Contamination of Tea and Tea Infusion with Polycyclic Aromatic Hydrocarbons
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Reagents
2.3. Apparatus
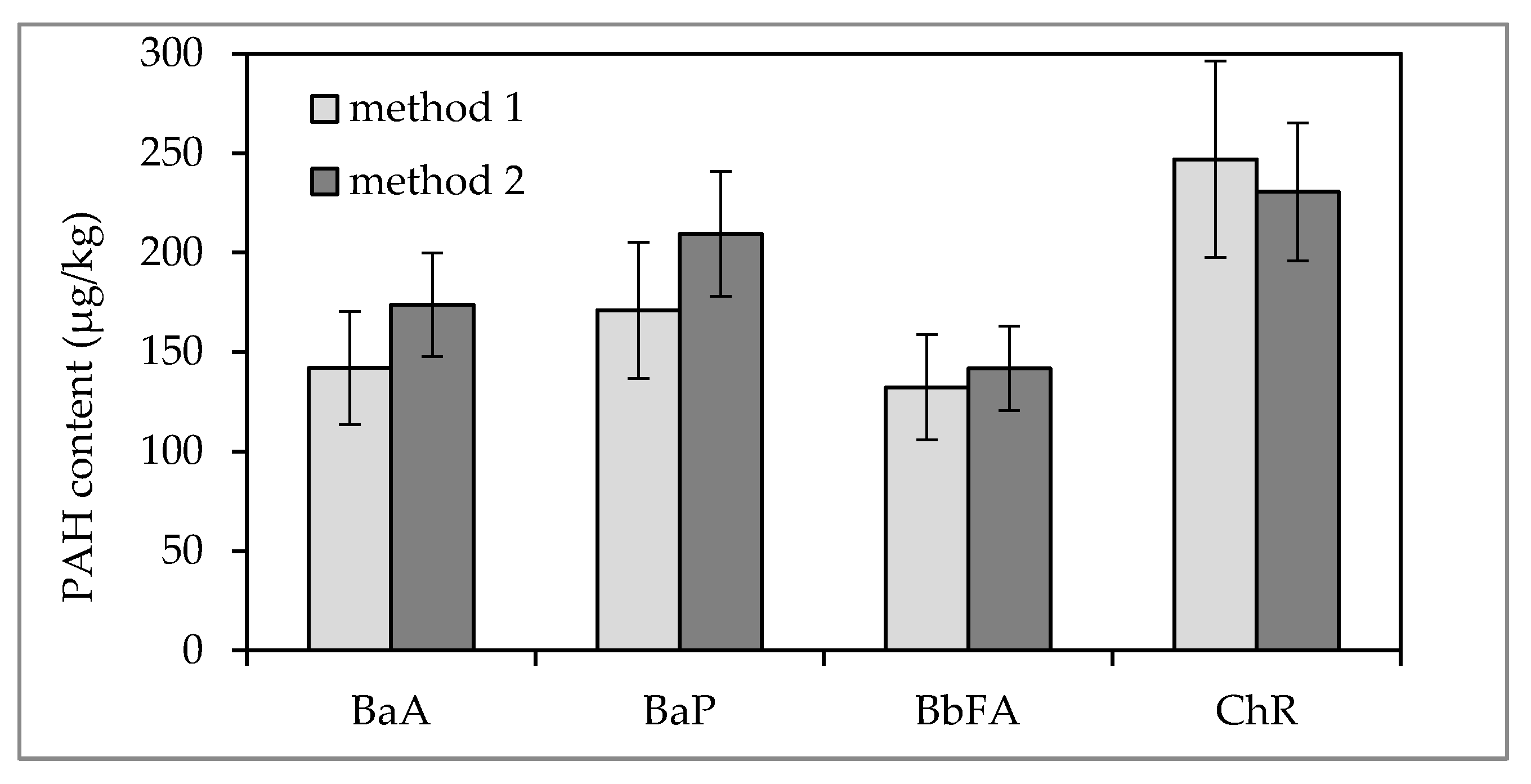
2.4. Methods
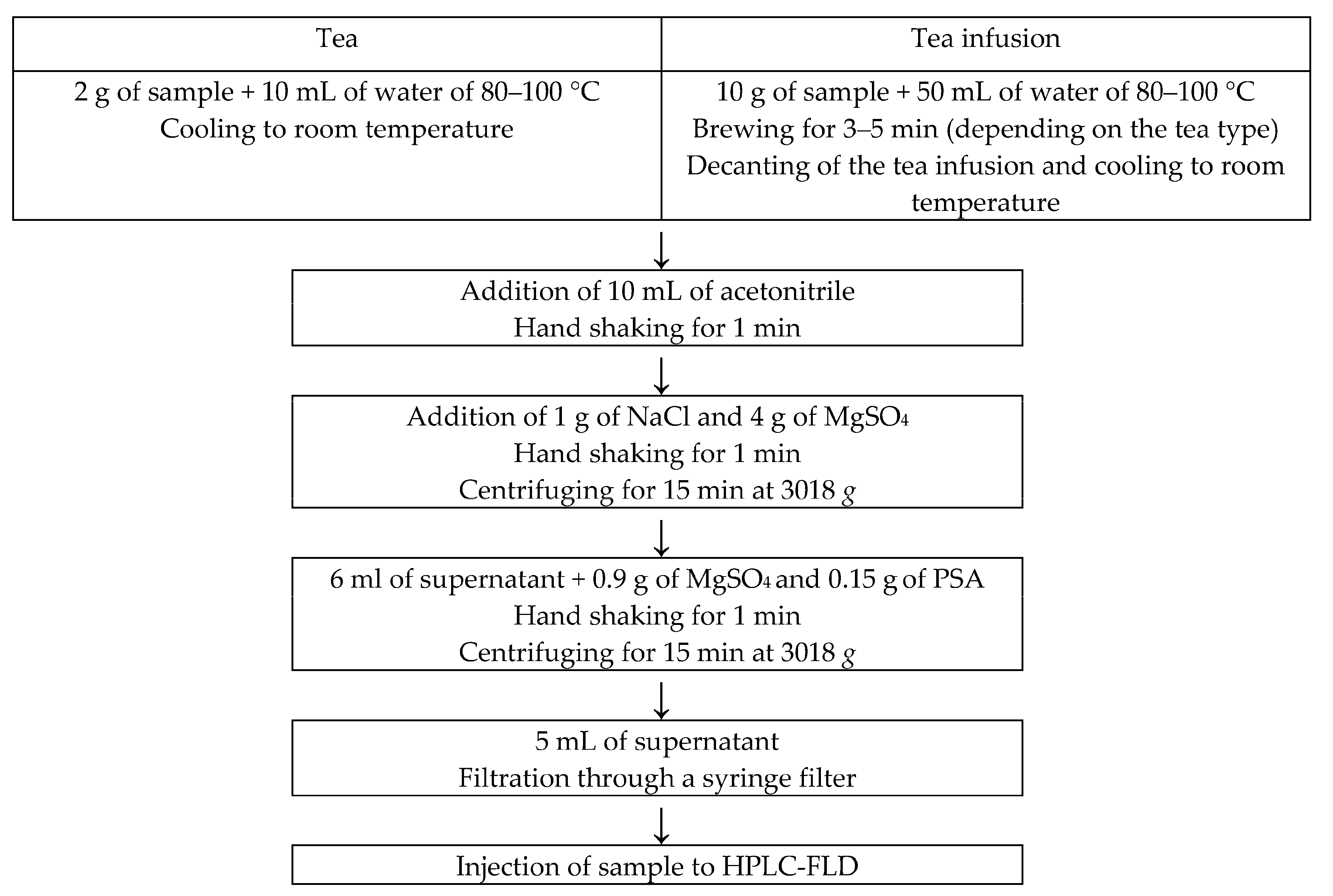
2.4.1. Sample Preparation
2.4.2. Preparing the Tea Infusion
2.4.3. Extraction and Purification of the Hydrocarbon Fraction
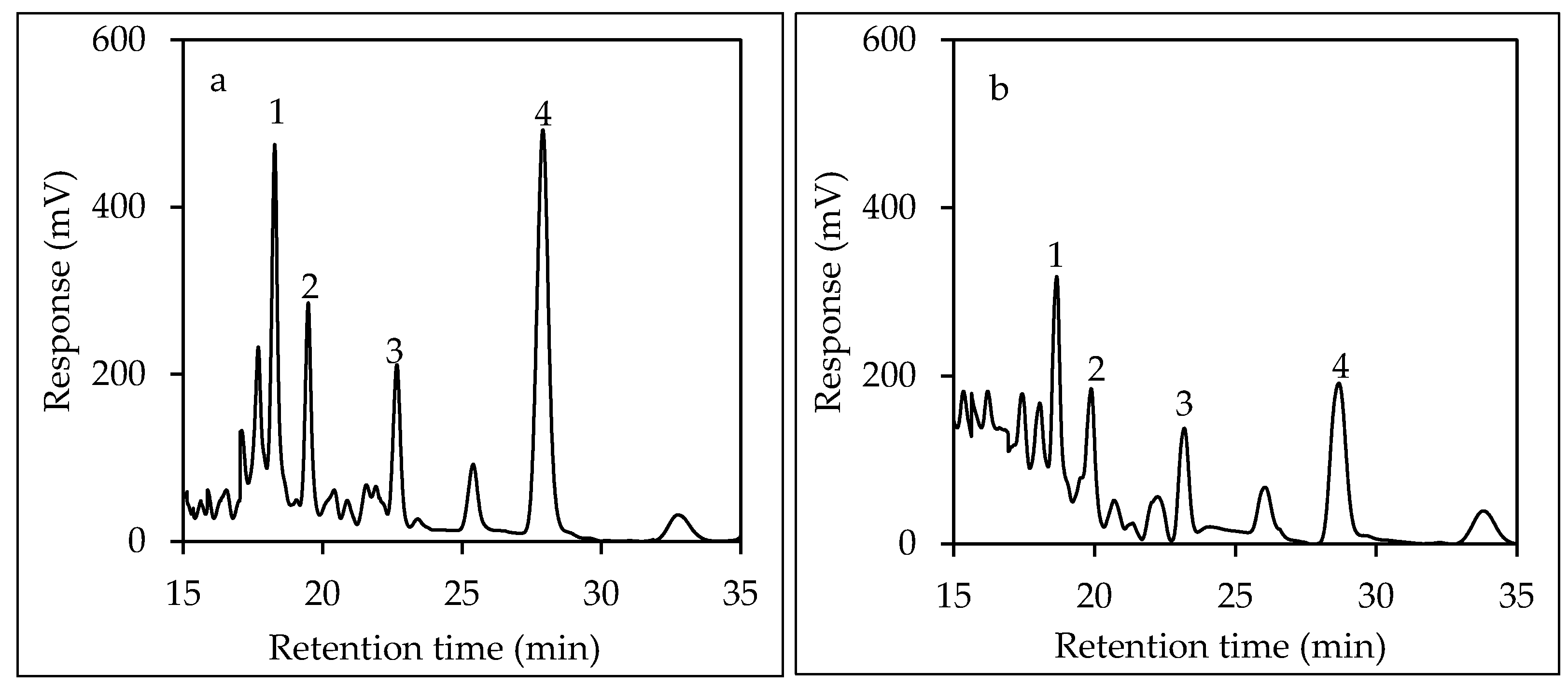
2.4.4. HPLC-FLD Analysis
Preparation of Calibration Curves
Determination of the Working Range and Linearity of the Method
Measurement of Samples
2.5. Statistical Analysis
3. Results and Discussion
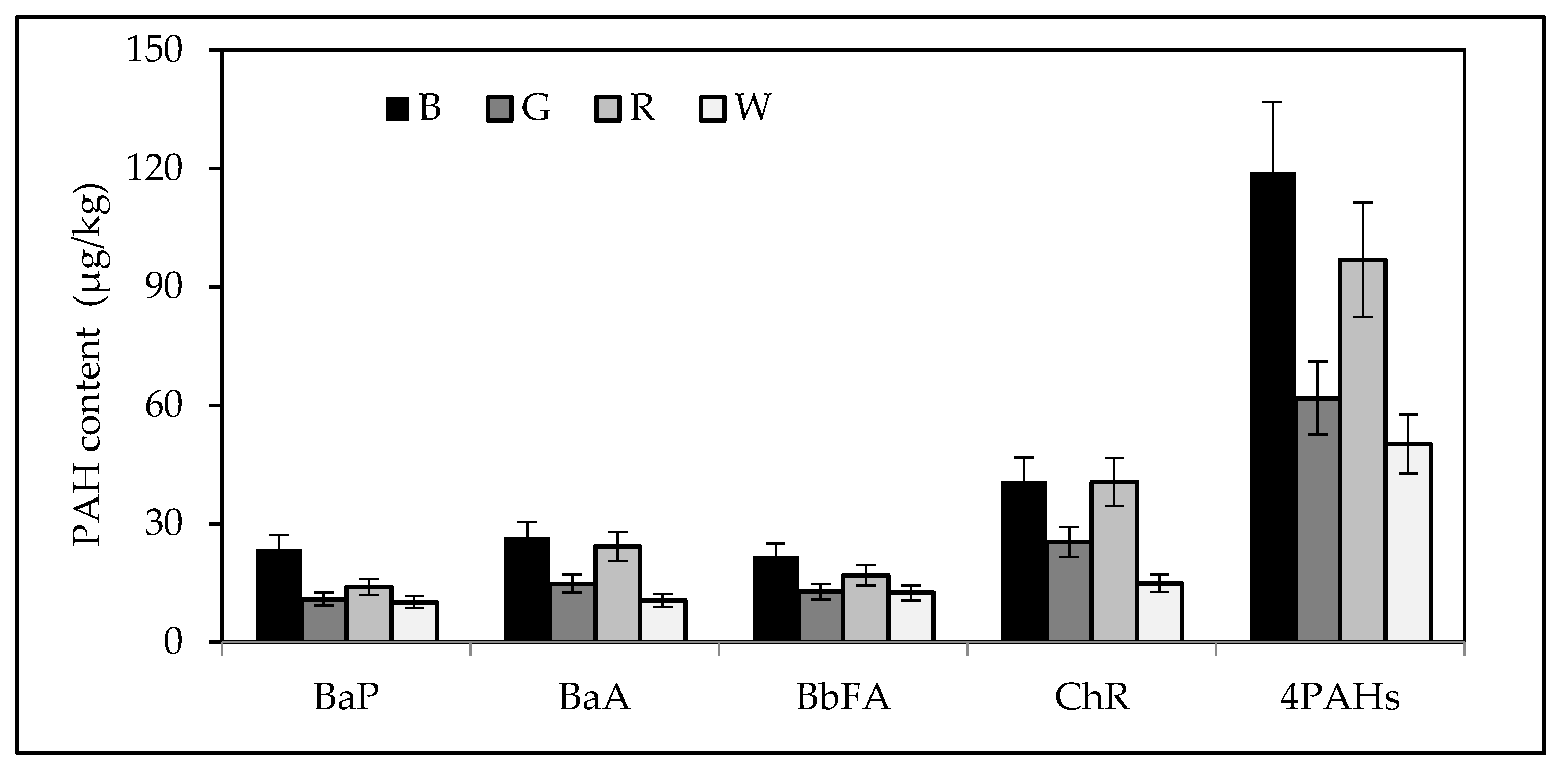
3.1. PAH Content of Tea
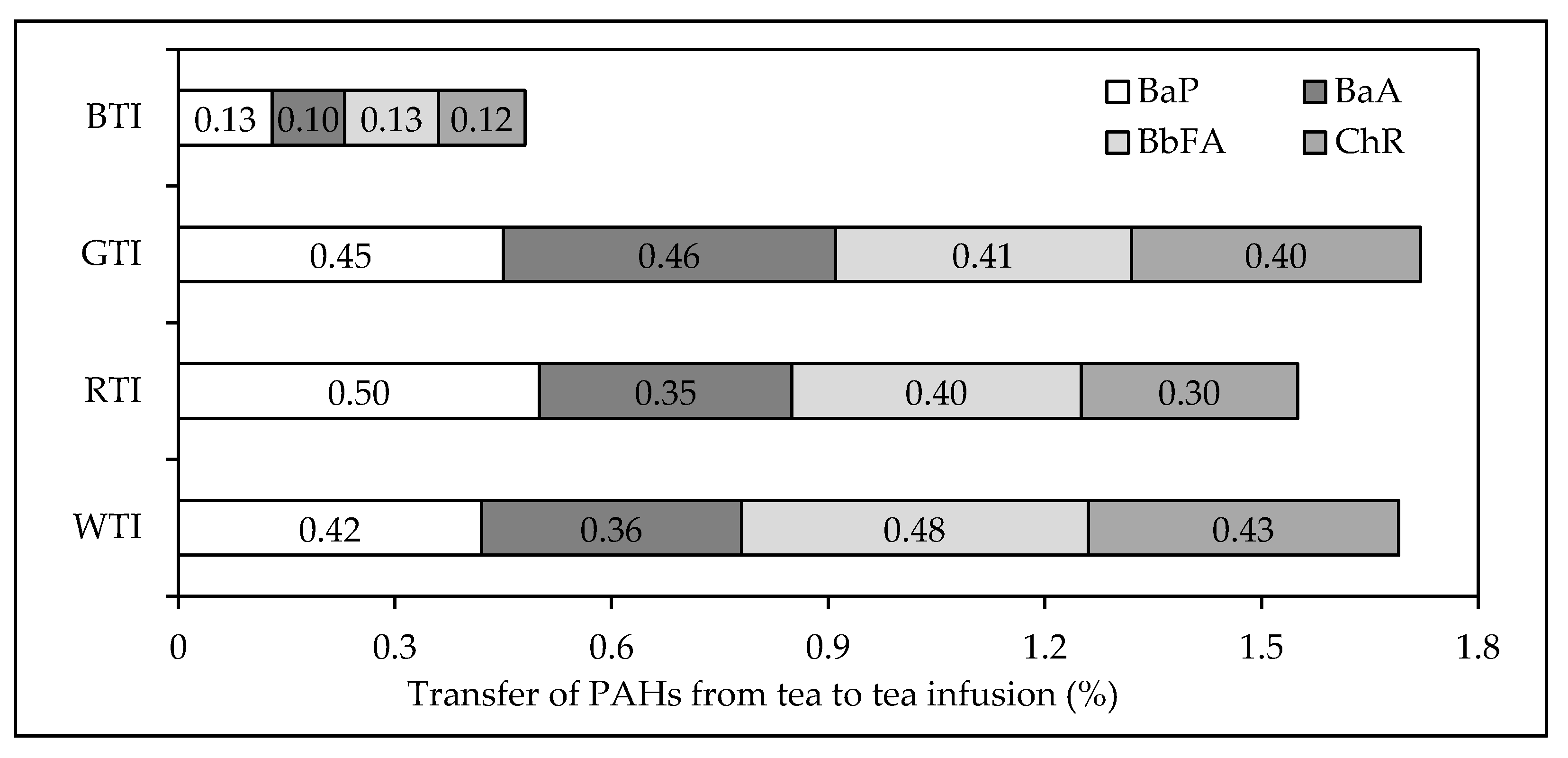
3.2. PAH Content of Tea Infusion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Plust, D.; Czerniejewska-Surma, B.; Domiszewski, Z.; Bienkiewicz, G.; Subda, R.; Wesołowski, T. Quality of selected white tea types. Food Sci. Qual. Technol. 2011, 3, 90–97. [Google Scholar] [CrossRef]
- Ishizaki, A.; Sito, K.; Kataoka, H. Analysis of contaminant polycyclic aromatic hydrocarbons in tea products and crude drugs. Anal. Methods 2011, 3, 299–305. [Google Scholar] [CrossRef]
- Rey-Salgueiro, L.; Martínez-Carballo, E.; García-Falcón, M.S.; Simal-Gándara, J. Effects of a chemical company fire on the occurrence of polycyclic aromatic hydrocarbons in plant foods. Food Chem. 2008, 108, 347–353. [Google Scholar] [CrossRef]
- Drabova, L.; Pulkrabova, J.; Kalachova, K.; Tomaniova, M.; Kocourek, V.; Hajslova, J. Rapid determination of polycyclic aromatic hydrocarbons (PAHs) in tea using two-dimensional gas chromatography coupled with time of flight mass spectrometry. Talanta 2012, 100, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Jánská, M.; Hajslová, J.; Tomaniová, M.; Kocourek, V.; Vávrová, M. Polycyclic aromatic hydrocarbons in fruits and vegetables grown in the Czech Republic. Bull. Environ. Contam. Toxicol. 2006, 77, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Zhu, L.; Luo, L. Factors affecting transfer of polycyclic aromatic hydrocarbons from made tea to tea infusion. J. Agric. Food Chem. 2006, 54, 4350–4354. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Zhu, L. Polycyclic aromatic hydrocarbons: Pollution and source analysis of a black tea. J. Agric. Food Chem. 2004, 52, 8268–8271. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Zhu, L.; He, W.; Tu, Y. Tea plant uptake and translocation of polycyclic aromatic hydrocarbons from water and around air. J. Agric. Food Chem. 2006, 54, 3658–3662. [Google Scholar] [CrossRef] [PubMed]
- Pincemaille, J.; Schummer, C.; Heinen, E.; Moris, G. Determination of polycyclic aromatic hydrocarbons in smoked and non-smoked black teas and tea infusions. Food Chem. 2014, 145, 807–813. [Google Scholar] [CrossRef] [PubMed]
- Ziegenhals, K.; Jira, W.; Speer, K. Polycyclic aromatic hydrocarbons (PAH) in various types of tea. Eur. Food Res. Technol. 2008, 228, 83–91. [Google Scholar] [CrossRef]
- European Food Safety Authority. Polycyclic aromatic hydrocarbons in food—Scientific Opinion of the Panel on Contaminants in the Food Chain (Question N° EFSA-Q-2007-136). EFSA J. 2008, 724, 1–114. [Google Scholar]
- Garcı́a-Falcón, M.S.; Simal-Gándara, J. Determination of polycyclic aromatic hydrocarbons in alcoholic drinks and identification of their potential sources. Food Addit. Contam. 2005, 22, 791–797. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization, International Agency for Research on Cancer. Monographs on the Evaluation of Carcinogenic Risks to Humans. Some Non-Heterocyclic Polycyclic Aromatic Hydrocarbons and Some Related Exposures; IARC: Lyon, France, 2010; Volume 92. [Google Scholar]
- Olatunji, O.S.; Fatoki, O.S.; Opeolu, B.O.; Ximba, B.J. Benzo[a]pyrene and benzo[k]fluoranthene in some processed fish and fish products. Int. J. Environ. Res. Public Health 2015, 12, 940–951. [Google Scholar] [CrossRef] [PubMed]
- Nowak, A.; Libudzisz, Z. Carcinogens in human gastrointestinal tract. Food Sci. Technol. Qual. 2008, 4, 9–25. [Google Scholar]
- Official Journal of the European Union. Commission Regulation (EU) 2015/1125 of 10 July 2015 amending Regulation (EC) No 1881/2006 as regards maximum levels for polycyclic aromatic hydrocarbons in Katsuobushi (dried bonito) and certain smoked Baltic herring. Off. J. Eur. Union 2015, L 184, 7–10. [Google Scholar]
- Official Journal of the European Union. Commission Regulation (EU) 2015/1933 of 27 October 2015 amending Regulation (EC) No 1881/2006 as regards maximum levels for polycyclic aromatic hydrocarbons in cocoa fibre, banana chips, food supplements, dried herbs and dried spices. Off. J. Eur. Union 2015, L 282, 11–13. [Google Scholar]
- EFSA A Report from the Unit of Data Collection and Exposure on a Request from the European Commission Findings of the EFSA Data Collection on Polycyclic Aromatic Hydrocarbons in Food. First Issued on 29 June 2007 and Revised on 31 July 2008. Available online: http://www.efsa.europa.eu/en/scdocs/doc/33r.pdf (accessed on 25 April 2016).
- Zachara, A.; Juszczak, L. Food contamination by polycyclic aromatic hydrocarbons—Legal requirements and monitoring. Food Sci. Technol. Qual. 2016, 3, 5–20. [Google Scholar] [CrossRef]
- Garcı́a-Falcón, M.S.; Cancho-Grande, B.; Simal-Gándara, J. Minimal clean-up and rapid determination of polycyclic aromatic hydrocarbons in instant coffee. Food Chem. 2005, 90, 643–647. [Google Scholar] [CrossRef]
- Sadowska-Rociek, A.; Surma, M.; Cieślik, E. Comparison of different modifications on QuEChERS sample preparation method for PAHs determination in black, green, red and white tea. Environ. Sci. Pollut. Res. Int. 2014, 21, 1326–1338. [Google Scholar] [CrossRef] [PubMed]
- Wenzl, T.; Simon, R.; Anklan, E.; Kleiner, J. Analytical methods of polycyclic aromatic hydrocarbons (PAHs) in food and the environment needed for new food legislation in the European Union. Trends Anal. Chem. 2006, 26, 716–725. [Google Scholar] [CrossRef]
- Zelinkova, Z.; Wenzl, T. The occurrence of 16 EPA PAHs in food—A review. Polycycl. Aromat. Compd. 2015, 35, 248–284. [Google Scholar] [CrossRef] [PubMed]
- González-Curbelo, M.Á.; Socas-Rodríguez, B.; Herrera-Herrera, A.V.; González-Sálamo, J.; Hernández-Borges, J.; Rodríguez-Delgado, M.Á. Evolution and applications of the QuEChERS method. Trends Anal. Chem. 2015, 71, 169–185. [Google Scholar] [CrossRef]
- Magnusson, B.; Örnemark, U. Eurachem Guide: The Fitness for Purpose of Analytical Methods—A Laboratory Guide to Method Validation and Related Topics, 2014. ISBN 978-91-87461-59-0. Available online: www.eurachem.org (accessed on 25 April 2016).
- Zachara, A.; Gałkowska, D.; Juszczak, L. Method validation and determination of polycyclic aromatic hydrocarbons in vegetable oils by HPLC-FLD. Food Anal. Methods 2017, 10, 1078–1086. [Google Scholar] [CrossRef]
- Cajka, T.; Sandy, C.; Bachanova, V.; Drabova, L.; Kalachova, K.; Pulkrabova, J.; Hajslova, J. Streamlining sample preparation and gas chromatography–tandem mass spectrometry analysis of multiple pesticide residues in tea. Anal. Chim. Acta 2012, 743, 51–60. [Google Scholar] [CrossRef] [PubMed]
- The Polish Committee for Standardization. Foods of Plant Origin—Determination of Pesticide Residues Using GC-MS and/or LC-MS/MS Following Acetonitrile Extraction/Partitioning and Clean-Up by Dispersive SPE-QuEChERS-Method; PN-EN 15662; The Polish Committee for Standardization: Warsaw, Poland, 2008. [Google Scholar]
- Wenzyl, T.; Haedrich, J.; Schaechtele, A.; Robouch, P.; Stroka, J. Guidance Document on the Estimation of LOD and LOQ for Measurements in the Field of Contaminants in Feed and Food; Publications Office of the European Union: Luxemburg, 2016. [Google Scholar]
- Official Journal of the European Union. Commission regulation (EU) No 836/2011 of 19 August 2011 amending regulation (EC) No 333/2007 laying down the methods of sampling and analysis for the official control of the levels of lead, cadmium, mercury, inorganic tin, 3-MCPD and benzo(a)pyrene in foodstuffs. Off. J. Eur. Union 2011, L 215, 9–16. [Google Scholar]
- Iwegbue, C.M.A.; Agadaga, H.; Bassey, F.I.; Overah, L.C.; Tesi, G.O.; Nwajei, G.E. Concentrations and profiles of polycyclic aromatic hydrocarbons in some commercial brands of tea-, coffee-, and cocoa-based food drinks in Nigeria. Int. J. Food Prop. 2015, 18, 2124–2133. [Google Scholar] [CrossRef]
- Lin, D.; Tu, Y.; Zhu, L. Concentrations and health risk of polycyclic aromatic hydrocarbons in tea. Food Chem. Toxicol. 2005, 43, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Londoño, V.A.G.; Reynoso, C.M.; Resnik, S.L. Polycyclic aromatic hydrocarbons (PAHs) survey on tea (Camellia sinensis) commercialized in Argentina. Food Control 2015, 50, 31–37. [Google Scholar] [CrossRef]
- Ciemniak, A.; Mocek, K. Polycyclic aromatic hydrocarbons in tea and tea infusions. Ann. Natl. Inst. Hyg. 2010, 3, 243–248. [Google Scholar]
- Prajapati, S.K.; Tripathi, B.D. Biomonitoring seasonal variation of urban air polycyclic aromatic hydrocarbons (PAHs) using Ficus benghalensis leaves. Environ. Pollut. 2008, 151, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Duedahl-Olesen, L.; Navaratnam, M.A.; Jewula, J.; Jensen, A.H. PAH in some brands of tea and coffee. Polycycl. Aromat. Compd. 2015, 35, 74–90. [Google Scholar] [CrossRef]
- Viñas, P.; Campillo, N.; Aguinaga, N.; Pérez-Cánovas, E.; Herández-Córdoba, M. Use of headspace solid-phase microextraction coupled to liquid chromatography for the analysis of polycyclic aromatic hydrocarbons in tea infusions. J. Chromatogr. A 2007, 1164, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Cacho, J.I.; Campillo, N.; Viñas, P.; Hernández-Córdoba, M. Use of headspace sorptive extraction coupled to gas chromatography–mass spectrometry for the analysis of volatile polycyclic aromatic hydrocarbons in herbal infusions. J. Chromatogr. A 2014, 1356, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Schulz, C.M.; Fritz, H.; Ruthenschrör, A. Occurrence of 15 + 1 EU priority polycyclic aromatic hydrocarbons (PAH) in various types of tea (Camellia sinensis) and herbal infusions. Food Addit. Contam. A 2014, 31, 1723–1735. [Google Scholar] [CrossRef] [PubMed]

| Kind of PAH | PAH Content of Tea (μg/kg) | PAH Content of Tea Infusion (ng/mL) | |||
|---|---|---|---|---|---|
| Tea Weight (g)/Water Volume (mL) | |||||
| 2/250 | 20/250 | 10/100 | 10/50 | ||
| black tea | |||||
| BaP | 209 | <LOQ | <LOQ | 0.16 | 0.25 |
| BaA | 174 | <LOQ | <LOQ | 0.11 | 0.18 |
| BbFA | 142 | <LOQ | <LOQ | 0.13 | 0.19 |
| Chr | 231 | <LOQ | 0.18 | 0.20 | 0.33 |
| white tea | |||||
| BaP | 23.9 | <LOQ | <LOQ | <LOQ | 0.15 |
| BaA | 38.4 | <LOQ | <LOQ | <LOQ | 0.15 |
| BbFA | 25.6 | <LOQ | <LOQ | <LOQ | 0.11 |
| Chr | 42.7 | <LOQ | <LOQ | <LOQ | 0.16 |
| Parameter | Kind of PAH | ||||
|---|---|---|---|---|---|
| BaP | BaA | BbFA | Chr | ||
| Linearity—correlation coefficient | 0.9999 | 1.0000 | 0.9999 | 1.0000 | |
| Sensitivity (slope) by regression equation | 2.451 | 9.050 | 7.398 | 3.667 | |
| Limit of detection (LOD) (μg/kg) (n = 10) | 0.25 | 0.15 | 0.15 | 0.25 | |
| Limit of quantification (LOQ) (μg/kg) (n = 10) | 0.75 | 0.50 | 0.50 | 0.75 | |
| Recovery (%) | Level I (5.00 μg/kg) (n = 6) | 82.25 | 93.30 | 75.00 | 71.35 |
| Level II (40.00 μg/kg) (n = 6) | 50.75 | 66.70 | 57.05 | 64.70 | |
| Level III (250 μg/kg) (n = 6) | 67.20 | 76.20 | 69.90 | 75.80 | |
| Repeatability RSDr (%) (n = 20) | 8.8 | 5.5 | 2.2 | 3.6 | |
| HORRATr | 0.61 | 0.34 | 0.15 | 0.25 | |
| Tea Type | Kind of PAH | ||||
|---|---|---|---|---|---|
| BaP | BaA | BbFA | Chr | Σ4PAHs | |
| Black tea (n = 10) | 3.96–209.36 | 7.58–187.00 | 4.51–145.10 | 14.80–280.00 | 33.12–770.10 |
| 51.13 | 51.53 | 41.08 | 75.06 | 218.80 | |
| Green tea (n = 6) | 4.30–24.82 | 5.62–23.03 | 7.27–46.53 | 9.80–61.20 | 29.62–153.27 |
| 13.34 | 15.10 | 18.25 | 28.91 | 75.96 | |
| Red tea (n = 6) | 7.00–18.09 | 15.81–30.99 | 9.13–22.89 | 23.80–51.61 | 55.74–120.41 |
| 12.93 | 23.24 | 16.25 | 37.79 | 90.21 | |
| White tea (n = 6) | 0.76–26.55 | 1.01–38.41 | 0.85–25.61 | 2.89–42.73 | 5.51–130.74 |
| 11.52 | 14.87 | 13.11 | 18.34 | 57.40 | |
| Tea Type | Sampling Market | Number of Samples | Kind of PAH | Reference | ||||
|---|---|---|---|---|---|---|---|---|
| BaP | BaA | BbFA | Chr | Σ4PAHs | ||||
| Black tea | Poland | 10 | 3.9–209 | 7.6–187 | 4.5–145 | 14.8–280 | 33–770 | present study |
| Poland | 7 | n.d. | 2.4–47 | 1.9–8.1 | 1.6–18 | - | [21] | |
| Poland | 9 | 2.9–63 | - | - | - | - | [34] | |
| Czech Republic | 18 | 0.2–152 | 1.4–196 | 0.9–123 | 3.9–229 | 7.4–699 | [4] | |
| Argentina | 27 | 0.2–93 | 0.2–63 | 0.1–68 | 2.5–109 | 4.1–332 | [33] | |
| Denmark | 10 | 0.30–32 | - | - | - | 2.8–115 | [36] | |
| China | 2 | 20.1–246 | - | - | - | - | [6] | |
| Nigeria | 4 | n.d.–137 | n.d.–44 | n.d.–27 | n.d.–55 | - | [31] | |
| Germany | 11 | 0.8–14 | 1.3–13 | 1.5–8.1 | 3.4–18 | 9.0–44 | [10] | |
| Green tea | Poland | 6 | 4.3–25 | 5.6–23 | 7.3–47 | 9.8–61 | 29–153 | present study |
| Poland | 7 | n.d | 11–19 | 1.8–2.0 | 2.8–3.7 | - | [21] | |
| Poland | 3 | 5.6–31 | - | - | - | - | [34] | |
| Czech Republic | 18 | 0.2–18 | 0.7–28 | 0.7–24 | 2.9–42 | 4.5–102 | [4] | |
| Argentina | 14 | 0.4–61 | 0.7–74 | 0.15–67 | 4.6–154 | 8.0–356 | [33] | |
| China | 1 | 6.8 | - | - | - | - | [6] | |
| Nigeria | 3 | n.d. | n.d. | n.d.–27 | n.d.–55 | - | [31] | |
| Germany | 11 | 1.6–33 | 1.8–40 | 2.2–33 | 6.7–62 | 12–168 | [10] | |
| Red tea | Poland | 6 | 7.0–18 | 16–31 | 9.1–23 | 24–52 | 55–120 | present study |
| Poland | 3 | n.d | n.d.–33 | n.d. | 3.2–12 | - | [21] | |
| Poland | 3 | 9.7–15 | - | - | - | - | [34] | |
| Argentina | 7 | 0.7–16 | 0.5–41 | 0.5–25 | 5.8–64 | 7.4–127 | [33] | |
| White tea | Poland | 6 | 0.8–27 | 1.0–38 | 0.8–26 | 2.9–42 | 5.5–131 | present study |
| Poland | 5 | n.d. | 2.4–17 | 2.2 | 12–19 | - | [21] | |
| Poland | 3 | 7.6–49 | - | - | - | - | [34] | |
| Argentina | 1 | 22 | 16 | 19 | 35 | 92 | [33] | |
| Germany | 3 | 11 | 14–80 | 14–45 | 19–95 | 59–80 | [10] | |
| Parameter | Kind of PAH | |||
|---|---|---|---|---|
| BaP | BaA | BbFA | Chr | |
| Linearity—correlation coefficient | 0.9999 | 0.9999 | 0.9999 | 0.9999 |
| Limit of detection (LOD) (ng/mL) (n = 10) | 0.05 | 0.03 | 0.03 | 0.05 |
| Limit of quantification (LOQ) (ng/mL) (n = 10) | 0.15 | 0.10 | 0.10 | 0.15 |
| Recovery Level I (0.18 ng/mL) (n = 6) (%) | 110.56 | 105.00 | 107.22 | 99.44 |
| Repeatability RSDr (%) (n = 10) | 8.0 | 4.4 | 5.1 | 6.5 |
| HORRATr | 0.55 | 0.30 | 0.35 | 0.45 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zachara, A.; Gałkowska, D.; Juszczak, L. Contamination of Tea and Tea Infusion with Polycyclic Aromatic Hydrocarbons. Int. J. Environ. Res. Public Health 2018, 15, 45. https://doi.org/10.3390/ijerph15010045
Zachara A, Gałkowska D, Juszczak L. Contamination of Tea and Tea Infusion with Polycyclic Aromatic Hydrocarbons. International Journal of Environmental Research and Public Health. 2018; 15(1):45. https://doi.org/10.3390/ijerph15010045
Chicago/Turabian StyleZachara, Alicja, Dorota Gałkowska, and Lesław Juszczak. 2018. "Contamination of Tea and Tea Infusion with Polycyclic Aromatic Hydrocarbons" International Journal of Environmental Research and Public Health 15, no. 1: 45. https://doi.org/10.3390/ijerph15010045




