Shawona, Z.B.Z.; Daniela, T.W.J.; Hidajata, K.; Uddinb, M.S. Fe3O4/cyclodextrin polymer nanocomposites for selective heavy metals removal from industrial wastewater. Carbohyd. Polym. 2013, 91, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Y.; Fang, H.X.; Yu, L.P. Molecularly imprinted photonic polymer based on β-cyclodextrin for amino acid sensing. Talanta 2013, 116, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.L.; Zhang, J.; Dai, C.M.; Zhou, X.F.; Liu, S.G. Sorption of carbamazepine from water by magnetic molecularly imprinted polymer based on chitosan-Fe3O4. Carbohyd. Polym. 2013, 97, 809–816. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Wang, Y.; Li, X.; Sun, B.; Wang, C. Synthesis of β-cyclodextrin-based electrospun nanofiber membranes for highly efficient adsorption and separation of methylene blue. ACS Appl. Mater. Interfaces 2015, 7, 26649–26657. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.M.; Shang, C.; Liu, Z.R.; Huang, G.L.; Adesina, A.A. Selective adsorption of uranium(VI) from aqueous solutions using the ion-imprinted magnetic chitosan resins. J. Colloid Interface Sci. 2012, 366, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Freundlich, H.M.F. Over the adsorption in solution. J. Phys. Chem. 1906, 57, 385–471. [Google Scholar]
- Kadhirvel, P.; Machado, C.; Freitas, A.; Oliveira, T.; Dias, R.C.; Costa, M.R. Molecular imprinting in hydrogels using reversible addition-fragmentation chain transfer polymerization and continuous flow micro-reactor. Chem. Technol. Biotechnol. 2015, 90, 1552–1564. [Google Scholar] [CrossRef]
- Lagergren, S. About the theory of so-called adsorption of soluble substances. K. Sven. Vetenskapsakad. Handl. 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S. Adsorption of Heavy Metals from Waste Streams by Peat. Ph.D. Thesis, University of Birmingham, Birmingham, UK, January 1995. [Google Scholar]
- Weber, W.J.; Morris, J.C. Kinetics of adsorption on carbon from solution. Am. Soc. Civ. Eng. 1963, 89, 31–59. [Google Scholar]
- Wu, F.C.; Tseng, R.L.; Juang, R.S. Comparisons of porous and adsorption properties of carbons activated by steam and KOH. J. Colloid Interface Sci. 2005, 283, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Lorenc-Grabowska, E.; Gryglewicz, G. Adsorption characteristics of Congo Red on coal-based mesoporous activated carbon. Dyes Pigm. 2007, 74, 34–40. [Google Scholar] [CrossRef]
- Lou, S.; Chen, Z.B.; Liu, Y.F.; Ye, H.L.; Di, D.L. Synthesis of functional adsorption resin and its adsorption properties in purification of flavonoids from Hippophae rhamnoides L. Leaves. Ind. Eng. Chem. Res. 2012, 51, 2682–2696. [Google Scholar] [CrossRef]
- Kumara, A.S.K.; Jiang, S.J. Chitosan-functionalized graphene oxide: A novel adsorbent an efficient adsorption of arsenic from aqueous solution. J. Environ. Chem. Eng. 2016, 4, 1698–1713. [Google Scholar] [CrossRef]
- Zhu, F.; Li, L.W.; Xing, J.D. Selective adsorption behavior of Cd(II) ion imprinted polymers synthesized by microwave-assisted inverse emulsion polymerization: Adsorption performance and mechanism. J. Hazard. Mater. 2017, 321, 103–110. [Google Scholar] [CrossRef] [PubMed]




| Polymer | Langmuir Isotherm | R2 | Freundlich Isotherm | R2 | ||
|---|---|---|---|---|---|---|
| Qm (mg·g−1) | KL (mL·mg−1) | KF (mg1-1/n·mL1/n·g−1) | 1/n | |||
| Fe3O4-CTs@MIP | 39.22 ± 1.466 | 7.29 ± 0.682 | 0.998 | 64.22 ± 3.316 | 0.678 ± 0.0392 | 0.981 |
| Fe3O4-CTs@NIP | 19.88 ± 0.577 | 6.29 ± 0.414 | 0.998 | 28.51 ± 1.353 | 0.655 ± 0.0326 | 0.987 |
| Fe3O4-MAH-β-CD@MIP | 46.51 ± 1.314 | 10.24 ± 0.634 | 0.996 | 81.85 ± 3.797 | 0.629 ± 0.0277 | 0.989 |
| Fe3O4-MAH-β-CD@NIP | 22.52 ± 0.823 | 6.94 ± 0.483 | 0.997 | 33.11 ± 2.113 | 0.641 ± 0.0468 | 0.987 |
| Adsorbents | Target | Qm (mg·g−1) | References |
|---|---|---|---|
| Bulk polymerized imprinted polymer | 2-aminopyridine | 12.8 | [16] |
| Hybrid MIP with NIPA hydrogel | 4-aminopyridine | 6.0 | [27] |
| MIH-FRP hydrogel | 3-aminopyridine | 600.0 | [36] |
| Magnetic chitosan-based imprinted polymer | 2-aminopyridine | 39.2 | This study |
| Magnetic β-cyclodextrin based imprinted polymer | 2-aminopyridine | 46.5 | This study |
| MIPs | Adsorption Capacity (Qe, mg·g−1) of Fe3O4-CTs@MIP | Adsorption Capacity (Qe, mg·g−1) of Fe3O4-MAH-β-CD@MIP |
|---|---|---|
| Batch#1 | 9.28 | 12.19 |
| Batch#2 | 10.04 | 12.65 |
| Batch#3 | 8.71 | 14.06 |
| Average ± SD | 9.34 ± 0.657 | 12.97 ± 0.978 |
| Adsorption Isotherm Models | Constants | Fe3O4-CTs@MIP | Fe3O4-MAH-β-CD@MIP | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 288 K | 298 K | 308 K | 318 K | 328 K | 288 K | 298 K | 308 K | 318 K | 328 K | ||
| Langmuir isotherm | Qm (mg·g−1) | 41.32 ± 1.917 | 39.22± 1.466 | 34.60 ± 0.977 | 30.68 ± 0.872 | 27.25 ± 0.967 | 48.78 ± 1.737 | 46.51 ± 1.374 | 41.67 ± 1.407 | 37.45 ± 1.139 | 33.56 ± 0.854 |
| KL (mL·mg−1) | 8.3 ± 0.591 | 7.29 ± 0.682 | 5.67 ± 0.375 | 4.87 ± 0.297 | 4.32 ± 0.268 | 12.81 ± 0.908 | 10.24 ± 0.634 | 7.27 ± 0.481 | 5.68 ± 0.339 | 4.89 ± 0.289 | |
| R2 | 0.998 | 0.998 | 0.998 | 0.998 | 0.998 | 0.994 | 0.996 | 0.998 | 0.999 | 0.999 | |
| Freundlich isotherm | KF (mg1-1/n·mL1/n·g−1) | 69.86 ± 3.474 | 64.22 ± 3.316 | 53.06 ± 3.285 | 44.59 ± 2.701 | 37.82 ± 2.276 | 87.42 ± 3.474 | 81.85 ± 3.797 | 68.16 ± 4.349 | 57.29 ± 3.598 | 48.68 ± 2.887 |
| 1/n | 0.66 ± 0.0482 | 0.678 ± 0.0392 | 0.71 ± 0.0519 | 0.72 ± 0.0517 | 0.74 ± 0.0511 | 0.59 ± 0.0482 | 0.63 ± 0.0277 | 0.68 ± 0.0497 | 0.70 ± 0.0507 | 0.72 ± 0.0481 | |
| R2 | 0.981 | 0.981 | 0.982 | 0.982 | 0.982 | 0.989 | 0.989 | 0.988 | 0.988 | 0.988 | |
| Polymers | T (K) | Thermodynamic Parameters | R2 | ||
|---|---|---|---|---|---|
| ΔG (kJ·mol−1) | ΔH (kJ·mol−1) | ΔS (J·mol−1·k−1) | |||
| Fe3O4-CTs@MIP | 288 | −2.81 | −11.26 | −29.31 | 0.991 |
| 298 | −2.64 | −28.89 | |||
| 308 | −2.23 | −29.28 | |||
| 318 | −1.94 | −29.29 | |||
| 328 | −1.70 | −29.12 | |||
| Fe3O4-MAH-β-CD@MIP | 288 | −3.76 | −16.14 | −42.98 | 0.990 |
| 298 | −3.47 | −42.50 | |||
| 308 | −2.85 | −43.15 | |||
| 318 | −2.44 | −43.08 | |||
| 328 | −2.13 | −42.69 | |||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Zhu, Z.; Zhang, H.; Qiu, Y. Selective Removal of the Genotoxic Compound 2-Aminopyridine in Water using Molecularly Imprinted Polymers Based on Magnetic Chitosan and β-Cyclodextrin. Int. J. Environ. Res. Public Health 2017, 14, 991. https://doi.org/10.3390/ijerph14090991
Zhang W, Zhu Z, Zhang H, Qiu Y. Selective Removal of the Genotoxic Compound 2-Aminopyridine in Water using Molecularly Imprinted Polymers Based on Magnetic Chitosan and β-Cyclodextrin. International Journal of Environmental Research and Public Health. 2017; 14(9):991. https://doi.org/10.3390/ijerph14090991
Chicago/Turabian StyleZhang, Wei, Zhiliang Zhu, Hua Zhang, and Yanling Qiu. 2017. "Selective Removal of the Genotoxic Compound 2-Aminopyridine in Water using Molecularly Imprinted Polymers Based on Magnetic Chitosan and β-Cyclodextrin" International Journal of Environmental Research and Public Health 14, no. 9: 991. https://doi.org/10.3390/ijerph14090991






