Contact to Nature Benefits Health: Mixed Effectiveness of Different Mechanisms
Abstract
:1. Introduction
1.1. Health and Stress
1.2. Well-Being Effects of Contact to Nature
- improvement in micro-climatic conditions (e.g., air quality),
- stimulation of physical activity (e.g., exercise),
- facilitation of social cohesion, and,
- restoration from mental fatigue.
1.3. Aims and Hypotheses
- … taking place in contact with nature are more effective than indoor activities.
- … involving physical activity are more effective than passive recreation.
- … involving social interaction are more effective than those executed alone.
- … allowing for restoration from mental fatigue are more effective than those that do not.
2. Methods
2.1. Study Overview
2.2. Participants
2.3. Questionnaire
2.4. Measurements of Hair Cortisol Concentration
2.5. Statistical Analyses
3. Results
3.1. Stressors, Well-Being Status, and Hair Cortisol Levels
3.2. Recreational Activities and Time Spent in Nature
3.3. Correlations
3.4. Regression Model for Hair Cortisol Prediction
4. Discussion
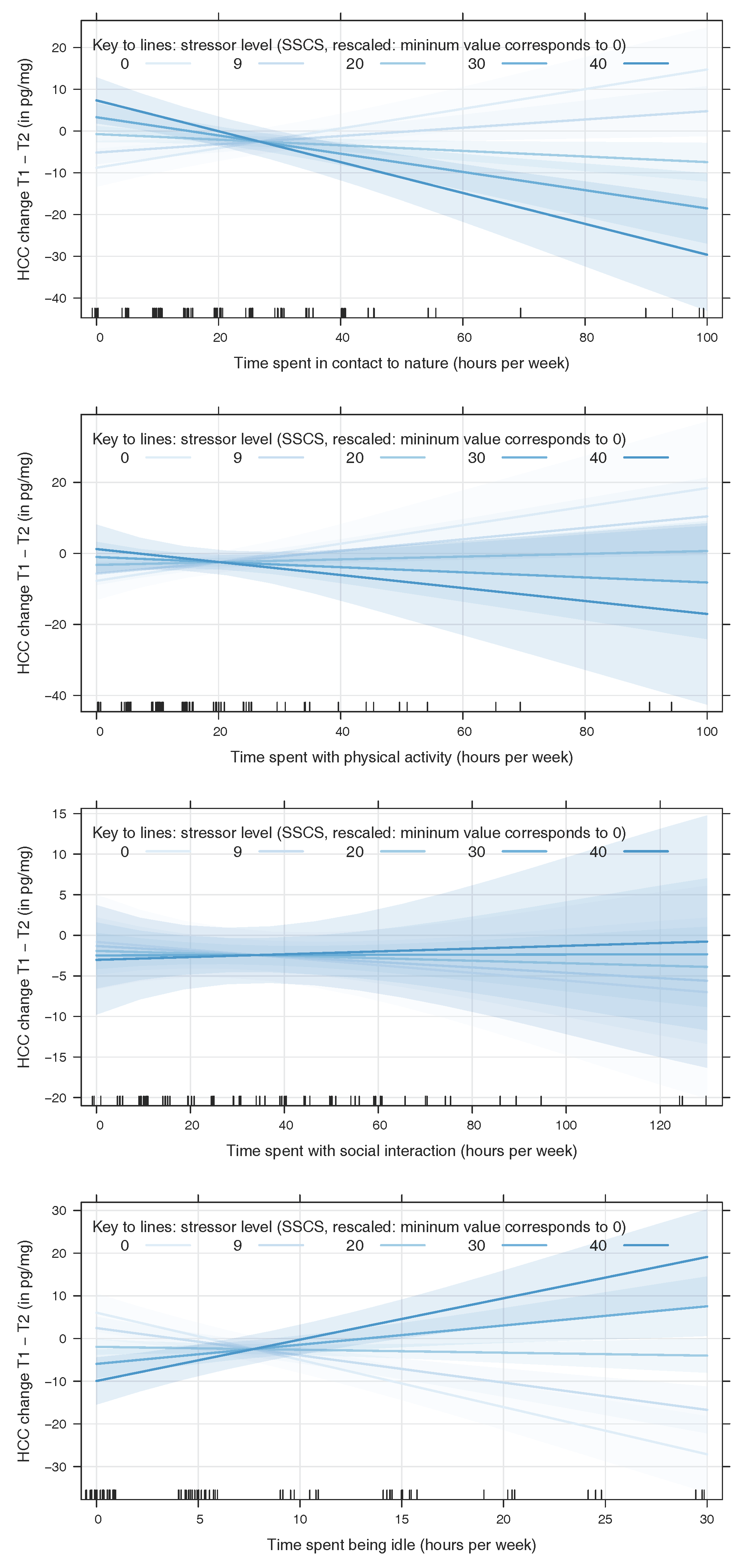
4.1. Contact to Nature and Physical Activity Mitigate Stress While Idleness Intensifies Stress Outcomes
4.2. Inverse Effects of Nature Contact and Idleness at High Levels of Stress
4.3. No Effects of Social Interaction
4.4. No Main Effect of Present Stressors
4.5. Representativeness of the Participants
4.6. Limitations and Consequences for Future Research
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- World Health Organization. Constitution; Basic Documents; World Health Organization: Genève, Switzerland, 1948. [Google Scholar]
- Selye, H. Stress and disease. Laryngoscope 1955, 65, 500–514. [Google Scholar] [CrossRef] [PubMed]
- Toates, F.M. Biological Psychology: An Integrative Approach; Pearson Education: Harlow, UK, 2001. [Google Scholar]
- Staufenbiel, S.M.; Penninx, B.W.J.H.; Spijker, A.T.; Elzinga, B.M.; van Rossum, E.F.C. Hair cortisol, stress exposure, and mental health in humans: A systematic review. Psychoneuroendocrinology 2013, 38, 1220–1235. [Google Scholar] [CrossRef] [PubMed]
- Korte, S.M.; Koolhaas, J.M.; Wingfield, J.C.; McEwen, B.S. The Darwinian concept of stress: Benefits of allostasis and costs of allostatic load and the trade-offs in health and disease. Neurosci. Biobehav. Rev. 2005, 29, 3–38. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S.; Wingfield, J.C. The concept of allostasis in biology and biomedicine. Horm. Behav. 2003, 43, 2–15. [Google Scholar] [CrossRef]
- Cohen, S.; Janicki-Deverts, D.; Miller, G.E. Psychological Stress and Disease. JAMA 2007, 298, 1685. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.E.; Chen, E.; Zhou, E.S. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychol. Bull. 2007, 133, 25–45. [Google Scholar] [CrossRef] [PubMed]
- Bundesamt für Statistik. Schweizerische Gesundheitsbefragung 2012; Technical Report; Bundesamt für Statistik: Neuchâtel, Switzerland, 2014. [Google Scholar]
- Hapke, U.; Maske, U.E.; Scheidt-Nave, C.; Bode, L.; Schlack, R.; Busch, M.A. Chronischer Stress bei Erwachsenen in Deutschland. Bundesgesundheitsblatt 2013, 56, 749–754. [Google Scholar] [CrossRef] [PubMed]
- Sharpley, C.F.; McFarlane, J.R.; Slominski, A. Stress-linked cortisol concentrations in hair: What we know and what we need to know. Rev. Neurosci. 2012, 23, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Izawa, S.; Miki, K.; Tsuchiya, M.; Mitani, T.; Midorikawa, T.; Fuchu, T.; Komatsu, T.; Togo, F. Cortisol level measurements in fingernails as a retrospective index of hormone production. Psychoneuroendocrinology 2015, 54, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Russell, E.; Koren, G.; Rieder, M.; Van Uum, S. Hair cortisol as a biological marker of chronic stress: Current status, future directions and unanswered questions. Psychoneuroendocrinology 2012, 37, 589–601. [Google Scholar] [CrossRef] [PubMed]
- Stalder, T.; Kirschbaum, C. Analysis of cortisol in hair—State of the art and future directions. Brain Behav. Immun. 2012, 26, 1019–1029. [Google Scholar] [CrossRef] [PubMed]
- Karlén, J.; Ludvigsson, J.; Frostell, A.; Theodorsson, E.; Faresjö, T. Cortisol in hair measured in young adults—A biomarker of major life stressors? BMC Clin. Pathol. 2011, 11, 12. [Google Scholar] [CrossRef] [PubMed]
- Gidlow, C.J.; Randall, J.; Gillman, J.; Smith, G.R.; Jones, M.V. Natural environments and chronic stress measured by hair cortisol. Landsc. Urban Plan. 2016, 148, 61–67. [Google Scholar] [CrossRef]
- Honold, J.; Lakes, T.; Beyer, R.; van der Meer, E. Restoration in Urban Spaces: Nature Views From Home, Greenways, and Public Parks. Environ. Behav. 2015, 48, 796–825. [Google Scholar] [CrossRef]
- United Nations. World Urbanization Prospects: The 2014 Revision; United Nations, Department of Economic and Social Affairs, Population Division: New York, NY, USA, 2014. [Google Scholar]
- Honold, J.; Beyer, R.; Lakes, T.; van der Meer, E. Multiple environmental burdens and neighborhood-related health of city residents. J. Environ. Psychol. 2012, 32, 305–317. [Google Scholar] [CrossRef]
- Lederbogen, F.; Kirsch, P.; Haddad, L.; Streit, F.; Tost, H.; Schuch, P.; Wüst, S.; Pruessner, J.C.; Rietschel, M.; Deuschle, M.; et al. City living and urban upbringing affect neural social stress processing in humans. Nature 2011, 474, 498–501. [Google Scholar] [CrossRef] [PubMed]
- Thompson, C.W. Linking landscape and health: The recurring theme. Landsc. Urban Plan. 2011, 99, 187–195. [Google Scholar] [CrossRef]
- Haluza, D.; Schönbauer, R.; Cervinka, R. Green Perspectives for Public Health: A Narrative Review on the Physiological Effects of Experiencing Outdoor Nature. Int. J. Environ. Res. Public Health 2014, 11, 5445–5461. [Google Scholar] [CrossRef] [PubMed]
- Hartig, T.; Mitchell, R.; de Vries, S.; Frumkin, H. Nature and Health. Annu. Rev. Public Health 2014, 35, 207–228. [Google Scholar] [CrossRef] [PubMed]
- Maas, J.; Verheij, R.A.; Groenewegen, P.P.; de Vries, S.; Spreeuwenberg, P. Green space, urbanity, and health: How strong is the relation? J. Epidemiol. Community Health 2006, 60, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Martens, D.; Gutscher, H.; Bauer, N. Walking in “wild” and “tended” urban forests: The impact on psychological well-being. J. Environ. Psychol. 2011, 31, 36–44. [Google Scholar] [CrossRef]
- Bonaiuto, M.; Aiello, A.; Perugini, M.; Bonnes, M.; Ercolani, A.P. Multidimensional perception of residential environment quality and neighbourhood attachment in the urban environment. J. Environ. Psychol. 1999, 19, 331–352. [Google Scholar] [CrossRef]
- Amérigo, M.; Aragonés, J.I. A theoretical and methodological approach to the study of residential satisfaction. J. Environ. Psychol. 1997, 17, 47–57. [Google Scholar] [CrossRef]
- Kaplan, S. Meditation, restoration, and the management of mental fatigue. Environ. Behav. 2001, 33, 480–506. [Google Scholar] [CrossRef]
- Faber Taylor, A.; Kuo, F.E.; Sullivan, W.C. Coping with ADD: The Surprising Connection to Green Play Settings. Environ. Behav. 2001, 33, 54–77. [Google Scholar] [CrossRef]
- Berry, M.S.; Sweeney, M.M.; Morath, J.; Odum, A.L.; Jordan, K.E. The Nature of Impulsivity: Visual Exposure to Natural Environments Decreases Impulsive Decision-Making in a Delay Discounting Task. PLoS ONE 2014, 9, e97915. [Google Scholar] [CrossRef] [PubMed]
- Faber Taylor, A.; Kuo, F.E.; Sullivan, W.C. Views of nature and self-discipline: Evidence from inner city children. J. Environ. Psychol. 2002, 22, 49–63. [Google Scholar] [CrossRef]
- Degenhardt, B.; Buchecker, M. Exploring Everyday Self-Regulation in Nearby Nature: Determinants, Patterns, and a Framework of Nearby Outdoor Recreation Behavior. Leisure Sci. 2012, 34, 450–469. [Google Scholar] [CrossRef]
- Hartig, T.; Evans, G.W.; Jamner, L.D.; Davis, D.S.; Gärling, T. Tracking restoration in natural and urban field settings. J. Environ. Psychol. 2003, 23, 109–123. [Google Scholar] [CrossRef]
- Ulrich, R.S. View through a window may influence recovery from surgery. Science 1984, 224, 420–421. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, R.; Popham, F. Effect of exposure to natural environment on health inequalities: An observational population study. Lancet 2008, 372, 1655–1660. [Google Scholar] [CrossRef]
- De Vries, S. Nearby nature and human health: looking at mechanisms and their implications. In Innovative Approaches to Researching Landscape and Health; Ward Thompson, C., Aspinall, P., Bell, S., Eds.; Routledge: London, UK, 2010; pp. 77–96. [Google Scholar]
- Groenewegen, P.P.; van den Berg, A.E.; Maas, J.; Verheij, R.A.; de Vries, S. Is a Green Residential Environment Better for Health? If So, Why? Ann. Assoc. Am. Geogr. 2012, 102, 996–1003. [Google Scholar] [CrossRef]
- Triguero-Mas, M.; Dadvand, P.; Cirach, M.; Martínez, D.; Medina, A.; Mompart, A.; Basagaña, X.; Grazuleviciene, R.; Nieuwenhuijsen, M.J. Natural outdoor environments and mental and physical health: Relationships and mechanisms. Environ. Int. 2015, 77, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Dadvand, P.; Bartoll, X.; Basagaña, X.; Dalmau-Bueno, A.; Martinez, D.; Ambros, A.; Cirach, M.; Triguero-Mas, M.; Gascon, M.; Borrell, C.; et al. Green spaces and General Health: Roles of mental health status, social support, and physical activity. Environ. Int. 2016, 91, 161–167. [Google Scholar] [CrossRef] [PubMed]
- De Vries, S.; van Dillen, S.M.; Groenewegen, P.P.; Spreeuwenberg, P. Streetscape greenery and health: Stress, social cohesion and physical activity as mediators. Soc. Sci. Med. 2013, 94, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Shanahan, D.F.; Fuller, R.A.; Bush, R.; Lin, B.B.; Gaston, K.J. The Health Benefits of Urban Nature: How Much Do We Need? BioScience 2015, 65, 476–485. [Google Scholar] [CrossRef]
- Bundesamt für Statistik. Die Bevölkerung der Schweiz 2015; Technical Report 348-1500; Bundesamt für Statistik: Neuchâtel, Switzerland, 2016. [Google Scholar]
- Bundesamt für Statistik. Alter, Zivilstand, Staatsangehörigkeit; Technical Report; Bundesamt für Statistik: Neuchâtel, Switzerland, 2016. [Google Scholar]
- Bundesamt für Statistik. Ständige Wohnbevölkerung ab 15 Jahren nach Migrationsstatus und Verschiedenen Soziodemografischen Merkmalen; Technical Report su-d-01.05.03.01.01; Bundesamt für Statistik: Neuchâtel, Switzerland, 2016. [Google Scholar]
- Bundesamt für Statistik. Privathaushalte nach Haushaltstyp, 2013-2015 Kumuliert; Technical Report su-d-40.02.01.02.02-2015; Bundesamt für Statistik: Neuchâtel, Switzerland, 2016. [Google Scholar]
- Bundesamt für Statistik. Bildungsstand 2015; Technical Report; Bundesamt für Statistik: Neuchâtel, Switzerland, 2016. [Google Scholar]
- Bundesamt für Statistik. Erwerbsstatus der Ständigen Wohnbevölkerung ab 15 Jahren; Technical Report gr-d-03.02.00.02.02-je; Bundesamt für Statistik: Neuchâtel, Switzerland, 2016. [Google Scholar]
- Bundesamt für Statistik. Subjektive Einschätzung der Lebensqualität, nach Verschiedenen Soziodemografischen Merkmalen; Technical Report je-d-20.03.04.01.02; Bundesamt für Statistik: Neuchâtel, Switzerland, 2016. [Google Scholar]
- Schulz, P.; Schlotz, W.; Becker, P. TICS: Trierer Inventar zum Chronischen Stress; Hogrefe: Göttingen, Germany, 2004. [Google Scholar]
- Satow, L. Stress- und Coping-Inventar (SCI): Test- und Skalendokumentation. Available online: https://www.drsatow.de/tests/stress-und-coping-inventar/ (accessed on 21 December 2017).
- Ware, J.E.J.; Kosinski, M.; Keller, S.D. A 12-Item Short-Form Health Survey: Construction of Scales and Preliminary Tests of Reliability and Validity. Med. Care 1996, 34, 220–233. [Google Scholar] [CrossRef] [PubMed]
- Morfeld, M.; Kirchberger, I.; Bullinger, M. Fragebogen zum Gesundheitszustand: SF-36, 2nd ed.; Hogrefe: Göttingen, Germany, 2011. [Google Scholar]
- Dalbert, C. Aktuelle Stimmungsskala (ASTS); Universität Tübingen, Abteilung Pädagogische Psychologie: Tübingen, Germany, 1992. [Google Scholar]
- McNair, D.M.; Lorr, M.; Droppleman, L.F. Manual for the Profile of Mood States; Educational and Industrial Testing Service: San Diego, CA, USA, 1971. [Google Scholar]
- Binz, T.M.; Braun, U.; Baumgartner, M.R.; Kraemer, T. Development of an LC–MS/MS method for the determination of endogenous cortisol in hair using 13C3-labeled cortisol as surrogate analyte. J. Chromatogr. B 2016, 1033–1034, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Van Buuren, S. Flexible Imputation of Missing Data; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Belsley, D.A.; Kuh, E.; Welsch, R.E. Regression Diagnostics—Identifying Influential Data and Sources of Collinearity; Wiley: New York, NY, USA, 1980. [Google Scholar]
- Belsley, D.A. A Guide to using the collinearity diagnostics. Comput. Sci. Econ. Manag. 1991, 4, 33–50. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Wien, Austria, 2015. [Google Scholar]
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 2nd ed.; Sage: Thousand Oaks, CA, USA, 2011. [Google Scholar]
- Abell, J.G.; Stalder, T.; Ferrie, J.E.; Shipley, M.J.; Kirschbaum, C.; Kivimäki, M.; Kumari, M. Assessing cortisol from hair samples in a large observational cohort: The Whitehall II study. Psychoneuroendocrinology 2016, 73, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Mezentsef, A.; Jackson, P.; Baig, U.; Fairclough, J.; Brooks, C.; Mitroka, J. Hair Cortisol and Perceived Stress in Health-studies Students during Summer Break and Fall Term. J. Steroids Horm. Sci. 2017, 8. [Google Scholar] [CrossRef]
- Smith, M.N.; Wilder, C.S.; Griffith, W.C.; Workman, T.; Thompson, B.; Dills, R.; Onstad, G.; Vredevoogd, M.; Vigoren, E.M.; Faustman, E.M. Seasonal variation in cortisol biomarkers in Hispanic mothers living in an agricultural region. Biomarkers 2015, 20, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Sirois, F.; Pychyl, T. Procrastination and the Priority of Short-Term Mood Regulation: Consequences for Future Self. Soc. Personal. Psychol. Compass 2013, 7, 115–127. [Google Scholar] [CrossRef]
- DeLongis, A.; Holtzman, S. Coping in Context: The Role of Stress, Social Support, and Personality in Coping. J. Personal. 2005, 73, 1633–1656. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.; Wills, T.A. Stress, social support, and the buffering hypothesis. Psychol. Bull. 1985, 98, 310–357. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S. Social Relationships and Health. Am. Psychol. 2004, 59, 676–684. [Google Scholar] [CrossRef] [PubMed]
- Mancini, A.D.; Littleton, H.L.; Grills, A.E. Can People Benefit From Acute Stress? Social Support, Psychological Improvement, and Resilience after the Virginia Tech Campus Shootings. Clin. Psychol. Sci. 2016, 4, 401–417. [Google Scholar] [CrossRef]
- Uchino, B.N. Social Support and Health: A Review of Physiological Processes Potentially Underlying Links to Disease Outcomes. J. Behav. Med. 2006, 29, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Berg, A.; Palomaki, H.; Lonnqvist, J.; Lehtihalmes, M.; Kaste, M. Depression among Caregivers of Stroke Survivors. Stroke 2005, 36, 639–643. [Google Scholar] [CrossRef] [PubMed]
- Martens, L.; Addington, J. The psychological well-being of family members of individuals with schizophrenia. Soc. Psychiatry Psychiatr. Epidemiol. 2001, 36, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Solomon, P.; Draine, J. Subjective burden among family members of mentally ill adults: Relation to stress, coping, and adaptation. Am. J. Orthopsychiatr. 1995, 65, 419–427. [Google Scholar] [CrossRef]
- Bundesamt für Statistik. Domestic and Family Work; Technical Report; Bundesamt für Statistik: Neuchâtel, Switzerland, 2016. [Google Scholar]
| Parameter | T1 | T2 | MT1 = MT2 | Norm | MT2 = MNorm | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| MD | M | SD | MD | M | SD | tDFp | M | SD | tDFp | |
| Stressors (SSCS) inv. | 54 | 54.2 | 8.1 | 52 | 52.5 | 7.8 | 1.32157.8 | 50 | 10 | 2.979 ** |
| Stress symptoms (from SCI) inv. | 36 | 36.4 | 6.5 | 34 | 35 | 5.6 | 1.43154.9 | 34 | n/a | 1.679 |
| Physical health (SF12) | 52.8 | 49.9 | 8.7 | 54.2 | 52.1 | 6.2 | −1.87143.5 | 49.03 | 9.35 | 4.479 *** |
| Mental health (SF12) | 51.1 | 49.1 | 8.8 | 52 | 49.8 | 7.3 | −0.55152.8 | 52.24 | 8.1 | −2.979 ** |
| Mood (ASTS) inv. | 0 | 1.2 | 15.9 | −4 | −2.5 | 13.2 | 1.61152.7 | n/a | n/a | |
| HCC (pg/mg) inv. | 6.2 | 8.5 | 10.3 | 7.6 | 10.8 | 11.2 | −1.35156.8 | n/a | n/a |
| Activity | T1 | T2 | ||||
|---|---|---|---|---|---|---|
| Time (hours/week) spent … | MD | M | SD | MD | M | SD |
| … just being there, doing nothing (idleness) | 5 | 6.9 | 10 | 5 | 7.8 | 8.6 |
| … with physical activity (combined) | 12.5 | 18.2 | 19 | 15 | 19.7 | 18.6 |
| … with light physical activity | 10 | 13.2 | 15.1 | 10 | 12.4 | 13 |
| … with intense physical activity | 5 | 5 | 9 | 5 | 7.3 | 10.9 |
| … with social interaction (combined) | 20 | 35.8 | 32 | 30 | 37.1 | 29.2 |
| … with friends | 10 | 15.9 | 17 | 10 | 16.6 | 16.7 |
| … with family | 10 | 19.9 | 19.7 | 15 | 20.6 | 19.6 |
| … in natural environments (combined) | 15 | 23.4 | 25.2 | 20 | 26.3 | 22.9 |
| … in the forest | 5 | 3.8 | 4.4 | 5 | 3.9 | 4.5 |
| … in urban nature (e.g., in a garden) | 5 | 9.1 | 10.3 | 5 | 8.8 | 9.2 |
| … in open landscape | 5 | 5 | 7.4 | 5 | 5.9 | 7.4 |
| … at the water | 5 | 5.6 | 8.7 | 5 | 8.4 | 11.4 |
| … outdoors (other environments) | 5 | 12.6 | 15.1 | 10 | 13.2 | 13.2 |
| Idleness | Nature Contact | Social Interaction | Physical Activity | HCCT1 | HCCT2 | |
|---|---|---|---|---|---|---|
| Stressors (SSCS) | −0.03 | 0.03 | −0.09 | −0.05 | 0.06 | 0.03 |
| Idleness | 0.16 | 0.13 | 0.37 *** | −0.04 | 0.15 | |
| Nature contact | 0.19 | 0.36 *** | 0.16 | 0.12 | ||
| Social interaction | 0.44 *** | −0.23 * | −0.1 | |||
| Physical activity | 0.01 | 0.11 | ||||
| HCCT1 | 0.63 *** |
| SE | t | p | ||
|---|---|---|---|---|
| Intercept | −3.5191 | 2.6935 | −1.3065 | 0.1957 |
| Stressors | 0.1565 | 0.1337 | 1.1706 | 0.2457 |
| Nature contact | 0.2354 | 0.0656 | 3.5894 | 0.0006 *** |
| Physical activity | 0.2612 | 0.1154 | 2.2623 | 0.0268 * |
| Social interaction | −0.0476 | 0.0687 | −0.6928 | 0.4907 |
| Idleness | −1.1058 | 0.1974 | −5.6018 | 0.0000 *** |
| Stressors × Nature contact | −0.0151 | 0.0035 | −4.2682 | 0.0001 *** |
| Stressors × Physical activity | −0.0111 | 0.0064 | −1.7389 | 0.0864 |
| Stressors × Social interaction | 0.0016 | 0.0035 | 0.4602 | 0.6468 |
| Stressors × Idleness | 0.0519 | 0.0104 | 4.9776 | 0.0000 *** |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hofmann, M.; Young, C.; Binz, T.M.; Baumgartner, M.R.; Bauer, N. Contact to Nature Benefits Health: Mixed Effectiveness of Different Mechanisms. Int. J. Environ. Res. Public Health 2018, 15, 31. https://doi.org/10.3390/ijerph15010031
Hofmann M, Young C, Binz TM, Baumgartner MR, Bauer N. Contact to Nature Benefits Health: Mixed Effectiveness of Different Mechanisms. International Journal of Environmental Research and Public Health. 2018; 15(1):31. https://doi.org/10.3390/ijerph15010031
Chicago/Turabian StyleHofmann, Mathias, Christopher Young, Tina M. Binz, Markus R. Baumgartner, and Nicole Bauer. 2018. "Contact to Nature Benefits Health: Mixed Effectiveness of Different Mechanisms" International Journal of Environmental Research and Public Health 15, no. 1: 31. https://doi.org/10.3390/ijerph15010031





