Neighborhood Perceptions and Cumulative Impacts of Low Level Chronic Exposure to Fine Particular Matter (PM2.5) on Cardiopulmonary Health
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Chronic Fine Particulate Matter (PM2.5) Exposures
2.3. Cardiopulmonary Outcomes
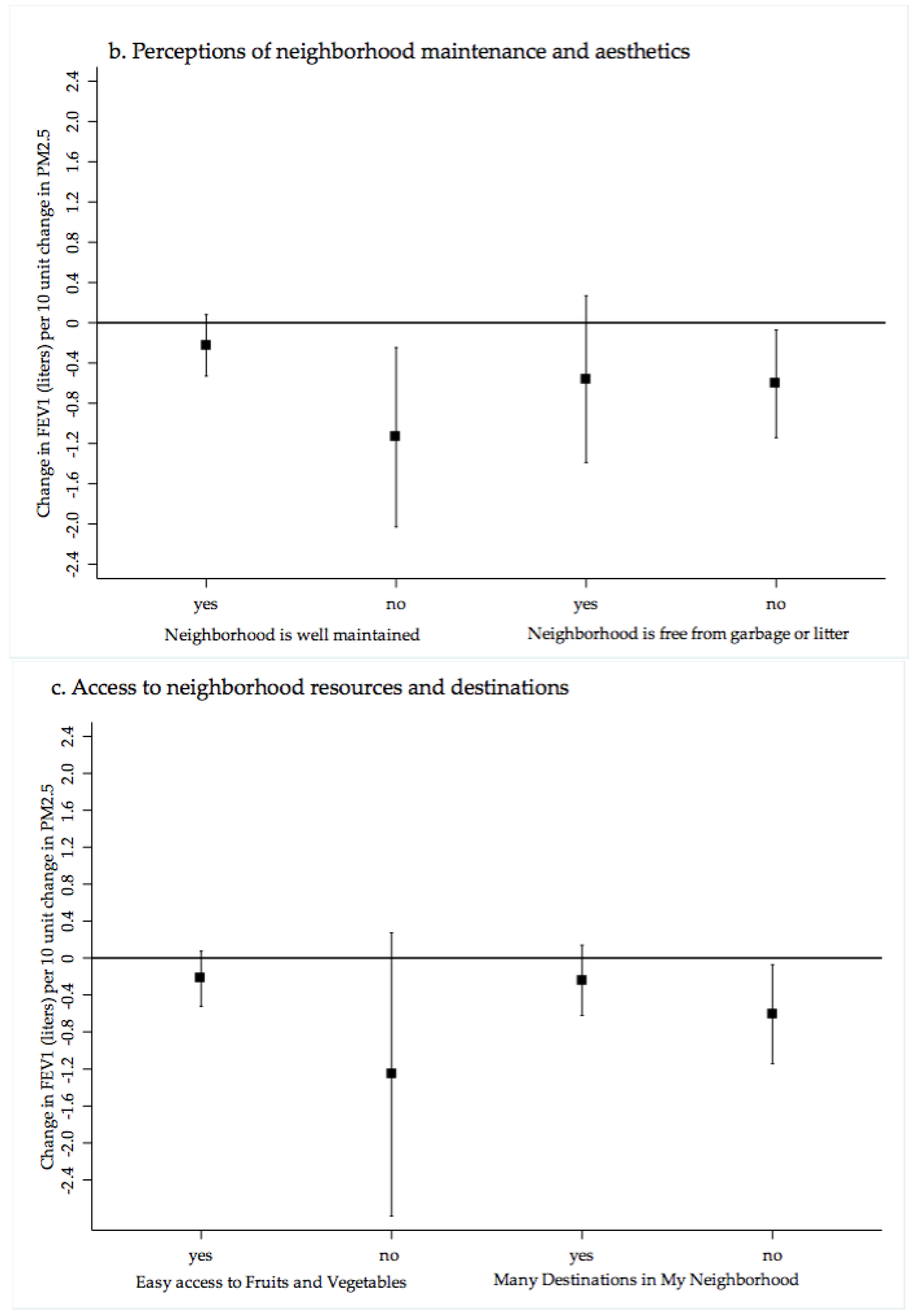
2.4. Neighborhood Stressors
2.5. Predictors and Covariates
2.6. Statistical Analyses
3. Results
4. Discussion
4.1. PM2.5 Exposure, Cardiopulmonary Health and Neighborhood Perceptions of Quality and Crime
4.2. Strengths and Limitations
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bell, M.L.; Ebisu, K. Environmental inequality in exposures to airborne particulate matter components in the United States. Environ. Health Perspect. 2012, 120, 1699–1704. [Google Scholar] [CrossRef] [PubMed]
- Morello-Frosch, R.; Jesdale, B.M. Separate and unequal: residential segregation and estimated cancer risks associated with ambient air toxics in U.S. metropolitan areas. Environ. Health Perspect. 2006, 114, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Diez Roux, A.V. Estimating neighborhood health effects: The challenges of causal inference in a complex world. Soc. Sci. Med. 2004, 58, 1953–1960. [Google Scholar] [CrossRef]
- Chi, G.C.; Hajat, A.; Bird, C.E.; Cullen, M.R.; Griffin, B.A.; Miller, K.A.; Shih, R.A.; Stefanick, M.L.; Vedal, S.; Whitsel, E.A.; et al. Individual and neighborhood socioeconomic status and the association between air pollution and cardiovascular disease. Environ. Health Perspect. 2016, 124, 1840–1847. [Google Scholar] [CrossRef] [PubMed]
- Clougherty, J.E.; Kubzansky, L.D. A framework for examining social stress and susceptibility to air pollution in respiratory health. Environ. Health Perspect. 2009, 117, 1351–1358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diez Roux, A.V.; Merkin, S.S.; Arnett, D.; Chambless, L.; Massing, M.; Nieto, F.J.; Sorlie, P.; Szklo, M.; Tyroler, H.A.; Watson, R.L. Neighborhood of residence and incidence of coronary heart disease. N. Engl. J. Med. 2001, 345, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, J.D.; Adar, S.D.; Allen, R.W.; Barr, R.G.; Budoff, M.J.; Burke, G.L.; Casillas, A.M.; Cohen, M.A.; Curl, C.L.; Daviglus, M.L.; et al. Prospective study of particulate air pollution exposures, subclinical atherosclerosis, and clinical cardiovascular disease: The Multi-Ethnic Study of Atherosclerosis and Air Pollution (MESA Air). Am. J. Epidemiol. 2012, 176, 825–837. [Google Scholar] [CrossRef] [PubMed]
- Landrigan, P.J.; Fuller, R.; Acosta, N.J.R.; Adeyi, O.; Arnold, R.; Basu, N.N.; Balde, A.B.; Bertollini, R.; Bose-O’Reilly, S.; Boufford, J.I.; et al. The Lancet Commission on pollution and health. Lancet 2017. [Google Scholar] [CrossRef]
- Hajat, A.; Hsia, C.; O’Neill, M.S. Socioeconomic disparities and air pollution exposure: A global review. Curr. Environ. Health Rep. 2015, 2, 440–450. [Google Scholar] [CrossRef] [PubMed]
- Clark, L.P.; Millet, D.B.; Marshall, J.D. National patterns in environmental injustice and inequality: Outdoor NO2 air pollution in the United States. PLoS ONE 2014, 9, e94431. [Google Scholar] [CrossRef] [PubMed]
- Clougherty, J.E.; Rossi, C.A.; Lawrence, J.; Long, M.S.; Diaz, E.A.; Lim, R.H.; McEwen, B.; Koutrakis, P.; Godleski, J.J. Chronic social stress and susceptibility to concentrated ambient fine particles in rats. Environ. Health Perspect. 2010, 118, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.R.; Diez-Roux, A.V.; Hajat, A.; Kershaw, K.N.; O’Neill, M.S.; Guallar, E.; Post, W.S.; Kaufman, J.D.; Navas-Acien, A. Race/ethnicity, residential segregation, and exposure to ambient air pollution: The Multi-Ethnic Study of Atherosclerosis (MESA). Am. J. Public Health 2014, 104, 2130–2137. [Google Scholar] [CrossRef] [PubMed]
- Morello-Frosch, R.; Lopez, R. The riskscape and the color line: Examining the role of segregation in environmental health disparities. Environ. Res. 2006, 102, 181–196. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J.D. Environmental inequality: Air pollution exposures in California’s South Coast Air Basin. Atmos. Environ. 2008, 42, 5499–5503. [Google Scholar] [CrossRef]
- Hajat, A.; Diez-Roux, A.V.; Adar, S.D.; Auchincloss, A.H.; Lovasi, G.S.; O’Neill, M.S.; Sheppard, L.; Kaufman, J.D. Air pollution and individual and neighborhood socioeconomic status: Evidence from the Multi-Ethnic Study of Atherosclerosis (MESA). Environ. Health Perspect. 2013, 121, 1325–1333. [Google Scholar] [CrossRef] [PubMed]
- Hicken, M.T.; Adar, S.D.; Diez Roux, A.V.; O’Neill, M.S.; Magzamen, S.; Auchincloss, A.H.; Kaufman, J.D. Do psychosocial stress and social disadvantage modify the association between air pollution and blood pressure? The multi-ethnic study of atherosclerosis. Am. J. Epidemiol. 2013, 178, 1550–1562. [Google Scholar] [CrossRef] [PubMed]
- Hicken, M.T.; Adar, S.D.; Hajat, A.; Kershaw, K.N.; Do, D.P.; Barr, R.G.; Kaufman, J.D.; Diez Roux, A.V. Air Pollution, Cardiovascular Outcomes, and Social Disadvantage: The Multi-ethnic Study of Atherosclerosis. Epidemiology 2016, 27, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Hicken, M.T.; Dvonch, J.T.; Schulz, A.J.; Mentz, G.; Max, P. Fine particulate matter air pollution and blood pressure: The modifying role of psychosocial stress. Environ. Res. 2014, 133, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Diez Roux, A.V.; Mair, C. Neighborhoods and health. Ann. N. Y. Acad. Sci. 2010, 1186, 125–145. [Google Scholar] [CrossRef] [PubMed]
- Djuric, Z.; Bird, C.E.; Furumoto-Dawson, A.; Rauscher, G.H.; Ruffin, M.T.T.; Stowe, R.P.; Tucker, K.L.; Masi, C.M. Biomarkers of psychological stress in health disparities research. Open Biomarks J. 2008, 1, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Epel, E.S.; Blackburn, E.H.; Lin, J.; Dhabhar, F.S.; Adler, N.E.; Morrow, J.D.; Cawthon, R.M. Accelerated telomere shortening in response to life stress. Proc. Natl. Acad. Sci. USA 2004, 101, 17312–17315. [Google Scholar] [CrossRef] [PubMed]
- Evans, G.W. A multimethodological analysis of cumulative risk and allostatic load among rural children. Dev. Psychol. 2003, 39, 924–933. [Google Scholar] [CrossRef] [PubMed]
- Seeman, T.; Epel, E.; Gruenewald, T.; Karlamangla, A.; McEwen, B.S. Socio-economic differentials in peripheral biology: Cumulative allostatic load. Ann. N. Y. Acad. Sci. 2010, 1186, 223–239. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S. Stress, adaptation, and disease. Allostasis and allostatic load. Ann. N. Y. Acad. Sci. 1998, 840, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.; Schreier, H.M.; Strunk, R.C.; Brauer, M. Chronic traffic-related air pollution and stress interact to predict biologic and clinical outcomes in asthma. Environ. Health Perspect. 2008, 116, 970–975. [Google Scholar] [CrossRef] [PubMed]
- Hajat, A.; Allison, M.; Diez-Roux, A.V.; Jenny, N.S.; Jorgensen, N.W.; Szpiro, A.A.; Vedal, S.; Kaufman, J.D. Long-term exposure to air pollution and markers of inflammation, coagulation, and endothelial activation: A repeat-measures analysis in the Multi-Ethnic Study of Atherosclerosis (MESA). Epidemiology 2015, 26, 310–320. [Google Scholar] [CrossRef] [PubMed]
- National Research Council. Phthalates and Cumulative Risk Assessment: The Tasks Ahead; The National Academies Press: Washington, DC, USA, 2008; Available online: https://doi.org/10.17226/12528 (accessed on 5 December 2017).
- Clougherty, J.E.; Levy, J.I.; Kubzansky, L.D.; Ryan, P.B.; Suglia, S.F.; Canner, M.J.; Wright, R.J. Synergistic effects of traffic-related air pollution and exposure to violence on urban asthma etiology. Environ. Health Perspect. 2007, 115, 1140–1146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, M.R.; Diez-Roux, A.V.; O’Neill, M.S.; Guallar, E.; Sharrett, A.R.; Post, W.; Kaufman, J.D.; Navas-Acien, A. Ambient air pollution and racial/ethnic differences in carotid intima-media thickness in the Multi-Ethnic Study of Atherosclerosis (MESA). J. Epidemiol. Community Health 2015, 69, 1191–1198. [Google Scholar] [CrossRef] [PubMed]
- Kristiansson, M.; Sorman, K.; Tekwe, C.; Calderon-Garciduenas, L. Urban air pollution, poverty, violence and health—Neurological and immunological aspects as mediating factors. Environ. Res. 2015, 140, 511–513. [Google Scholar] [CrossRef] [PubMed]
- Ranjit, N.; Diez-Roux, A.V.; Shea, S.; Cushman, M.; Seeman, T.; Jackson, S.A.; Ni, H. Psychosocial factors and inflammation in the multi-ethnic study of atherosclerosis. Arch. Intern. Med. 2007, 167, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Wright, R.J.; Mitchell, H.; Visness, C.M.; Cohen, S.; Stout, J.; Evans, R.; Gold, D.R. Community violence and asthma morbidity: The Inner-City Asthma Study. Am. J. Public Health 2004, 94, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Sandberg, S.; Jarvenpaa, S.; Penttinen, A.; Paton, J.Y.; McCann, D.C. Asthma exacerbations in children immediately following stressful life events: A Cox’s hierarchical regression. Thorax 2004, 59, 1046–1051. [Google Scholar] [CrossRef] [PubMed]
- Wright, R.J.; Subramanian, S.V. Advancing a multilevel framework for epidemiologic research on asthma disparities. Chest 2007, 132, 757S–769S. [Google Scholar] [CrossRef] [PubMed]
- Shmool, J.L.; Kubzansky, L.D.; Newman, O.D.; Spengler, J.; Shepard, P.; Clougherty, J.E. Social stressors and air pollution across New York City communities: A spatial approach for assessing correlations among multiple exposures. Environ. Health 2014, 13, 91. [Google Scholar] [CrossRef] [PubMed]
- Evans, G.W.; Kim, P. Multiple risk exposure as a potential explanatory mechanism for the socioeconomic status-health gradient. Ann. N. Y. Acad. Sci. 2010, 1186, 174–189. [Google Scholar] [CrossRef] [PubMed]
- Nieto, F.J.; Peppard, P.E.; Engelman, C.D.; McElroy, J.A.; Galvao, L.W.; Friedman, E.M.; Bersch, A.J.; Malecki, K.C. The Survey of the Health of Wisconsin (SHOW), a novel infrastructure for population health research: Rationale and methods. BMC Public Health 2010, 10, 785. [Google Scholar] [CrossRef] [PubMed]
- US Environmental Protection Agency. RSIG-Related Downloadable Data Files. Technical Information about Fused Air Quality Surface Using Downscaling Tool: Metadata Description. Available online: https://www.epa.gov/sites/production/files/2016-07/documents/data_fusion_meta_file_july_2016.pdf (accessed on 4 January 2018).
- US Environmental Protection Agency. RSIG-Related Downloadable Data Files. Available online: https://www.epa.gov/hesc/rsig-related-downloadable-data-files (accessed on 15 September 2016).
- World Health Organization (WHO). Obesity and Overweight. 2016. Available online: http://www.who.int/mediacentre/factsheets/fs311/en/ (accessed on 29 August 2017).
- Richter, K.; Kanniess, F.; Mark, B.; Jorres, R.A.; Magnussen, H. Assessment of accuracy and applicability of a new electronic peak flow meter and asthma monitor. Eur. Respir. J. 1998, 12, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Hankinson, J.L.; Odencrantz, J.R.; Fedan, K.B. Spirometric reference values from a sample of the general U.S. population. Am. J. Respir. Crit. Care Med. 1999, 159, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Morrill, R.; Cromartie, J.; Hart, L. Metropolitan, urban, and rural commuting area: Toward a better depiction of the US settlement system. Urban Geogr. 1999, 20, 727–748. [Google Scholar] [CrossRef]
- Block, G.; Gillespie, C.; Rosenbaum, E.H.; Jenson, C. A rapid food screener to assess fat and fruit and vegetable intake. Am. J. Prev. Med. 2000, 18, 284–288. [Google Scholar] [CrossRef]
- StataCorp. Stata Statistical Software: Release 14; StataCorp.: College Station, TX, USA, 2015. [Google Scholar]
- Cuzick, J. A Wilcoxon-type test for trend. Stat. Med. 1985, 4, 87–90. [Google Scholar] [CrossRef] [PubMed]
- US Environmental Protection Agency (USEPA). National Ambient Air Quality Standards Table. Available online: https://www.epa.gov/criteria-air-pollutants/naaqs-table (accessed on 29 July 2016).
- World Health Organization (WHO). WHO Expert Consultation: Available Evidence for the Future Update of the WHO Global Air Quality Guidelines (AQGs); WHO: Geneva, Switzerland, 2016. [Google Scholar]
- Montiel, L.M.; Nathan, R.P.; Wright, D.J. An Update on Urban Hardship; The Nelson A Rockefeller Institute of Government: Albany, NY, USA, 2004. [Google Scholar]
- Adam, M.; Schikowski, T.; Carsin, A.E.; Cai, Y.; Jacquemin, B.; Sanchez, M.; Vierkotter, A.; Marcon, A.; Keidel, D.; Sugiri, D.; et al. Adult lung function and long-term air pollution exposure. ESCAPE: A multicentre cohort study and meta-analysis. Eur. Respir. J. 2015, 45, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Forbes, L.J.; Kapetanakis, V.; Rudnicka, A.R.; Cook, D.G.; Bush, T.; Stedman, J.R.; Whincup, P.H.; Strachan, D.P.; Anderson, H.R. Chronic exposure to outdoor air pollution and lung function in adults. Thorax 2009, 64, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Sunyer, J. Lung function effects of chronic exposure to air pollution. Thorax 2009, 64, 645–646. [Google Scholar] [CrossRef] [PubMed]
- Ackermann-Liebrich, U.; Leuenberger, P.; Schwartz, J.; Schindler, C.; Monn, C.; Bolognini, G.; Bongard, J.P.; Brandli, O.; Domenighetti, G.; Elsasser, S.; et al. Lung function and long term exposure to air pollutants in Switzerland. Study on Air Pollution and Lung Diseases in Adults (SAPALDIA) Team. Am. J. Respir. Crit. Care Med. 1997, 155, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Peters, J.M.; Avol, E.; Gauderman, W.J.; Linn, W.S.; Navidi, W.; London, S.J.; Margolis, H.; Rappaport, E.; Vora, H.; Gong, H., Jr.; et al. A study of twelve Southern California communities with differing levels and types of air pollution. II. Effects on pulmonary function. Am. J. Respir. Crit. Care Med. 1999, 159, 768–775. [Google Scholar] [CrossRef] [PubMed]
- Beyer, K.M.; Kaltenbach, A.; Szabo, A.; Bogar, S.; Nieto, F.J.; Malecki, K.M. Exposure to neighborhood green space and mental health: Evidence from the survey of the health of Wisconsin. Int. J. Environ. Res. Public Health 2014, 11, 3453–3472. [Google Scholar] [CrossRef] [PubMed]
- Bogar, S.; Beyer, K.M. Green Space, Violence, and Crime: A Systematic Review. Trauma Violence Abuse 2016, 17, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Lorenc, T.; Clayton, S.; Neary, D.; Whitehead, M.; Petticrew, M.; Thomson, H.; Cummins, S.; Sowden, A.; Renton, A. Crime, fear of crime, environment, and mental health and wellbeing: Mapping review of theories and causal pathways. Health Place 2012, 18, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Mair, C.; Diez Roux, A.V.; Morenoff, J.D. Neighborhood stressors and social support as predictors of depressive symptoms in the Chicago Community Adult Health Study. Health Place 2010, 16, 811–819. [Google Scholar] [CrossRef] [PubMed]
- Lorenc, T.; Petticrew, M.; Whitehead, M.; Neary, D.; Clayton, S.; Wright, K.; Thomson, H.; Cummins, S.; Sowden, A.; Renton, A. Fear of crime and the environment: Systematic review of UK qualitative evidence. BMC Public Health 2013, 13, 496. [Google Scholar] [CrossRef] [PubMed]
- Lorenc, T.; Petticrew, M.; Whitehead, M.; Neary, D.; Clayton, S.; Wright, K.; Thomson, H.; Cummins, S.; Sowden, A.; Renton, A. Environmental interventions to reduce fear of crime: Systematic review of effectiveness. Syst. Rev. 2013, 2, 30. [Google Scholar] [CrossRef] [PubMed]
- Schneider, R.H.; Kitchen, T. Crime Prevention in the Built Environment; Routledge, Taylor & Francis Group: New York, NY, USA, 2007; Volume xxii, 274p. [Google Scholar]
- Kerr, Z.; Evenson, K.R.; Moore, K.; Block, R.; Diez Roux, A.V. Changes in walking associated with perceived neighborhood safety and police-recorded crime: The multi-ethnic study of atherosclerosis. Prev. Med. 2015, 73, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Al-Bayan, M.; Islam, N.; Edwards, S.; Duncan, D.T. Neighborhood perceptions and hypertension among low-income black women: A qualitative study. BMC Public Health 2016, 16, 1075. [Google Scholar] [CrossRef] [PubMed]
- Morello-Frosch, R.; Shenassa, E.D. The environmental “riskscape” and social inequality: Implications for explaining maternal and child health disparities. Environ. Health Perspect. 2006, 114, 1150–1153. [Google Scholar] [CrossRef] [PubMed]
- Braun, L. Race, ethnicity and lung function: A brief history. Can. J. Respir. Ther. 2015, 51, 99–101. [Google Scholar] [PubMed]
- Kumar, R.; Seibold, M.A.; Aldrich, M.C.; Williams, L.K.; Reiner, A.P.; Colangelo, L.; Galanter, J.; Gignoux, C.; Hu, D.; Sen, S.; et al. Genetic ancestry in lung-function predictions. N. Engl. J. Med. 2010, 363, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Rossiter, C.E.; Weill, H. Ethnic differences in lung function: Evidence for proportional differences. Int. J. Epidemiol. 1974, 3, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Van Sickle, D.; Magzamen, S.; Mullahy, J. Understanding socioeconomic and racial differences in adult lung function. Am. J. Respir. Crit. Care Med. 2011, 184, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Hegewald, M.J.; Crapo, R.O. Socioeconomic status and lung function. Chest 2007, 132, 1608–1614. [Google Scholar] [CrossRef] [PubMed]
- Cummins, S.; Curtis, S.; Diez-Roux, A.V.; Macintyre, S. Understanding and representing ‘place’ in health research: A relational approach. Soc. Sci. Med. 2007, 65, 1825–1838. [Google Scholar] [CrossRef] [PubMed]
- Lokke, H. Novel methods for integrated risk assessment of cumulative stressors—Results from the NoMiracle project. Sci. Total Environ. 2010, 408, 3719–3724. [Google Scholar] [CrossRef] [PubMed]
- Fox, M.A. Evaluating cumulative risk assessment for environmental justice: A community case study. Environ. Health Perspect. 2002, 110 (Suppl. 2), 203–209. [Google Scholar] [CrossRef] [PubMed]
- National Research Council (U.S.). Committee on Improving Risk Analysis Approaches Used by the United States Environmental Protection Agency. In Science and Decisions: Advancing Risk Assessment; The National Academies Press: Washington, DC, USA, 2009; Available online: https://doi.org/10.17226/12209 (accessed on 4 January 2018).


| Quartile of Estimated 3 Year Annual Average Exposure to PM2.5 (μg/m3) | ||||||
|---|---|---|---|---|---|---|
| Study Sample $ n = 2230 % | μ (SD) | Q1 (7.0–9.4 μg/m3) n = 563, % | Q2 (9.5–10.3 μg/m3) n = 573 % | Q3 (10.3–10.9 μg/m3) n = 551 % | Q4 (10.9–14.0 μg/m3) n = 543 % | |
| Demographics | p-value for trend † | |||||
| Age, years | *** | |||||
| 21–39 | 36.7 | 10.5 (0.09) | 15.4 | 27.2 | 23.7 | 33.7 |
| 40–54 | 35.9 | 10.4 (0.08) | 18.5 | 28.4 | 20.2 | 32.8 |
| 55–74 | 27.4 | 10.2 (0.08) | 23.7 | 28.3 | 20.0 | 28.0 |
| Gender | ||||||
| Female | 48.2 | 10.4 (0.06) | 52.2 | 44.6 | 50.9 | 47.3 |
| Male | 51.7 | 10.4 (0.06) | 47.8 | 55.4 | 49.1 | 52.7 |
| Race/Ethnicity | *** | |||||
| White, Non-Hispanic | 90.0 | 10.2 (0.07) | 20.2 | 29.3 | 20.1 | 30.3 |
| Black, Non-Hispanic | 4.5 | 11.8 (0.10) | - | 6.7 | 48.0 | 45.3 |
| Hispanic | 1.4 | 11.1 (0.22) | 7.3 | 14.4 | 36.5 | 41.7 |
| Other | 4.1 | 11.1 (0.22) | 10.8 | 27.0 | 16.5 | 45.6 |
| Household Income (U.S. $, mid-point, before taxes) | 66,970.53 (1869.2) | ** | ||||
| 0–19,999 | 10.7 | 10.5 (0.14) | 25.0 | 18.3 | 23.0 | 33.7 |
| 20–49,999 | 27.2 | 10.2 (0.10) | 20.6 | 28.8 | 21.8 | 28.8 |
| 50–74,999 | 22.2 | 10.1 (0.11) | 20.9 | 23.8 | 22.0 | 33.2 |
| 75–99,999 | 14.6 | 10.4 (0.09) | 13.1 | 30.5 | 18.0 | 38.3 |
| >100,000 | 25.3 | 10.6 (0.10) | 15.6 | 33.2 | 22.0 | 29.2 |
| Education | *** | |||||
| <HS | 5.5 | 10.4 (0.15) | 18.4 | 19.1 | 23.5 | 39.0 |
| HS or some college | 58.0 | 10.2 (0.07) | 22.1 | 29.7 | 18.8 | 29.4 |
| College graduate or > | 36.5 | 10.6 (0.10) | 13.6 | 26.5 | 25.3 | 34.6 |
| Behaviors | ||||||
| Length of Residence in Household, years | 11.18 (0.41) | *** | ||||
| <5 years | 27.1 | 10.5 (0.09) | 16.7 | 22.3 | 20.3 | 40.6 |
| >5 years | 72.9 | 10.3 (0.08) | 19.6 | 30.0 | 21.8 | 28.5 |
| Physical Activity (MET-Min-Week) | ||||||
| ≥600 | 76.1 | 10.3 (0.09) | 19.7 | 31.9 | 18.2 | 30.2 |
| <600 | 23.8 | 10.3 (0.07) | 18.5 | 26.7 | 22.5 | 32.3 |
| Total Daily Saturated Fat Consumption (grams) & | 20.12 (0.32) g/day | |||||
| >20.0 | 43.8 | 10.4 (0.07) | 19.6 | 26.4 | 19.8 | 34.2 |
| ≤20.0 | 56.1 | 10.4 (0.07) | 18.1 | 29.2 | 22.8 | 29.9 |
| Total Daily Vegetable Consumption (cups) | 1.29 (0.02) cups/day | |||||
| >2 | 16.9 | 10.4 (0.10) | 20.2 | 25.9 | 22.1 | 31.8 |
| ≤2 | 83.1 | 10.4 (0.05) | 18.5 | 28.4 | 21.3 | 31.7 |
| Smoking Status | ||||||
| current | 17.3 | 10.5 (1.0) | 21.6 | 26.2 | 23.0 | 29.2 |
| former | 26.3 | 10.3 (0.09) | 19.0 | 30.4 | 20.0 | 30.5 |
| never | 56.4 | 10.3 (0.08) | 18.0 | 27.2 | 21.6 | 33.2 |
| Neighborhood Perceptions of Safety, Aesthetics, Access and Stress | ||||||
| Community safety from crime for walking and biking | *** | |||||
| <somewhat safe | 28.3 | 10.7 (0.08) | 15.4 | 18.2 | 28.3 | 38.0 |
| very safe | 71.7 | 10.2 (0.08) | 20.0 | 31.8 | 18.7 | 29.5 |
| Many Destinations in easy walking distance from home | *** | |||||
| Agree | 58.3 | 10.6 (0.08) | 16.2 | 23.0 | 24.8 | 36.0 |
| Disagree | 41.7 | 10.0 (0.09) | 22.1 | 35.0 | 16.8 | 26.1 |
| Easy Access to fresh fruits and vegetables in community | *** | |||||
| Agree | 91.9 | 10.4 (0.05) | 19.3 | 29.7 | 20.2 | 30.1 |
| Disagree | 8.1 | 10.8 (0.14) | 14.3 | 13.0 | 29.1 | 43.5 |
| Community Generally Free of Garbage, litter or broken glass | *** | |||||
| Agree | 87.4 | 10.4 (0.05) | 19.0 | 29.4 | 20.8 | 30.7 |
| Disagree | 12.6 | 10.6 (0.12) | 16.7 | 18.2 | 25.8 | 39.0 |
| Community is Well Maintained | ||||||
| Agree | 87.9 | 10.3 (0.12) | 18.4 | 29.7 | 21.1 | 30.9 |
| Disagree | 12.1 | 10.5 (0.07) | 20.3 | 16.2 | 24.5 | 38.9 |
| Neighborhood Stress | *** | |||||
| Yes | 19.4 | 10.7 (0.09) | 16.3 | 18.6 | 29.7 | 35.3 |
| No | 80.6 | 10.2 (0.07) | 19.4 | 30.2 | 19.4 | 31.0 |
| Contextual Neighborhood Level Factors | ||||||
| Economic Hardship | *** | |||||
| Low | 38.3 | 10.7 (0.12) | 11.2 | 25.9 | 20.8 | 41.2 |
| Med | 34.2 | 10.3 (0.12) | 18.2 | 39.0 | 17.2 | 25.5 |
| High | 27.4 | 10.1 (0.13) | 29.0 | 16.9 | 27.5 | 26.5 |
| Urbanicity | *** | |||||
| Urban | 52.5 | 10.8 (0.10) | 8.0 | 22.5 | 31.0 | 38.6 |
| Suburban | 17.0 | 10.2 (0.17) | 18.5 | 43.9 | 16.0 | 21.5 |
| Rural | 30.5 | 9.8 (0.11) | 37.8 | 28.4 | 8.0 | 25.8 |
| Cardiopulmonary Health Outcome | Quartile of Estimated 3 Year Annual Average Exposure to PM2.5 (μg/m3) | ||||||
|---|---|---|---|---|---|---|---|
| Total Sample (n = 2589) | Non-Asthmatic Adult Only Sample (n = 2230) | Q1 (7.0–9.4) n = 563 | Q2 (9.5–10.3) n = 573 | Q3 (10.3–10.9) n = 551 | Q4 (10.9–14.0) n = 543 | p for Trend † | |
| FEV1 (L/1 s) | 3.09 (3.04–3.14) | 3.10 (3.05–3.15) | 3.02 (2.91–3.12) | 3.19 (3.11–3.28) | 3.12 (3.04–3.20) | 3.07 (2.97–3.17) | 0.529 |
| FVC (L) | 3.75 (3.70–3.81) | 3.77 (3.71–3.84) | 3.59 (3.48–3.70) | 3.87 (3.76–3.99) | 3.82 (3.73–3.91) | 3.76 (3.64–3.88) | 0.056 |
| FEV1/FVC (%) *** | 0.83 (0.83–0.84) | 0.83 (0.83–0.84) | 0.85 (0.84–0.86) | 0.84 (0.82–0.85) | 0.83 (0.82–0.84) | 0.83 (0.81–0.84) | 0.001 |
| Body Mass Index * | 29.3 (28.9–29.7) | 29.1 (28.7–29.5) | 29.3 (28.3–30.3) | 29.6 (28.8–30.4) | 28.9 (28.1–29.6) | 28.8 (28.0–29.5) | 0.046 |
| Systolic BP | 122.4 (121.6–123.2) | 122.4 (121.6–123.3) | 121.5 (119.7–123.2) | 122.7 (121.0–124.4) | 120.6 (118.6–122.5) | 124.0 (122.4–125.6) | 0.779 |
| Diastolic BP | 76.3 (75.7–76.9) | 76.2 (75.5–76.9) | 75.5 (74.2–76.8) | 76.2 (75.0–77.4) | 75.2 (73.6–76.9) | 77.4 (76.1–78.6) | 0.324 |
| Total Cholesterol | 190.2 (188.0–192.4) | 189.9 (187.7–192.1) | 191.4 (187.6–195.1) | 192.4 (187.7–197.1) | 188.3 (184.4–192.2) | 187.8 (183.1–192.4) | 0.072 |
| HDL Cholesterol | 47.6 (46.6–48.5) | 47.4 (46.4–48.4) | 47.7 (46.1–49.3) | 47.5 (45.5–49.4) | 48.4 (46.4–50.4) | 46.3 (44.3–48.3) | 0.136 |
| Non-Asthmatic Adults (n = 2230) | Linear Spline Regression * | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate † | Adjusted * | <8 μg/m3 | 8–10 μg/m3 | >10 μg/m3 | ||||||||||||||||
| β | (95% CI) | p-Value | β | (95% CI) | p-Value | β | (95% CI) | p-Value | β | (95% CI) | p-Value | β | (95% CI) | p-Value | ||||||
| FEV1 (L/1 s) | −0.40 | −0.60 | −0.06 | −0.009 | −0.34 | −0.64 | −0.04 | 0.024 | −0.32 | −2.34 | 1.7 | 0.753 | 0.23 | −0.45 | 0.91 | 0.507 | −0.74 | −1.35 | −0.13 | 0.018 |
| FVC (L) | −0.02 | −0.03 | −0.02 | 0.000 | −0.09 | −0.50 | 0.31 | 0.647 | −1.30 | −4.63 | 2.02 | 0.439 | 1.14 | 0.079 | 2.19 | 0.035 | −0.83 | −1.69 | −0.07 | 0.033 |
| FEV1 to FVC Ratio | −0.49 | −0.58 | −0.42 | 0.000 | −0.07 | −0.13 | −0.01 | 0.018 | 0.35 | −0.05 | 0.76 | 0.090 | −0.19 | −0.34 | −0.05 | 0.010 | −0.03 | −0.14 | 0.08 | 0.575 |
| Model 1 Demographics | Model 2 + Behaviors | Model 3 + Perceptions of Safety for Walking and Biking | Model 4 + Perceptions of Safety + Race | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | (95% CI) | p-Value | β | (95% CI) | p-Value | β | (95% CI) | p-Value | β | (95% CI) | p-Value | |||||
| PM2.5 μg/m3 (10 unit change) | −0.40 | −0.60 | −0.06 | −0.009 | −0.34 | −0.64 | −0.05 | 0.024 | −0.30 | −0.60 | −0.02 | 0.048 | −0.14 | −0.04 | 0.02 | 0.354 |
| Age, (years) | −0.02 | −0.03 | −0.02 | 0.000 | −0.03 | −0.03 | −0.02 | 0.000 | −0.03 | −0.03 | −0.02 | 0.000 | −0.03 | −0.03 | −0.02 | 0.000 |
| Gender (female vs. male) | −0.49 | −0.58 | −0.42 | 0.000 | −0.50 | −0.58 | −0.42 | 0.000 | −0.52 | −0.58 | −0.41 | 0.000 | −0.53 | −0.62 | −0.44 | 0.000 |
| Education (years) | 0.03 | 0.01 | 0.04 | 0.000 | 0.02 | 0.01 | 0.04 | 0.007 | 0.02 | 0.01 | 0.04 | 0.011 | 0.01 | 0.001 | 0.03 | 0.037 |
| Household Income (mid US $) | 1.03 × 10−6 | 4.8 × 10−7 | 1.6 × 10−6 | 0.000 | 6.5 × 10−7 | 1.5 × 10−7 | 1.2 × 10−6 | 0.000 | 5.8 × 10−7 | 7.9 × 10−8 | 1.1 × 10−6 | 0.024 | 4.0 × 10−7 | −9.1 × 10−7 | 8.8 × 10−7 | 0.110 |
| height (cm) | 0.04 | 0.03 | 0.04 | 0.000 | 0.04 | 0.03 | 0.04 | 0.000 | 0.04 | 0.03 | 0.04 | 0.00 | 0.04 | 0.03 | 0.04 | 0.000 |
| BMI (mg/kg2 continuos) | −0.01 | −0.01 | 0.00 | 0.010 | −0.01 | −0.00 | 0.00 | 0.014 | −0.01 | −0.00 | −0.00 | 0.021 | ||||
| Smoking (per 10 pack-years) | −0.03 | −0.05 | 0.02 | 0.000 | −0.04 | −0.068 | 0.011 | 0.000 | −0.04 | −0.063 | 0.091 | 0.000 | ||||
| Allow smoke in home (yes vs. no) | −0.05 | −0.12 | 0.02 | 0.143 | −0.04 | −0.114 | 0.026 | 0.219 | −0.03 | −0.10 | 0.04 | 0.421 | ||||
| Physical Activity (guidelines yes vs. no) | 0.02 | −0.06 | 0.09 | 0.693 | 0.01 | −0.06 | 0.09 | 0.730 | 0.02 | −0.06 | 0.10 | 0.634 | ||||
| Saturated Fat (grams/day) | −0.004 | −0.006 | −0.001 | 0.006 | −0.004 | −0.006 | −0.001 | 0.006 | −0.004 | −0.006 | −0.001 | 0.009 | ||||
| Vegetables (cups/day) | 0.04 | 0.01 | 0.08 | 0.022 | 0.04 | 0.01 | 0.08 | 0.028 | 0.05 | 0.01 | 0.08 | 0.014 | ||||
| Resident Time (>5 years) | 0.10 | 0.02 | 0.17 | 0.015 | 0.09 | 0.01 | 0.16 | 0.02 | 0.07 | −0.00 | 0.15 | 0.06 | ||||
| Perceived Safety from Crime for Walking and Biking (<Somewhat safe vs. very safe) | −0.06 | −0.13 | 0.01 | 0.095 | −0.04 | −0.13 | 0.01 | 0.203 | ||||||||
| Race | ||||||||||||||||
| Non-Hispanic Black vs. White | −0.37 | −0.52 | −0.22 | 0.000 | ||||||||||||
| Hispanic vs. White | 0.04 | −0.21 | 0.28 | 0.768 | ||||||||||||
| Other vs. White | −0.14 | −0.31 | 0.03 | 0.096 | ||||||||||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malecki, K.M.C.; Schultz, A.A.; Bergmans, R.S. Neighborhood Perceptions and Cumulative Impacts of Low Level Chronic Exposure to Fine Particular Matter (PM2.5) on Cardiopulmonary Health. Int. J. Environ. Res. Public Health 2018, 15, 84. https://doi.org/10.3390/ijerph15010084
Malecki KMC, Schultz AA, Bergmans RS. Neighborhood Perceptions and Cumulative Impacts of Low Level Chronic Exposure to Fine Particular Matter (PM2.5) on Cardiopulmonary Health. International Journal of Environmental Research and Public Health. 2018; 15(1):84. https://doi.org/10.3390/ijerph15010084
Chicago/Turabian StyleMalecki, Kristen M. C., Amy A. Schultz, and Rachel S. Bergmans. 2018. "Neighborhood Perceptions and Cumulative Impacts of Low Level Chronic Exposure to Fine Particular Matter (PM2.5) on Cardiopulmonary Health" International Journal of Environmental Research and Public Health 15, no. 1: 84. https://doi.org/10.3390/ijerph15010084





