Community Characteristics and Leaf Stoichiometric Traits of Desert Ecosystems Regulated by Precipitation and Soil in an Arid Area of China
Abstract
:1. Introduction
2. Materials and Methods
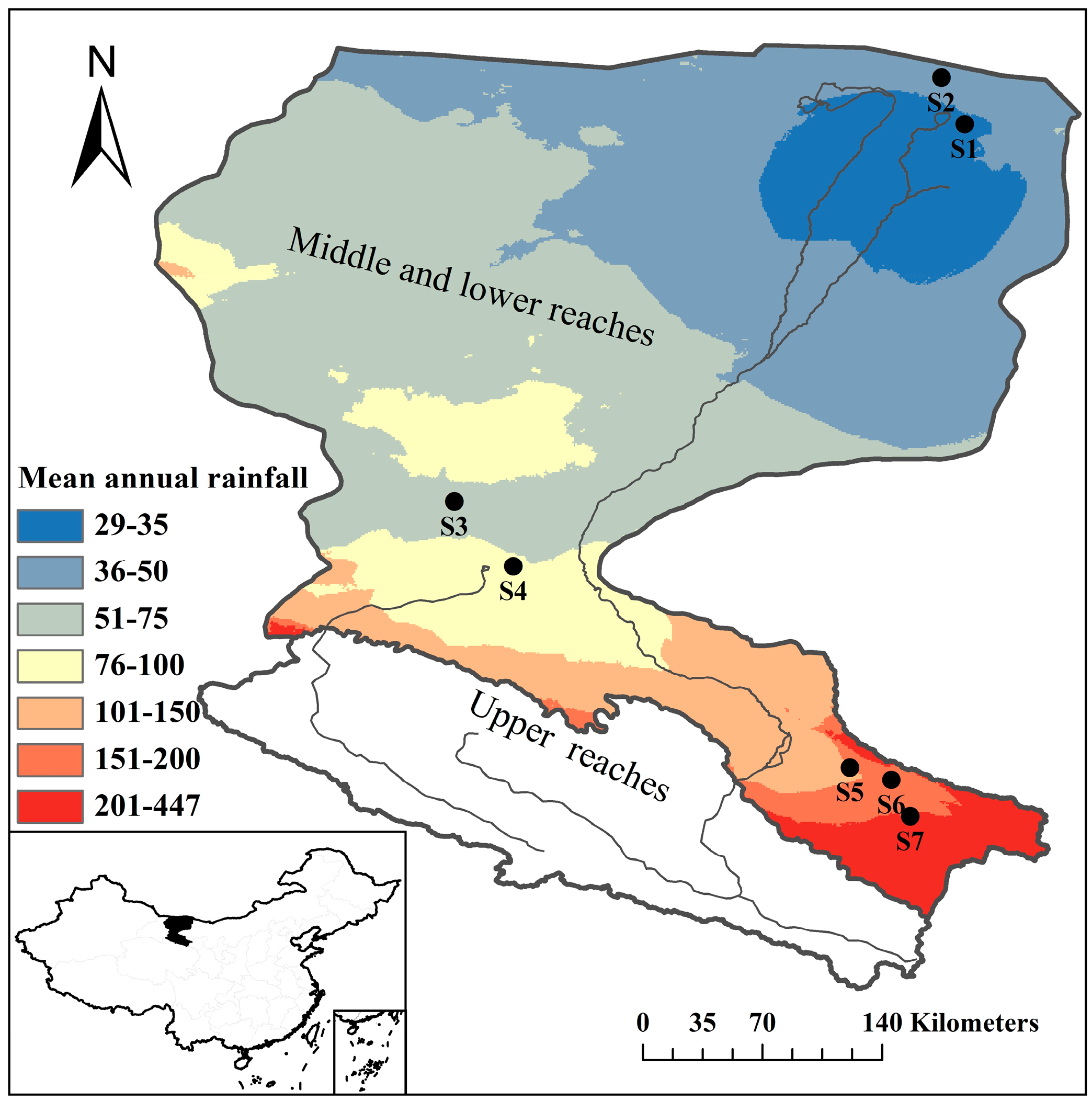
2.1. Study Area and Site Description
2.2. Experimental Design and Data Collection
2.3. Leaf Stoichiometric Traits
2.4. Statistical Analysis
3. Results
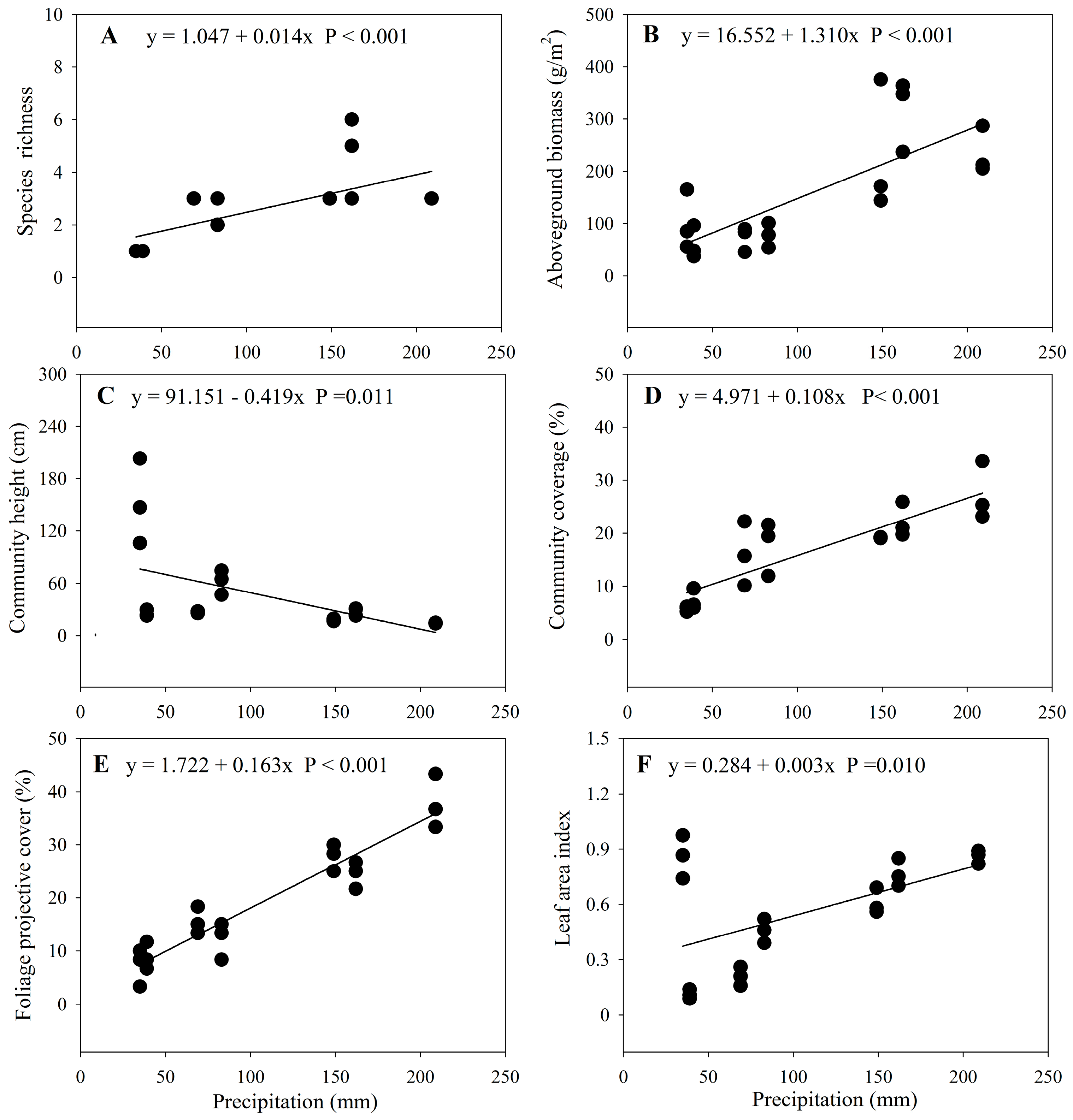
3.1. Changes in Community Characteristics along the Precipitation Gradient
3.2. Changes in Leaf Stoichiometric Traits along the Precipitation Gradient
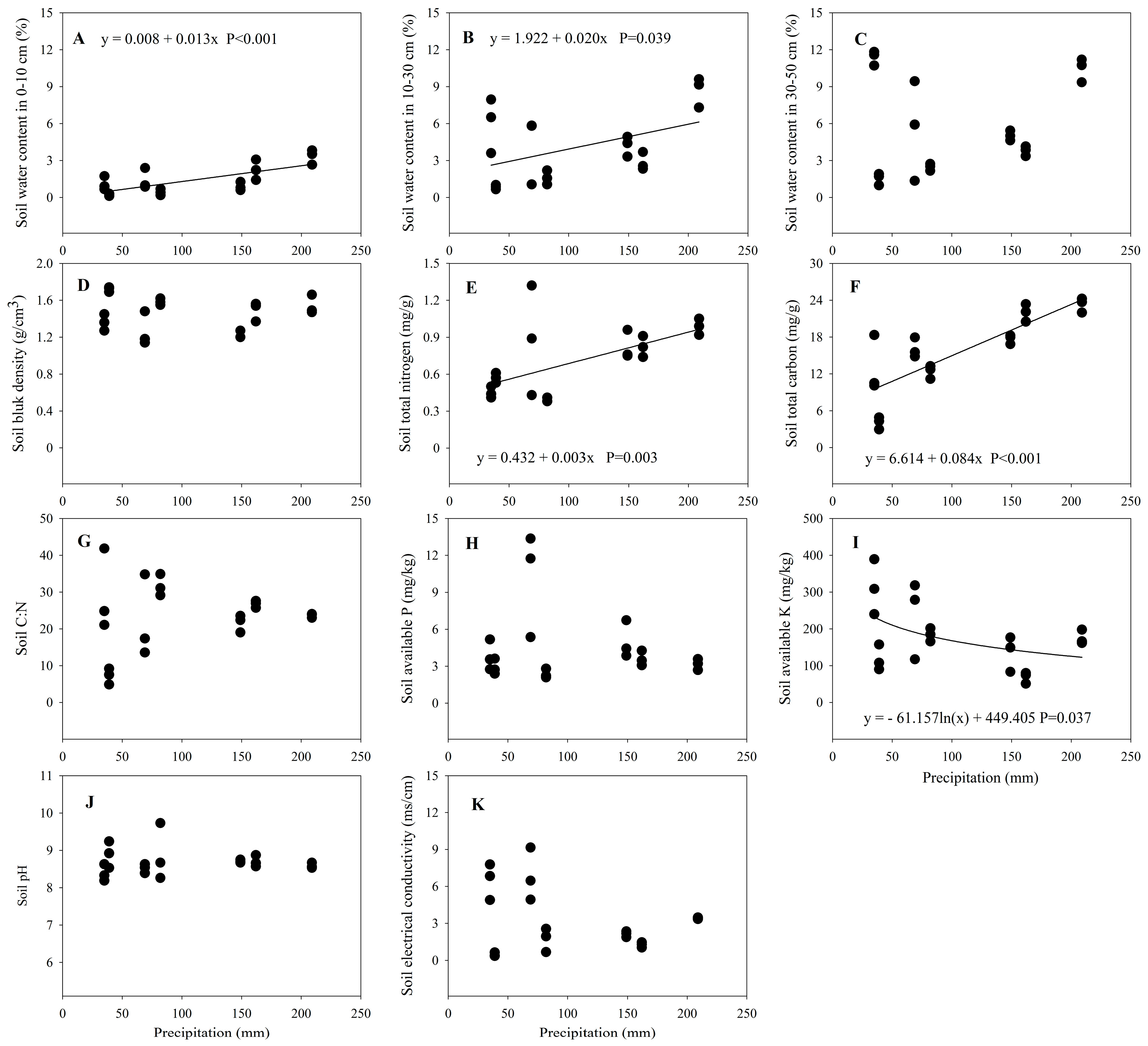
3.3. Changes in Soil Properties along the Precipitation Gradient
3.4. Relationships among Community Characteristics, Leaf Stoichiometric Traits and Environmental Factors
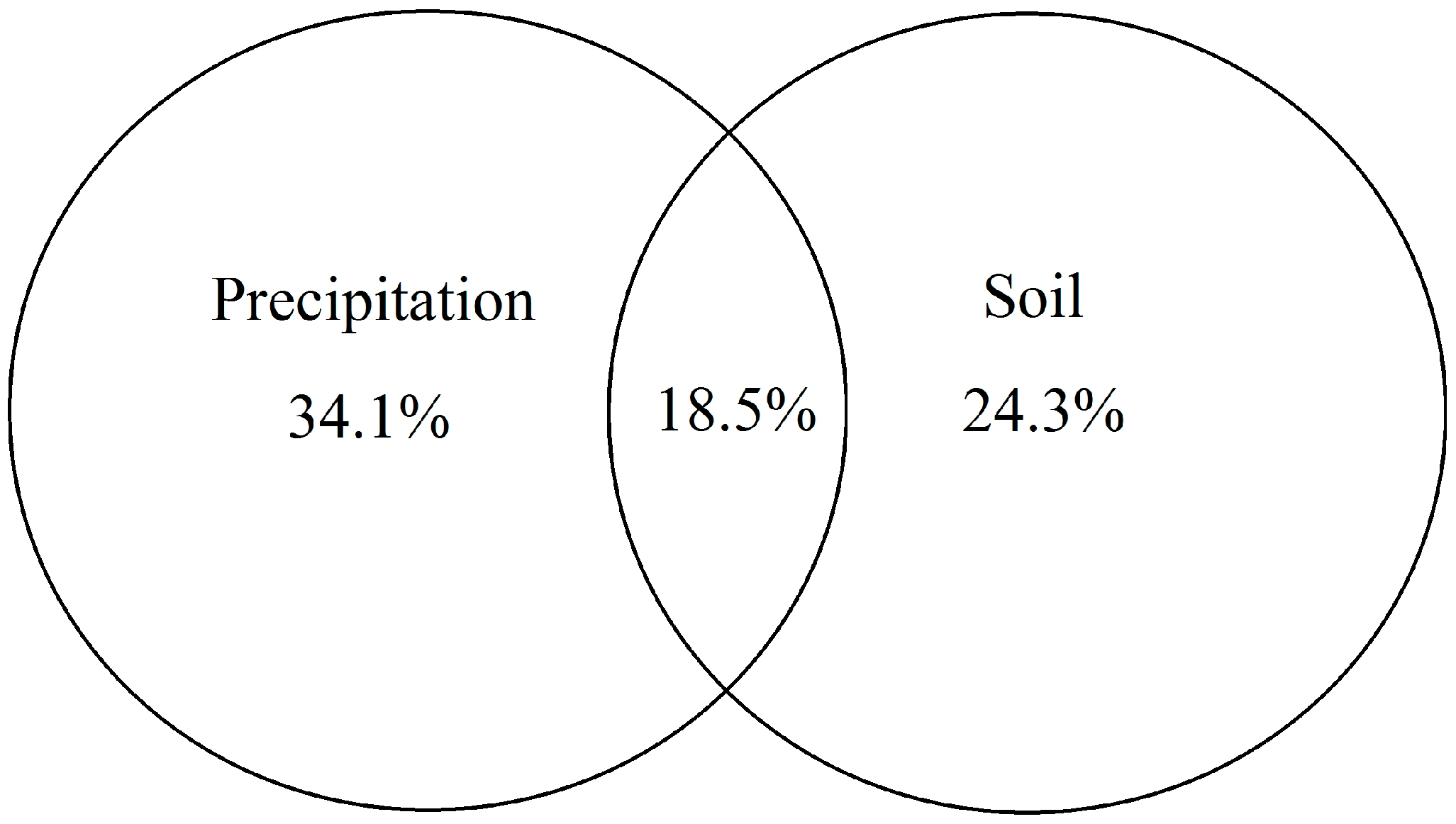
3.5. Controlling Factors of Community Characteristics and Leaf Stoichiometric Traits
4. Discussion
4.1. Community Characteristics and the Precipitation Gradient
4.2. Leaf Stoichiometric Traits and the Precipitation Gradient
4.3. Soil Properties and the Precipitation Gradient
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
| Sampling Sites | Family | Species |
|---|---|---|
| S1 (1 species including 1 shrub) | Chenopodiaceae | Haloxylon ammodendron (C. A. Mey.) Bunge |
| S2 (1 species including 1 shrub) | Zygophyllaceae | Nitraria sibirica Pall. |
| S3 (2 species including 2 shrubs) | Zygophyllaceae | Nitraria praevisa Bobr. |
| Solanaceae | Lycium ruthenicum Murr. | |
| S4 (3 species including 3 shrubs) | Asteraceae | Artemisia desertorum Spreng. |
| Ephedraceae | Ephedra przewalskii Stapf. | |
| Polygonaceae | Calligonum mongolicum Turcz. | |
| S5 (3 species including 3 shrubs) | Chenopodiaceae | Kalidium gracile Fenzl |
| Chenopodiaceae | Salsola passerina Bunge | |
| Chenopodiaceae | Sympegma regelii Bunge | |
| S6 (7 species including 5 shrubs and 2 herbages) | Chenopodiaceae | Kalidium gracile Fenzl |
| Chenopodiaceae | Salsola passerina Bunge | |
| Chenopodiaceae | Sympegma regelii Bunge | |
| Leguminosae | Caragana roborovskyi Kom. | |
| Zygophyllaceae | Nitraria roborowskii Kom. | |
| Chenopodiaceae | Agriophyllum squarrosum (L.) Moq. | |
| Chenopodiaceae | Halogeton glomeratus (Bieb.) C. A. Mey. | |
| S7 (4 species including 3 shrubs and 1 herbage) | Chenopodiaceae | Kalidium cuspidatum (Ung. Sternb.) Grub. |
| Chenopodiaceae | Salsola passerina Bunge | |
| Tamaricaceae | Reaumuria songarica (Pall.) Maxim. | |
| Zygophyllaceae | Zygophyllum fabago L. |
| Site | Annual Rainfall (mm) | Important Value of Major Species in Shrub Layer | Important Value of Major Species in Herb Layer | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HA | NS | NP | AD | KG | SP | SR | KC | AS | HG | ZF | ||
| S1 | 35 | 1.00 | ||||||||||
| S2 | 39 | 1.00 | ||||||||||
| S3 | 62 | 0.76 | ||||||||||
| S4 | 82 | 0.72 | ||||||||||
| S5 | 149 | 0.51 | 0.31 | |||||||||
| S6 | 162 | 0.61 | 0.22 | 0.85 | 0.15 | |||||||
| S7 | 209 | 0.26 | 0.69 | 1 | ||||||||
| Community Characteristics/Leaf Stoichiometric Traits | Sum of Squares | df | Mean Square | F | Sig. |
|---|---|---|---|---|---|
| Species richness | 1.153 | 6 | 0.192 | 38.79 | <0.001 *** |
| Aboveground biomass | 1.601 | 6 | 0.267 | 8.75 | <0.001 *** |
| Community coverage | 1.148 | 6 | 0.191 | 18.51 | <0.001 *** |
| Community height | 2.187 | 6 | 0.364 | 62.05 | <0.001 *** |
| Foliage projective cover | 1.403 | 6 | 0.234 | 14.80 | <0.001 *** |
| Leaf area index | 2.127 | 6 | 0.354 | 76.44 | <0.001 *** |
| Leaf C | 0.430 | 6 | 0.072 | 175.76 | <0.001 *** |
| Leaf N | 0.246 | 6 | 0.041 | 109.19 | <0.001 *** |
| Leaf P | 0.651 | 6 | 0.108 | 456.43 | <0.001 *** |
| Leaf K | 0.487 | 6 | 0.081 | 253.59 | <0.001 *** |
| Leaf C/N | 0.098 | 6 | 0.016 | 319.26 | <0.001 *** |
| Leaf C/P | 0.815 | 6 | 0.136 | 306.01 | <0.001 *** |
| Leaf N/P | 0.564 | 6 | 0.094 | 241.23 | <0.001 *** |
| Soil Properties | Sum of Squares | df | Mean Square | F | Sig. |
|---|---|---|---|---|---|
| Gravimetric soil water content (0–10 cm) | 2.596 | 6 | 0.433 | 3.24 | <0.033 * |
| Gravimetric soil water content (10–30 cm) | 4.144 | 6 | 0.691 | 12.62 | <0.001 ** |
| Gravimetric soil water content (30–50 cm) | 3.862 | 6 | 0.644 | 18.01 | <0.001 ** |
| Soil bulk density | 0.251 | 6 | 0.042 | 24.39 | <0.001 ** |
| Soil total nitrogen | 2.034 | 6 | 0.339 | 22.26 | <0.001 ** |
| Soil total carbon | 6.793 | 6 | 1.132 | 56.56 | <0.001 ** |
| Soil C/N | 4.751 | 6 | 0.792 | 28.68 | <0.001 ** |
| Soil available P | 3.665 | 6 | 0.611 | 23.76 | <0.001 ** |
| Soil available K | 4.215 | 6 | 0.702 | 24.02 | <0.001 ** |
| Soil pH | 0.007 | 6 | 0.001 | 2.330 | <0.041 * |
| Soil electrical conductivity | 19.360 | 6 | 3.227 | 25.36 | <0.001 ** |
| Community Characteristics and Leaf Stoichiometric Traits | GSWC10 | GSWC30 | GSWC50 | SBD | STN | STC | C/N | SAP | SAK | pH | EC |
|---|---|---|---|---|---|---|---|---|---|---|---|
| SR | 0.742 | 0.642 | 0.370 | −0.152 | 0.752 | 0.700 | −0.040 | 0.179 | −0.132 | −0.144 | 0.100 |
| AGB | 0.449 | 0.342 | 0.130 | −0.167 | 0.386 | 0.730 | 0.273 | −0.155 | −0.375 | −0.044 | −0.218 |
| COVER | 0.537 | 0.290 | 0.077 | −0.059 | 0.560 | 0.739 | 0.193 | 0.017 | −0.345 | 0.077 | −0.158 |
| HEIGHT | −0.248 | 0.189 | 0.414 | −0.131 | −0.539 | −0.177 | 0.469 | −0.179 | 0.628 | −0.249 | 0.477 |
| FPC | 0.678 | 0.463 | 0.226 | −0.149 | 0.698 | 0.752 | 0.003 | −0.046 | −0.359 | −0.136 | −0.157 |
| LAI | 0.573 | 0.581 | 0.674 | −0.196 | 0.103 | 0.623 | 0.496 | −0.285 | 0.112 | −0.232 | 0.133 |
| Leaf C | 0.067 | 0.122 | 0.170 | 0.071 | −0.242 | 0.075 | 0.517 | −0.033 | 0.429 | −0.069 | 0.333 |
| Leaf N | 0.037 | 0.090 | 0.180 | −0.245 | −0.070 | 0.064 | 0.383 | 0.416 | 0.534 | −0.242 | 0.649 |
| Leaf P | −0.407 | −0.518 | −0.548 | −0.126 | −0.191 | −0.122 | 0.243 | 0.315 | −0.011 | 0.159 | 0.027 |
| Leaf K | −0.541 | −0.524 | −0.357 | 0.122 | −0.607 | −0.366 | 0.375 | −0.096 | 0.200 | 0.110 | 0.022 |
| Leaf C/N | 0.171 | 0.173 | 0.148 | 0.431 | −0.257 | 0.114 | 0.413 | −0.538 | 0.104 | 0.147 | −0.170 |
| Leaf C/P | 0.596 | 0.724 | 0.746 | 0.143 | 0.145 | 0.326 | 0.174 | −0.257 | 0.340 | −0.246 | 0.288 |
| Leaf N/P | 0.583 | 0.748 | 0.813 | 0.035 | 0.172 | 0.286 | 0.104 | −0.140 | 0.411 | −0.335 | 0.413 |
References
- Yang, H.J.; Wu, M.Y.; Liu, W.X.; Zhang, Z.; Zhang, N.L.; Wan, S.Q. Community structure and composition in response to climate change in a temperate steppe. Glob. Chang. Biol. 2011, 17, 452–465. [Google Scholar] [CrossRef]
- Wang, L.; D’Odorico, P.; Evans, J.P.; Eldridge, D.J.; McCabe, M.F.; Caylor, K.K.; King, E.G. Dryland ecohydrology and climate change: Critical issues and technical advances. Hydrol. Earth Syst. Sci. 2012, 16, 2585–2603. [Google Scholar] [CrossRef] [Green Version]
- Deng, L.; Wang, K.B.; Li, J.P.; Zhao, G.W.; Shangguan, Z.P. Effect of soil moisture and atmospheric humidity on both plant productivity and diversity of native grasslands across the Loess Plateau, China. Ecol. Eng. 2016, 94, 525–531. [Google Scholar] [CrossRef]
- Noy-Meir, I. Desert ecosystems: Environment and producers. Annu. Rev. Ecol. Syst. 1973, 4, 25–51. [Google Scholar] [CrossRef]
- Arndt, S.K. Integrated research of plant functional traits is important for the understanding of ecosystem processes. Plant Soil 2006, 285, 1–3. [Google Scholar] [CrossRef]
- Huxman, T.E.; Cable, J.M.; Ignace, D.D.; Eits, J.A.; English, N.B.; Weltzin, J.; Williams, D.G. Response of net ecosystem gas exchange to a simulated precipitation pulse in a semi-arid grassland: The role of native versus non-native grasses and soil texture. Oecologia 2004, 141, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.H.; Talley, M.; Luo, Y.Q. Biomass, litter, and soil respiration along a precipitation gradient in Southern Great Plains, USA. Ecosystems 2009, 12, 1369–1380. [Google Scholar] [CrossRef]
- Smith, M.D.; Wilcox, K.R.; Power, S.A.; Tissue, D.T.; Knapp, A.K. Assessing community and ecosystem sensitivity to climate change-toward a more comparative approach. J. Veg. Sci. 2017, 28, 235–237. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T. The worldwide leaf economics spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Güsewell, S. N:P ratios in terrestrial plants: Variation and functional significance. New Phytol. 2004, 164, 243–266. [Google Scholar] [CrossRef]
- Song, L.L.; Fan, J.W.; Harris, W.; Wu, S.H.; Zhong, H.P.; Zhou, Y.C.; Wang, N.; Zhu, X.D. Adaptive characteristics of grassland community structure and leaf traits along an altitudinal gradient on a subtropical mountain in Chongqing, China. Plant Ecol. 2012, 213, 89–101. [Google Scholar] [CrossRef]
- Heisler, J.L.; Weltzin, J.F. Variability matters: Towards a perspective on the influence of precipitation on terrestrial ecosystems. Effects of precipitation change on ecosystems (EPRECOT)—A Terrestrial Ecosystem Response to Atmospheric and Climatic Change (TERACC) and European Commission sponsored precipitation workshop, Elsinore, Denmark, May 2006. New Phytol. 2006, 172, 189–192. [Google Scholar] [CrossRef] [PubMed]
- Ordoñez, J.C.; Van Bodegom, P.M.; Witte, J.M.; Wright, I.J.; Reich, P.B.; Aerts, R. A global study of relationships between leaf traits, climate and soil measures of nutrient fertility. Glob. Ecol. Biogeogr. 2009, 18, 137–149. [Google Scholar] [CrossRef]
- Xu, H.; Li, Y. Water-use strategy of three central Asian desert shrubs and their responses to rain pulse events. Plant Soil 2006, 285, 5–17. [Google Scholar] [CrossRef]
- Knapp, A.K.; Fay, P.A.; Blair, J.M.; Collins, S.L.; Smith, M.D.; Carlisle, J.D.; Harper, C.W.; Danner, B.T.; Lett, M.S.; McCarron, J.K. Rainfall variability, carbon cycling, and plant species diversity in a mesic grassland. Science 2002, 298, 2202–2205. [Google Scholar] [CrossRef] [PubMed]
- Misson, L.; Gershenson, A.; Tang, J.; Mckay, M.; Cheng, W.; Goldstein, A. Influences of canopy photosynthesis and summer rain pulses on root dynamics and soil respiration in a young ponderosa pine forest. Tree Physiol. 2006, 26, 833–844. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yu, T.F.; Li, X.Y.; Zhao, C.Y. Sap flow characteristics and their response to environmental variables in a desert riparian forest along lower Heihe River Basin, Northwest China. Environ. Monit. Assess. 2016, 188, 561. [Google Scholar] [CrossRef] [PubMed]
- Giladi, L.; Ziv, Y.; May, F.; Jeltsch, F. Scale-dependent determinants of plant species richness in a semi-arid fragmented agro-ecosystem. J. Veg. Sci. 2011, 22, 983–996. [Google Scholar] [CrossRef]
- Fry, E.L.; Manning, P.; Power, S.A. Ecosystem functions are resistant toextreme changes to rainfall regimes in a mesotrophic grassland. Plant Soil 2014, 381, 351–365. [Google Scholar] [CrossRef]
- Han, W.X.; Fang, J.Y.; Guo, D.L.; Zhang, Y. Leaf nitrogen and phosphorus stoichiometry across 753 terrestrial plant species in China. New Phytol. 2005, 168, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Elser, J.J.; Fagan, W.F.; Denno, R.F.; Dobberfuhl, D.R.; Folarin, A.; Huberty, A.; Interlandi, S.; Kilham, S.S.; McCauley, E.; Schulz, K.L.; et al. Nutritional constraints in terrestrial and freshwater food webs. Nature 2000, 408, 578–580. [Google Scholar] [CrossRef] [PubMed]
- Elser, J.J.; Fagan, W.F.; Kerkhoff, A.J.; Swenson, N.G.; Enquist, B.J. Biological stoichiometry of plant production: Metabolism, scaling and ecological response to global change. New Phytol. 2010, 186, 593–608. [Google Scholar] [CrossRef] [PubMed]
- Elser, J.J.; Sterner, R.W.; Gorokhova, E.; Fagan, W.F.; Markow, T.A.; Cotner, J.B.; Harrison, J.F.; Hobbie, S.E.; Odell, G.M.; Weider, L.J. Biological stoichiometry from genes to ecosystems. Ecol. Lett. 2000, 3, 540–550. [Google Scholar] [CrossRef]
- Sterner, R.W.; Elser, J.J. Ecological Stoichiometry: The Biology of Elements from Molecules to the Biosphere; Princeton University Press: Princeton, NJ, USA, 2002. [Google Scholar]
- Reich, P.B.; Oleksyn, J. Global patterns of plant leaf N and P in relation to temperature and latitude. Proc. Natl. Acad. Sci. USA 2004, 101, 11001–11006. [Google Scholar] [CrossRef] [PubMed]
- Han, W.X.; Fang, J.Y.; Reich, P.B.; Woodward, F.I.; Wang, Z.H. Biogeography and variability of eleven mineral elements in plant leaves across gradients of climate, soil and plant functional type in China. Ecol. Lett. 2011, 14, 788–796. [Google Scholar] [CrossRef] [PubMed]
- Posada, J.M.; Schuur, E.A.G. Relationships among precipitation regime, nutrient availability, and carbon turnover in tropical rain forests. Oecologia 2011, 165, 783–795. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; He, N.P.; Wang, Q.F.; Zhang, X.Y.; Wang, R.L.; Xu, Z.W.; Yu, G.R. The altitudinal patterns of leaf C:N:P stoichiometry are regulated by plant growth form, climate and soil on Changbai Mountain, China. PLoS ONE 2014, 9, e95196. [Google Scholar] [CrossRef]
- Zhu, Y.H.; Chen, Y.N.; Ren, L.L.; Lü, H.S.; Zhao, W.Z.; Yuan, F.; Xu, M. Ecosystem restoration and conservation in the arid inland river basins of Northwest China: Problems and strategies. Ecol. Eng. 2016, 94, 629–637. [Google Scholar] [CrossRef]
- Cheng, G.D. Study on the Integrated Management of the Water-Ecology-Economy System of Heihe River Basin; Science Press: Beijing, China, 2009. [Google Scholar]
- Zhu, J.T.; Yu, J.J.; Wang, P.; Zhang, Y.C.; Yu, Q. Interpreting the groundwater attributes influencing the distribution patterns of groundwater-dependent vegetation in northwestern China. Ecohydrology 2012, 5, 628–636. [Google Scholar] [CrossRef]
- Ding, J.Y.; Zhao, W.W.; Daryanto, S.; Wang, L.X.; Fan, H.; Feng, Q.; Wang, Y.P. The spatial distribution and temporal variation of desert riparian forests and their influencing factors in the downstream Heihe River basin, China. Hydrol. Earth Syst. Sci. 2017, 21, 2405–2419. [Google Scholar] [CrossRef]
- Zheng, Y.R.; Xie, Z.X.; Jiang, L.H.; Shimizu, H.; Rimmington, G.M.; Zhou, G.S. Vegetation responses along environmental gradients on the Ordos plateau, China. Ecol. Res. 2006, 21, 396–404. [Google Scholar] [CrossRef]
- Weltzin, J.F.; Loik, M.E.; Schwinning, S.; Williams, D.G.; Fay, P.A.; Haddad, B.M.; Harte, J.; Huxman, T.E.; Knapp, A.K.; Lin, G.H.; et al. Assessing the response of terrestrial ecosystems to potential changes in precipitation. Bioscience 2003, 53, 941–952. [Google Scholar] [CrossRef]
- Zheng, S.X.; Shuangguan, Z.P. Spatial patterns of leaf nutrient traits of the plants in the Loess Plateau of China. Trees 2007, 21, 357–370. [Google Scholar] [CrossRef]
- Wu, T.G.; Dong, Y.; Yu, M.K.; Wang, G.G.; Zeng, D.H. Leaf nitrogen and phosphorus stoichiometry of quercus species across China. For. Ecol. Manag. 2012, 284, 116–123. [Google Scholar] [CrossRef]
- Wang, Y.H.; Yang, H.B.; Yang, D.W.; Qin, Y.; Gao, B.; Cong, Z.T. Spatial interpolation of daily precipitation in a high mountainous watershed based on gauge observations and a regional climate model simulation. J. Hydrometeorol. 2017, 18, 845–862. [Google Scholar] [CrossRef]
- Zhang, X.L.; Zhou, J.H.; Zheng, Y.R. 1:100,000 Vegetation Map of Heihe River Basin (Version 2.0); Cold and Arid Regions Science Data Cente: Lanzhou, China, 2016. [Google Scholar]
- Jiang, L.H.; Wang, J.Z.; Zheng, Y.R. Foliage projective cover, effective tool for describing structure of plant communities. Acta Bot. Yunnanica 2004, 26, 166–172. [Google Scholar] [CrossRef]
- Zhu, Y.H.; Ren, L.L.; Skaggs, T.H.; Lü, H.S.; Yu, Z.B.; Wu, Y.Q.; Fang, X.Q. Simulation of Populus euphratica root uptake of groundwater in an arid woodland of the Ejina Basin, China. Hydrol. Process. 2009, 23, 2460–2469. [Google Scholar] [CrossRef]
- Zuo, X.A.; Wang, S.K.; Lv, P.; Zhou, X.; Zhao, X.Y.; Zhang, T.H.; Zhang, J. Plant functional diversity enhances associations of soil fungal diversity with vegetation and soil in the restoration of semiarid sandy grassland. Ecol. Evol. 2016, 6, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Köppler, M.R.; Kowarik, I.; Kühn, N.; Lippe, M.V. Enhancing wasteland vegetation by adding ornamentals: Opportunities and constraints for establishing steppe and prairie species on urban demolition sites. Landsc. Urban Plan. 2014, 126, 1–9. [Google Scholar] [CrossRef]
- ISSCAS (Institute of Soil Sciences, Chinese Academy of Sciences). Physical and Chemical Analysis Methods of Soils; Shanghai Science Technology Press: Shanghai, China, 1978. [Google Scholar]
- Seymour, C.L.; Milewski, A.V.; Mills, A.J.; Joseph, G.S.; Cumming, G.S.; Cumming, D.H.M.; Mahlangu, Z. Do the large termite mounds of macrotermes, concentrate micronutrients in addition to macronutrients in nutrient-poor African savannas? Soil Biol. Biochem. 2014, 68, 95–105. [Google Scholar] [CrossRef]
- Zhang, J.T.; Dong, Y. Factors affecting species diversity of plant communities and the restoration process in the Loess area of China. Ecol. Eng. 2010, 36, 345–350. [Google Scholar] [CrossRef]
- Ter Braak, C.J.F.; Smilauer, P. Canoco Reference Manual and User’s Guide: Software for Ordination, Version 5.0; Microcomputer Power, Ithaca: Wageningen, The Netherlands, 2012. [Google Scholar]
- Heikkinen, R.K.; Luoto, M.; Kuussaari, M.; Pöyry, J. New insights into Butterfly-environment relationships using partitioning methods. Proc. Biol. Sci. 2005, 272, 2203–2210. [Google Scholar] [CrossRef] [PubMed]
- Kardol, P.; Campany, C.E.; Souza, L.; Norby, R.J.; Weltzin, J.F.; Classen, A.T. Climate change effects on plant biomass alter dominance patterns and community evenness in an experimental old-field ecosystem. Glob. Chang. Biol. 2010, 16, 2676–2687. [Google Scholar] [CrossRef]
- Fu, A.H.; Chen, Y.N.; Li, W.H. Water use strategies of the desert riparian forest plant community in the lower reaches of Heihe River Basin, China. Sci. China Earth Sci. 2014, 57, 1293–1305. [Google Scholar] [CrossRef]
- Wu, G.L.; Ren, G.H.; Wang, D.; Shi, J.H.; Warrington, D. Above- and below-ground response to soil water change in an alpine wetland ecosystem on the Qinghai-Tibetan Plateau, China. J. Hydrol. 2013, 476, 120–127. [Google Scholar] [CrossRef]
- Ferreira, J.N.; Bustamante, M.; Garcia-Montiel, D.C.; Caylor, K.K.; Davidson, E.A. Spatial variation in vegetation structure coupled to plant available water determined by two-dimensional soil resistivity profiling in a Brazilian savanna. Oecologia 2007, 153, 417–430. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.C.; Zhang, J.N. Anatomical Structure and Analysis on leaves of Kalidium culpidatum (Umg-sternb) Grub. J. Agric. Sci. 2004, 25, 43–45. [Google Scholar] [CrossRef]
- Wu, G.L.; Zhang, Z.N.; Wang, D.; Shi, J.H.; Zhu, Y.J. Interactions of soil water content heterogeneity and species diversity patterns in semi-arid steppes on the Loess Plateau of China. J. Hydrol. 2014, 519, 1362–1367. [Google Scholar] [CrossRef]
- Bezemer, T.M.; Lawson, C.S.; Hedlund, K.; Edwards, A.R.; Brook, A.J.; Lgual, J.M.; Mortimer, S.R.; Van der Putten, W.H. Plant species and functional group effects on abiotic and microbial soil properties and plant-soil feedback responses in two grasslands. J. Ecol. 2006, 4, 893–904. [Google Scholar] [CrossRef]
- Wang, L.L.; Zhao, G.X.; Li, M.; Zhang, M.T.; Zhang, L.F.; Zhang, X.F.; An, L.Z.; Xu, S.J. C:N:P stoichiometry and leaf traits of halophytes in an arid saline environment, Northwest China. PLoS ONE 2015, 10, e0119935. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.L.; Mao, W.; Zhao, X.Y.; Zhang, T.H. Leaf nitrogen and phosphorus stoichiometry in typical desert and desertified regions, North China. Environ. Sci. 2010, 31, 1716–1725. [Google Scholar] [CrossRef]
- McCree, K. Whole-plant carbon balance during osmotic adjustment to drought and salinity stress. Funct. Plant Biol. 1986, 13, 33–43. [Google Scholar] [CrossRef]
- Killingbeck, K.T.; Whitford, W.G. High foliar nitrogen in desert shrubs: An important ecosystem trait or defective desert doctrine. Ecology 1996, 77, 1728–1737. [Google Scholar] [CrossRef]
- Wang, T.; Yang, Y.H.; Ma, W.H. Storage, patterns and environmental controls of soil phosphorus in China. Acta Sci. Nat. Univ. Pekin. 2008, 44, 945–952. [Google Scholar] [CrossRef]
- Koerselman, W.; Meuleman, A.F.M. The vegetation N:P ratio: A new tool to detect the nature of nutrient limitation. J. Appl. Ecol. 1996, 33, 1441–1450. [Google Scholar] [CrossRef]
- Vitousek, P.M.; Howarth, R.W. Nitrogen limitation on land and in the sea: How can it occur? Biogeochemistry 1991, 13, 87–115. [Google Scholar] [CrossRef]
- He, J.S.; Wang, L.; Flynn, D.F.B.; Wang, X.; Ma, W.; Fang, J.Y. Leaf nitrogen: Phosphorus stoichiometry across Chinese grassland biomes. Oecologia 2008, 155, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.Z.; Zhuang, H.L.; Wu, L.L.; Liu, Q.L.; Shen, G.G.; Berg, B.; Man, R.Z.; Liu, C.J. Variation in leaf nitrogen and phosphorus stoichiometry in Picea abies across Europe: An analysis based on local observations. For. Ecol. Manag. 2011, 261, 195–202. [Google Scholar] [CrossRef]
- Kerkhoff, A.J.; Enquist, B.J.; Elser, J.J.; Fagan, W.F. Plant allometry, stoichiometry and the temperature-dependence of primary productivity. Glob. Ecol. Biogeogr. 2005, 14, 585–598. [Google Scholar] [CrossRef]
- Rivas-Ubach, A.; Sardans, J.; Péreztrujillo, M.; Estiarte, M.; Peñuelas, J. Strong relationship between elemental stoichiometry and metabolome in plants. Proc. Natl. Acad. Sci. USA 2012, 109, 4181–4186. [Google Scholar] [CrossRef] [PubMed]
- Stirzaker, R.J.; Passioura, J.B.; Wilms, Y. Soil structure and plant growth: Impact of bulk density and biopores. Plant Soil 1996, 185, 151–162. [Google Scholar] [CrossRef]
- Ravi, S.; Breshears, D.D.; Huxman, T.E.; D’Odorico, P. Land degradation in drylands: Interactions among hydrologic-aeolian erosion and vegetation dynamics. Geomorphology 2010, 116, 236–245. [Google Scholar] [CrossRef]
- Thomey, M.L.; Collins, S.L.; Vargas, R.; Johnson, J.E.; Brown, R.F.; Natvig, D.O.; Friggens, M.T. Effect of precipitation varia bility on net primary production and soil respiration in a Chihuahuan Desert grassland. Glob. Chang. Biol. 2011, 17, 1505–1515. [Google Scholar] [CrossRef]
- Burke, I.C.; Lauenroth, W.K.; Parton, W.J. Regional and temporal variation in net primary production and nitrogen mineralization in grasslands. Ecology 1997, 78, 1330–1340. [Google Scholar] [CrossRef]
- Zhou, G.; Wang, Y.; Wang, S. Responses of grassland ecosystems to precipitation and land use along the Northeast China Transect. J. Veg. Sci. 2002, 13, 361–368. [Google Scholar] [CrossRef]
- Jones, M.L.; Sowerby, M.A.; Williams, D.L.; Jones, R.E. Factors controlling soil development in sand dunes: Evidence from a coastal dune soil chronosequence. Plant Soil 2008, 307, 219–234. [Google Scholar] [CrossRef] [Green Version]

| Site | Locations | Annual Rainfall (mm) | Altitude (m) | Dominant Species | Species Richness | Aboveground Biomass (g/m2) | Coverage (%) | Height (cm) | Foliage Projected Cover (%) | Leaf Area Index |
|---|---|---|---|---|---|---|---|---|---|---|
| S1 | 42°16.13′ N 101°22.46′ E | 35 | 920 | Haloxylon ammodendron | 1 | 101.8 ± 56.6 | 5.7 ± 0.5 | 152 ± 48.7 | 7.2 ± 3.5 | 0.8 ± 0.1 |
| S2 | 42°30.87′ N 101°15.07′ E | 39 | 982 | Nitraria sibirica Pall. | 1 | 60.3 ± 31.4 | 7.3 ± 2.0 | 25.4 ± 3.7 | 8.9 ± 2.5 | 0.1 ± 0.0 |
| S3 | 40°16.90′ N 98°41.03′ E | 69 | 1227 | Nitraria praevisa Bobr. | 2 | 72.7 ± 23.7 | 16.0 ± 6.0 | 26.6 ± 1.1 | 15.6 ± 2.5 | 0.2 ± 0.0 |
| S4 | 39°56.53′ N 98°59.91′ E | 82 | 1326 | Artemisia desertorum | 3 | 77.6 ± 23.5 | 17.6 ± 5.0 | 62.1 ± 14.1 | 12.2 ± 3.5 | 0.4 ± 0.1 |
| S5 | 39°56.53′ N 100°46.17′ E | 149 | 1655 | Kalidium gracile | 3 | 230.0 ± 126.6 | 19.1 ± 0.1 | 17.5 ± 1.5 | 27.8 ± 2.5 | 0.6 ± 0.1 |
| S6 | 38°49.09′ N 100°59.24′ E | 162 | 1714 | Salsola passerina | 5 | 316.0 ± 69.0 | 22.2 ± 3.3 | 27.9 ± 4.3 | 24.4 ± 2.5 | 0.8 ± 0.1 |
| S7 | 38°37.55′ N 101°5.25′ E | 209 | 2016 | Kalidium cuspidatum | 3 | 234.8 ± 45.1 | 27.3 ± 5.5 | 14.2 ± 0.3 | 37.8 ± 5.1 | 0.9 ± 0.1 |
| Site | C (mg/g) | N (mg/g) | P (mg/g) | K (mg/g) | C/N | C/P | N/P |
|---|---|---|---|---|---|---|---|
| S1 | 334.63 ± 11.39 | 21.06 ± 0.74 | 1.15 ± 0.01 | 20.29 ± 0.23 | 15.89 ± 0.03 | 291.03 ± 11.05 | 18.32 ± 0.73 |
| S2 | 193.69 ± 3.34 | 13.49 ± 0.21 | 1.34 ± 0.01 | 15.68 ± 0.09 | 14.35 ± 0.12 | 144.63 ± 2.52 | 10.08 ± 0.18 |
| S3 | 351.28 ± 28.75 | 26.74 ± 1.44 | 2.55 ± 0.11 | 18.43 ± 1.14 | 13.12 ± 0.19 | 138.18 ± 13.06 | 10.52 ± 0.71 |
| S4 | 467.27 ± 2.02 | 22.83 ± 0.45 | 2.84 ± 0.04 | 29.04 ± 0.43 | 20.48 ± 0.19 | 164.78 ± 3.00 | 8.05 ± 0.09 |
| S5 | 176.45 ± 2.43 | 12.86 ± 0.28 | 1.63 ± 0.03 | 12.82 ± 0.48 | 13.72 ± 0.11 | 108.19 ± 3.51 | 7.89 ± 0.32 |
| S6 | 238.86 ± 3.67 | 16.46 ± 0.45 | 1.83 ± 0.08 | 18.16 ± 0.92 | 14.51 ± 0.17 | 130.40 ± 7.49 | 8.99 ± 0.62 |
| S7 | 346.35 ± 27.43 | 18.19 ± 1.61 | 0.82 ± 0.05 | 8.70 ± 0.52 | 19.05 ± 0.24 | 420.55 ± 9.83 | 22.08 ± 0.80 |
| Mean | 301.22 ± 99.05 | 18.81 ± 4.86 | 1.74 ± 0.70 | 17.59 ± 6.08 | 15.88 ± 2.68 | 199.68 ± 108.61 | 12.27 ± 5.34 |
| Sites | GSWC10 (%) | GSWC30 (%) | GSWC50 (%) | SBD (g/cm−3) | TN (mg/g) | TC (mg/g) | C/N | AP (mg/kg) | AK (mg/kg) | pH | EC (ms/cm) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| S1 | 1.10 ± 0.56 | 6.02 ± 2.22 | 11.38 ± 0.59 | 1.36 ± 0.09 | 0.45 ± 0.05 | 12.98 ± 4.65 | 29.22 ± 11.06 | 3.82 ± 1.23 | 312.36 ± 74.79 | 8.38 ± 0.22 | 6.51 ± 1.47 |
| S2 | 0.22 ± 0.09 | 0.84 ± 0.18 | 1.53 ± 0.48 | 1.72 ± 0.09 | 0.57 ± 0.04 | 4.05 ± 0.99 | 7.18 ± 2.18 | 2.91 ± 0.63 | 118.28 ± 34.89 | 8.89 ± 0.36 | 0.46 ± 0.15 |
| S3 | 1.41 ± 0.85 | 4.23 ± 2.74 | 5.57 ± 4.06 | 1.27 ± 0.19 | 0.88 ± 0.45 | 16.10 ± 1.62 | 21.91 ± 11.32 | 10.16 ± 4.23 | 237.94 ± 106.37 | 8.52 ± 0.12 | 6.85 ± 2.14 |
| S4 | 0.42 ± 0.26 | 1.60 ± 0.57 | 2.47 ± 0.29 | 1.58 ± 0.04 | 0.39 ± 0.02 | 12.39 ± 1.08 | 31.69 ± 2.93 | 2.37 ± 0.37 | 183.90 ± 17.84 | 8.89 ± 0.76 | 1.72 ± 0.96 |
| S5 | 0.89 ± 0.34 | 4.22 ± 0.82 | 5.02 ± 0.40 | 1.25 ± 0.04 | 0.83 ± 0.12 | 17.69 ± 0.74 | 21.64 ± 2.34 | 5.00 ± 1.52 | 136.08 ± 47.98 | 8.71 ± 0.04 | 2.14 ± 0.24 |
| S6 | 2.24 ± 0.82 | 2.86 ± 0.73 | 3.79 ± 0.39 | 1.49 ± 0.01 | 0.82 ± 0.08 | 21.99 ± 1.43 | 26.73 ± 0.96 | 3.60 ± 0.60 | 68.40 ± 15.29 | 8.70 ± 0.16 | 1.26 ± 0.21 |
| S7 | 3.33 ± 0.60 | 8.69 ± 1.22 | 10.42 ± 0.96 | 1.54 ± 0.11 | 0.99 ± 0.77 | 23.31 ± 1.18 | 23.62 ± 0.55 | 3.16 ± 0.44 | 175.05 ± 19.84 | 8.59 ± 0.07 | 3.41 ± 0.06 |
| Marginal Effects | Conditional Effects | p Value | F Value | ||
|---|---|---|---|---|---|
| Environmental Variables | Eigenvalues | Environmental Variables | Eigenvalues | ||
| Precipitation | 60.5 | Precipitation | 60.5 | 0.001 | 29.1 |
| Soil total carbon | 42.5 | Soil C/N | 12.8 | 0.001 | 8.6 |
| Soil total nitrogen | 33.2 | GSWC50 | 5.1 | 0.002 | 4.5 |
| GSWC10 | 25.1 | Soil total nitrogen | 3.9 | 0.004 | 5.7 |
| Soil available K | 18.8 | Soil available P | 3.8 | 0.034 | 2.8 |
| Soil C/N | 11.7 | SEC | 2.2 | 0.082 | 2.2 |
| GSWC30 | 9.3 | Soil bulk density | 1.7 | 0.190 | 1.6 |
| SEC | 6.3 | Soil total carbon | 1.5 | 0.193 | 1.6 |
| GSWC50 | 4.3 | GSWC10 | 1.2 | 0.115 | 2.0 |
| Soil bulk density | 3.4 | GSWC30 | 1.1 | 0.395 | 1.0 |
| Soil available P | 1.8 | Soil available K | 0.7 | 0.391 | 1.0 |
| Soil pH | 1.6 | Soil pH | 0.6 | 0.460 | 0.9 |
| Marginal Effects | Conditional Effects | p Value | F Value | ||
|---|---|---|---|---|---|
| Environmental Variables | Eigenvalues | Environmental Variables | Eigenvalues | ||
| GSWC50 | 34.6 | GSWC50 | 34.6 | 0.001 | 10.1 |
| GSWC30 | 31.6 | Soil C/N | 11.7 | 0.002 | 7.4 |
| GSWC10 | 21.9 | Soil bulk density | 16.0 | 0.003 | 5.8 |
| Soil available K | 14.7 | GSWC30 | 5.9 | 0.007 | 4.5 |
| SEC | 12.9 | SEC | 12.4 | 0.010 | 5.7 |
| Precipitation | 10.1 | Soil available K | 3.6 | 0.038 | 3.5 |
| Soil C/N | 9.8 | Soil total carbon | 2.6 | 0.064 | 3.0 |
| Soil total nitrogen | 7.4 | Precipitation | 2.4 | 0.137 | 2.0 |
| Soil total carbon | 6.1 | Soil available P | 1.2 | 0.320 | 1.2 |
| Soil available P | 5.7 | GSWC10 | 1.0 | 0.376 | 1.0 |
| Soil pH | 5.3 | Soil total nitrogen | 0.9 | 0.449 | 0.8 |
| Soil bulk density | 2.3 | Soil pH | 0.4 | 0.658 | 0.5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Guan, T.; Zhou, J.; Cai, W.; Gao, N.; Du, H.; Jiang, L.; Lai, L.; Zheng, Y. Community Characteristics and Leaf Stoichiometric Traits of Desert Ecosystems Regulated by Precipitation and Soil in an Arid Area of China. Int. J. Environ. Res. Public Health 2018, 15, 109. https://doi.org/10.3390/ijerph15010109
Zhang X, Guan T, Zhou J, Cai W, Gao N, Du H, Jiang L, Lai L, Zheng Y. Community Characteristics and Leaf Stoichiometric Traits of Desert Ecosystems Regulated by Precipitation and Soil in an Arid Area of China. International Journal of Environmental Research and Public Health. 2018; 15(1):109. https://doi.org/10.3390/ijerph15010109
Chicago/Turabian StyleZhang, Xiaolong, Tianyu Guan, Jihua Zhou, Wentao Cai, Nannan Gao, Hui Du, Lianhe Jiang, Liming Lai, and Yuanrun Zheng. 2018. "Community Characteristics and Leaf Stoichiometric Traits of Desert Ecosystems Regulated by Precipitation and Soil in an Arid Area of China" International Journal of Environmental Research and Public Health 15, no. 1: 109. https://doi.org/10.3390/ijerph15010109





