Vertical Geochemical Variations and Speciation Studies of As, Fe, Mn, Zn, and Cu in the Sediments of the Central Gangetic Basin: Sequential Extraction and Statistical Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Area Description
2.2. Geomorphology and Hydrogeology of the Study Area
2.3. Sample Collection and Preparation
2.4. Chemical and Reagents
2.5. Sediment Sample Digestion and Sequential Extraction Procedure
2.6. Instrumentation and Analysis
2.7. Quality Assurance and Quality Control of the Analysis
2.8. Statistical Work
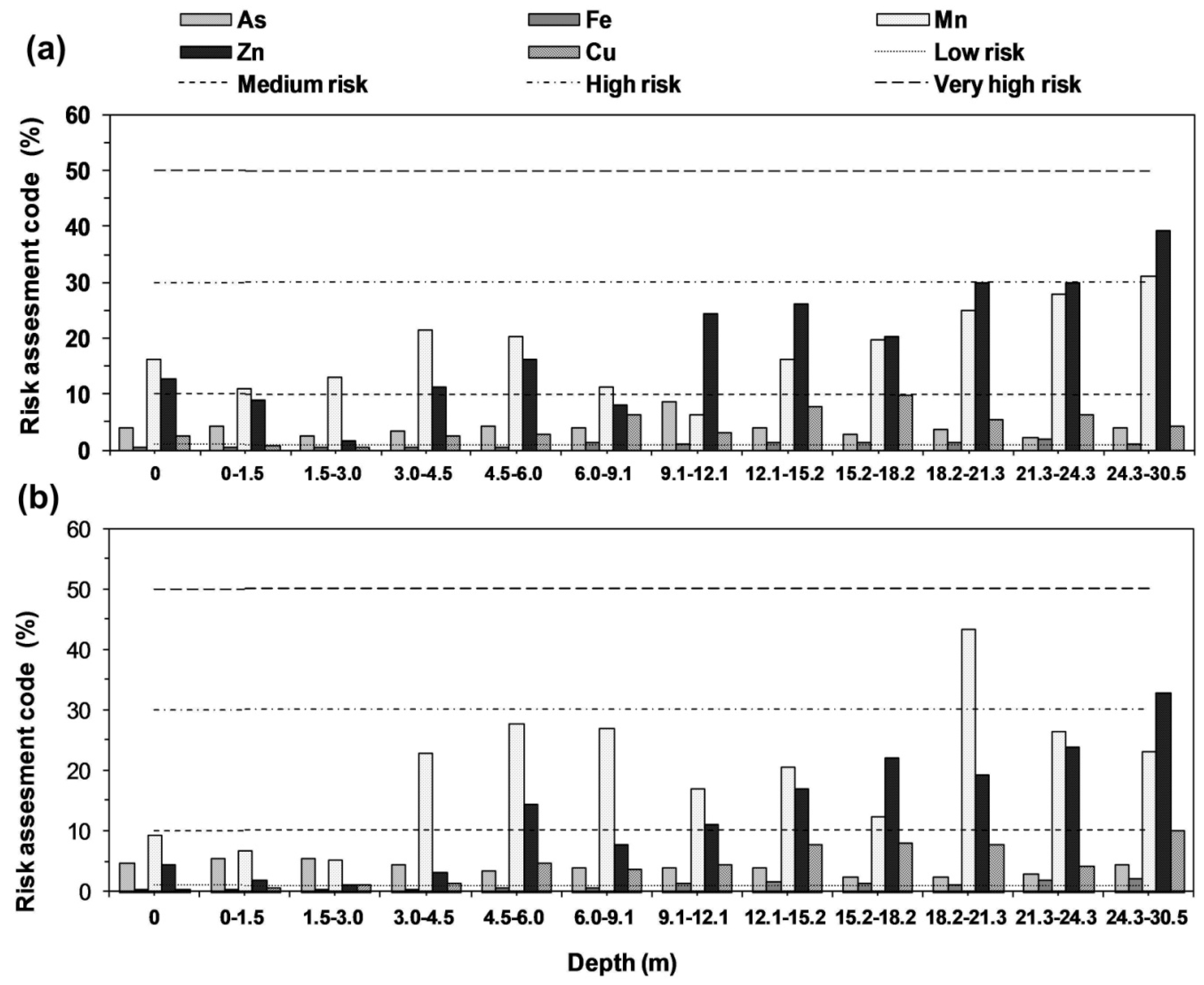
2.8.1. Risk Assessment Code (RAC)
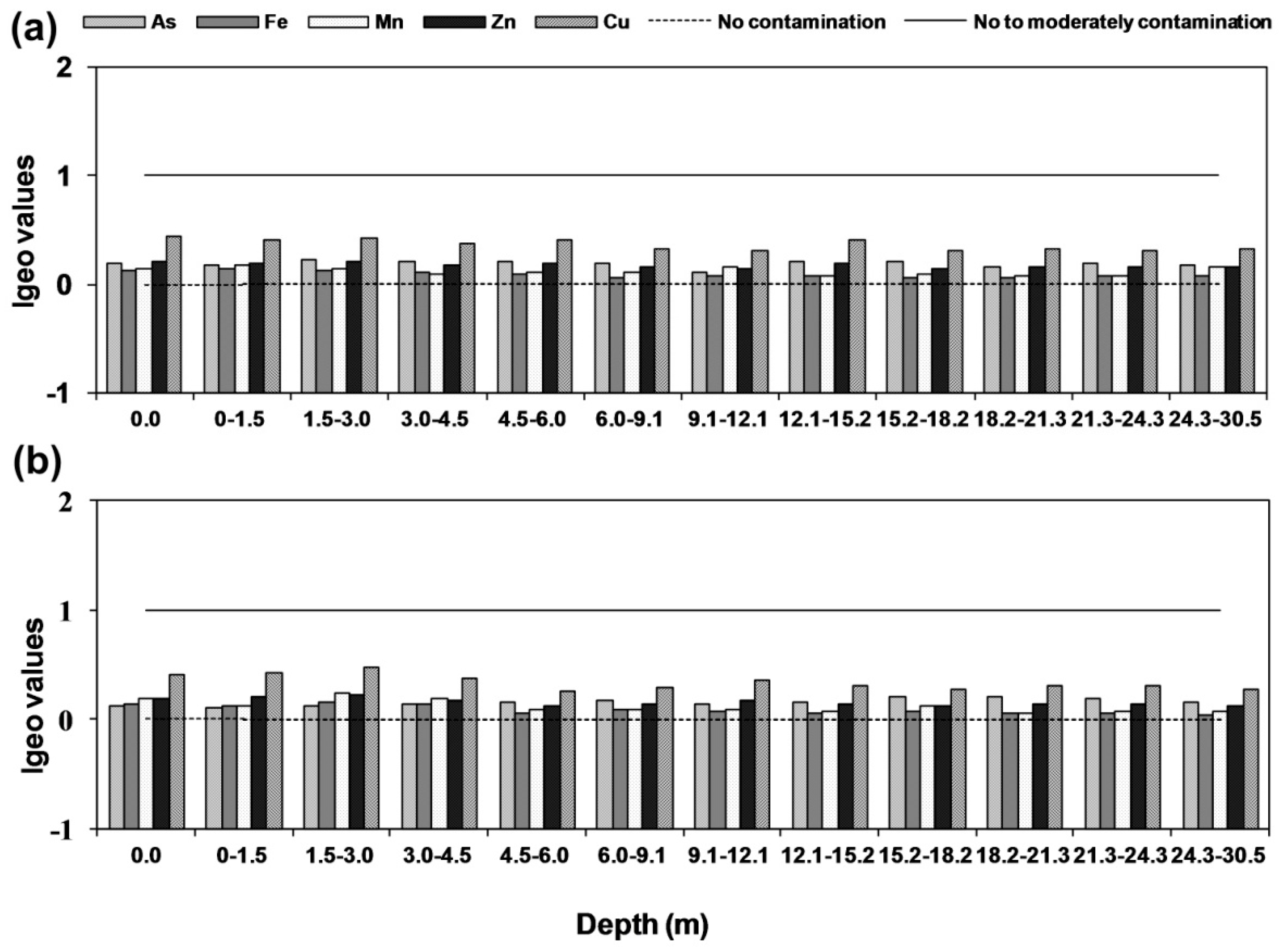
2.8.2. Geo-Accumulation Index (Igeo)
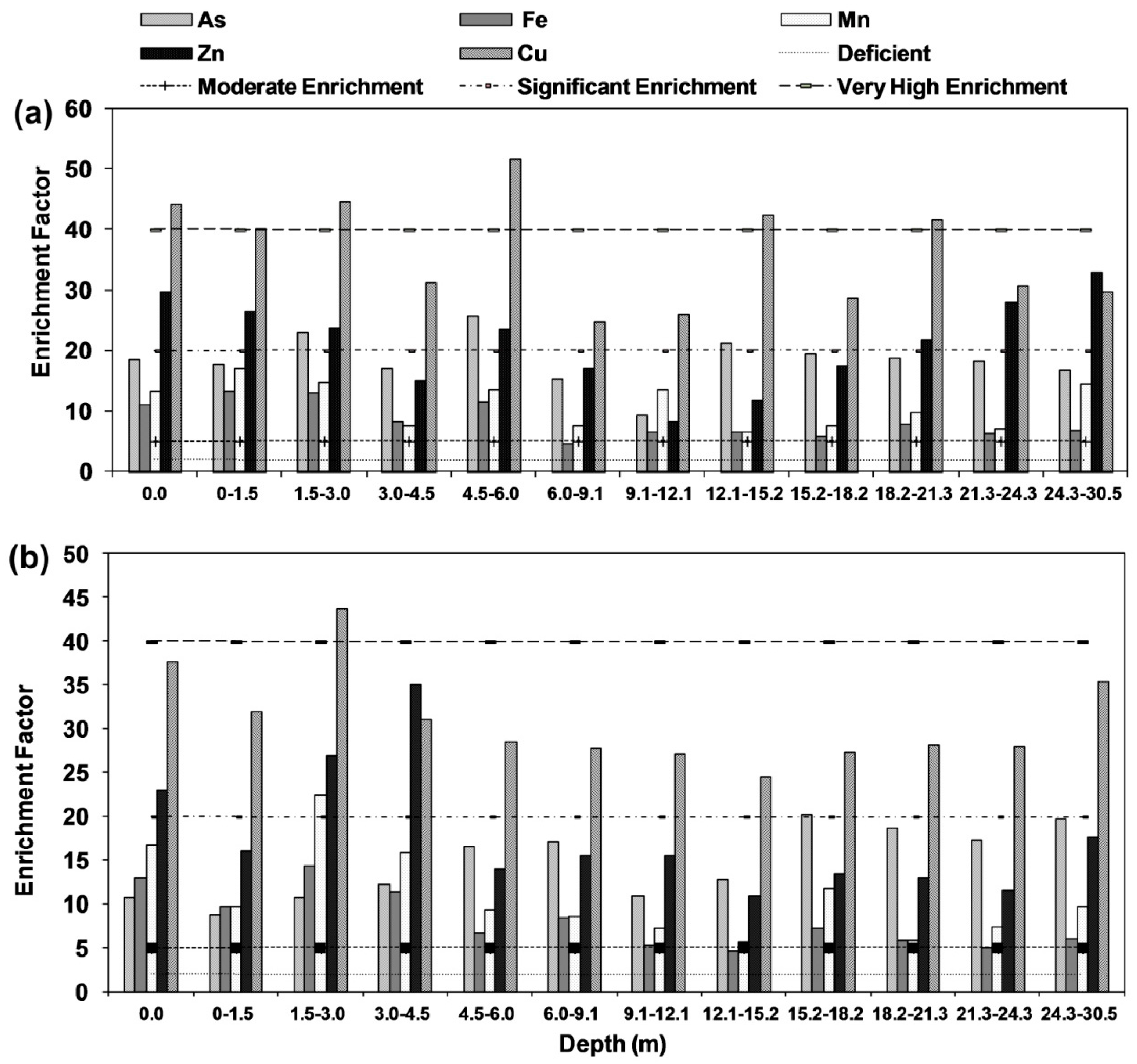
2.8.3. Enrichment Factor (EF)
3. Results and Discussion
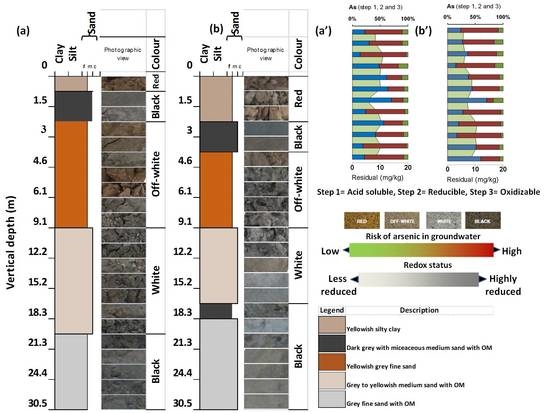
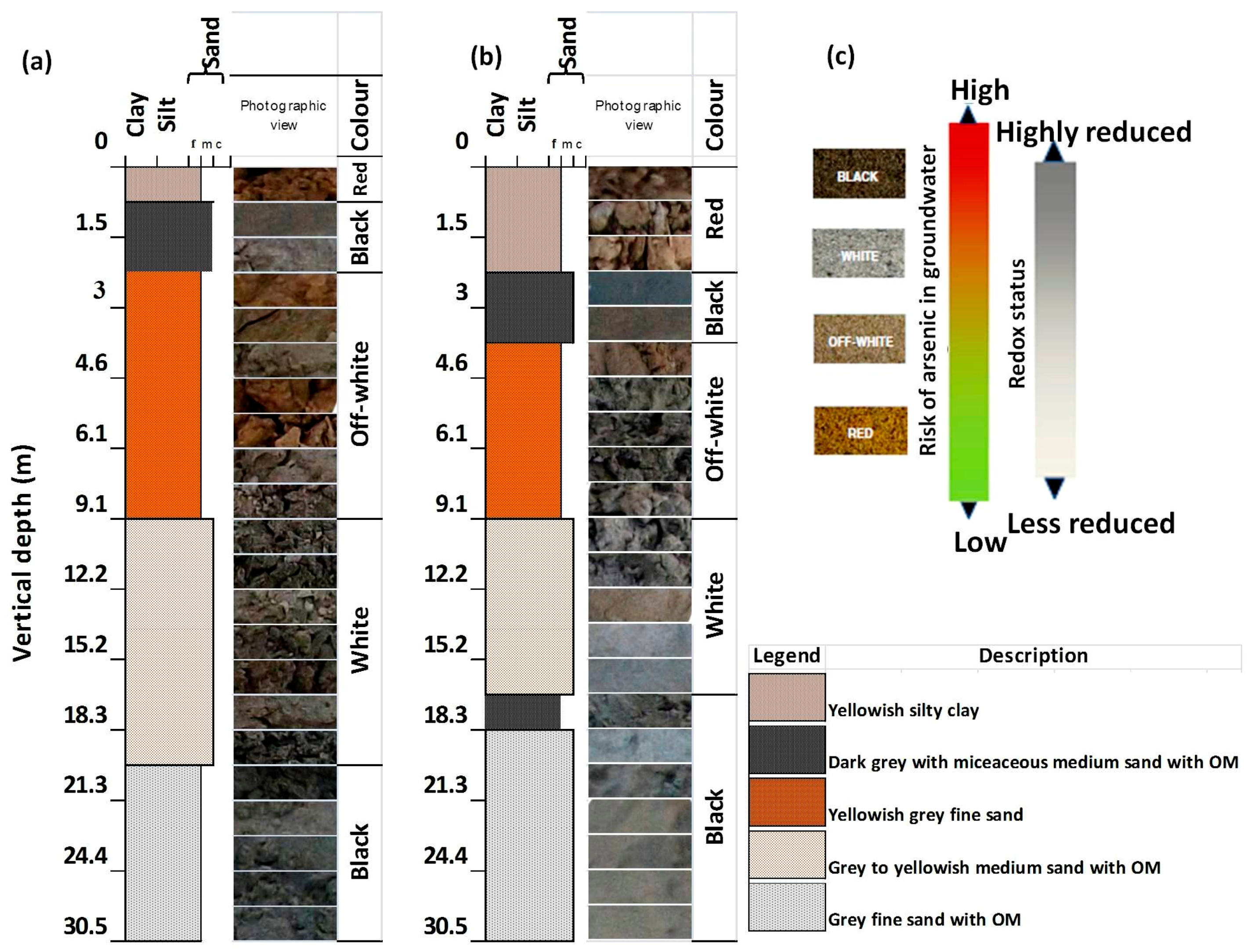
3.1. The Grain Size of the Core Sediments and Lithographs Reconstructions
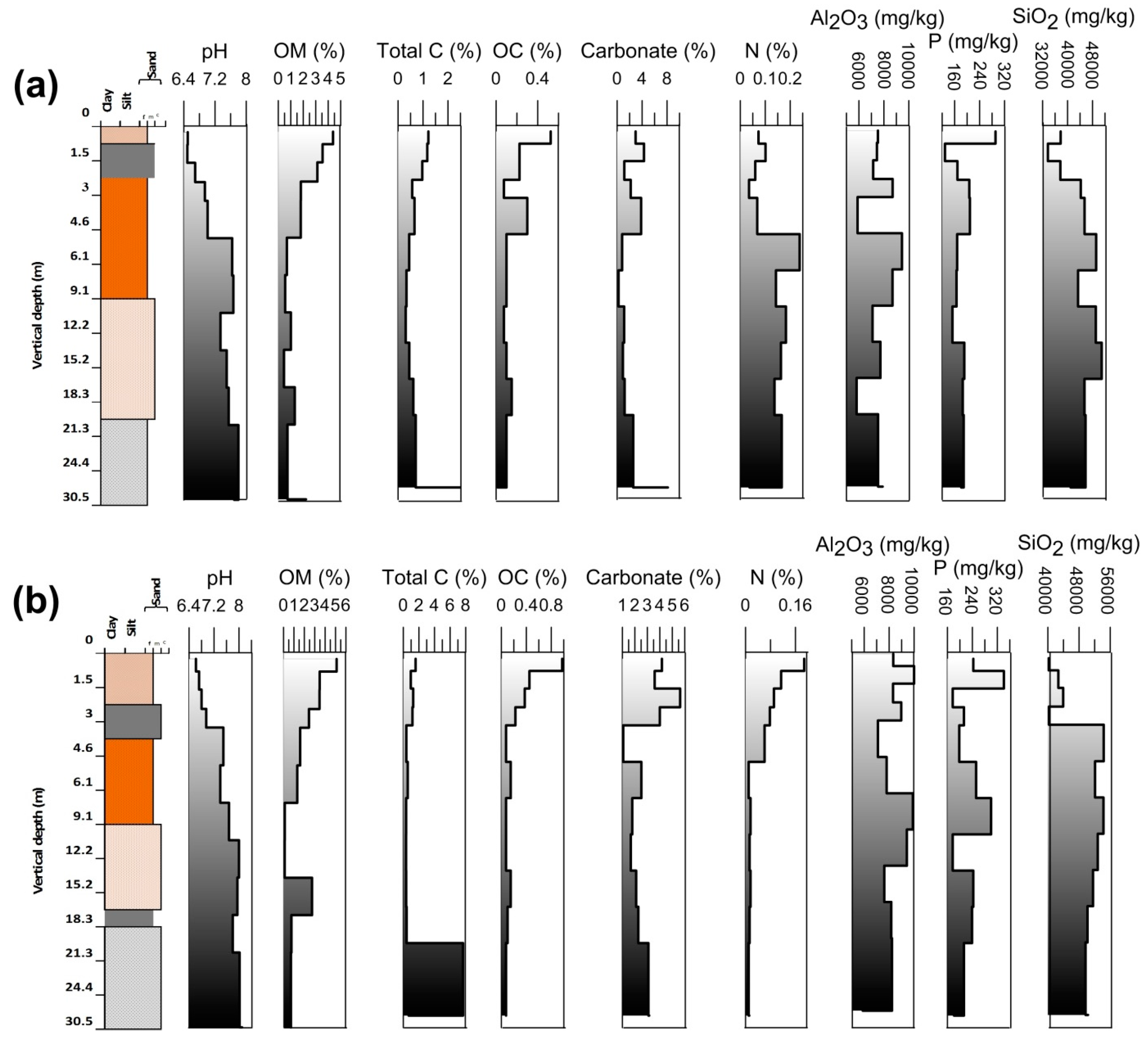
3.2. Physical Characteristics of the Vertically Subsurface Sediments
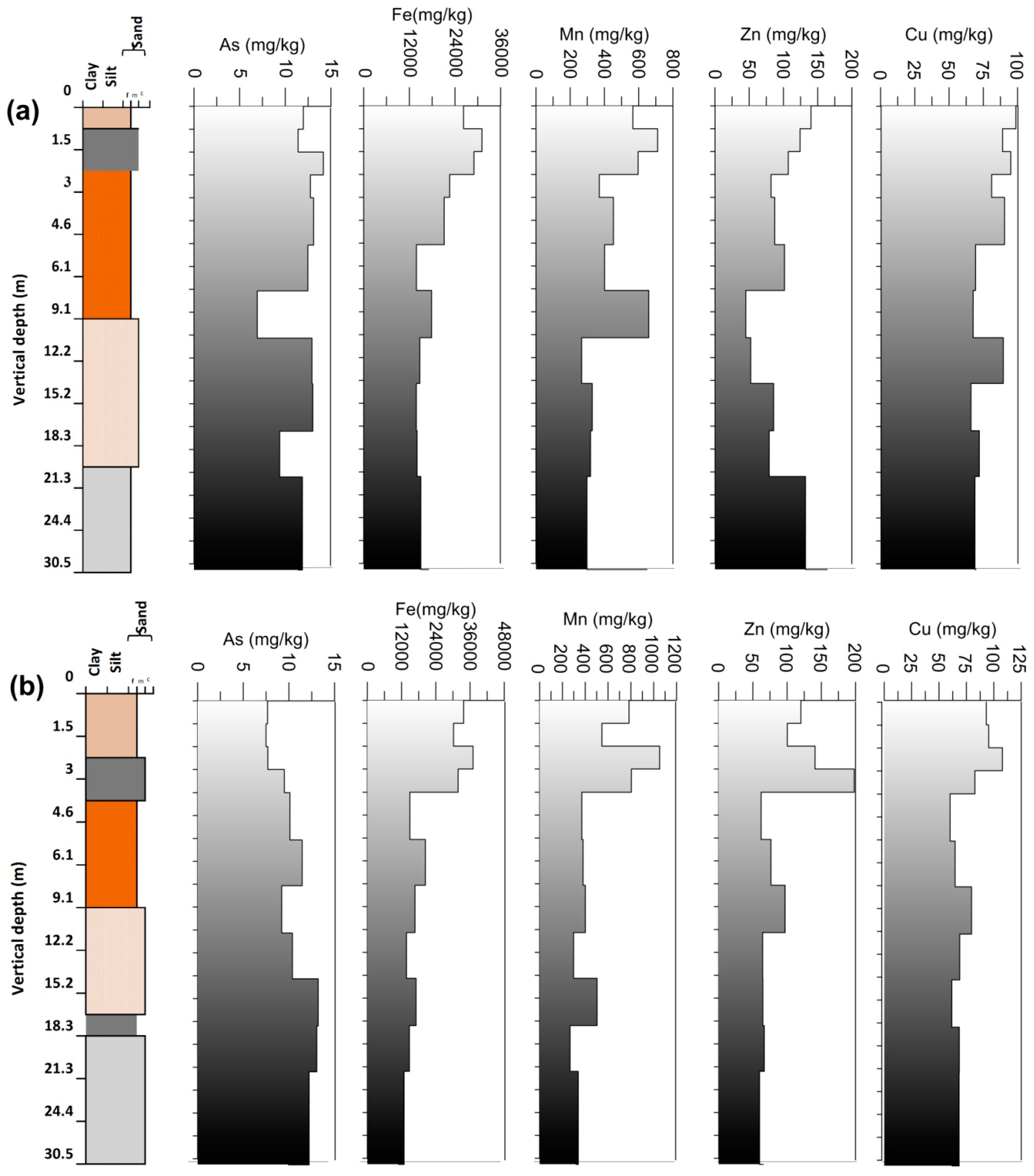
3.3. Arsenic and Other Elements in Vertical Profile
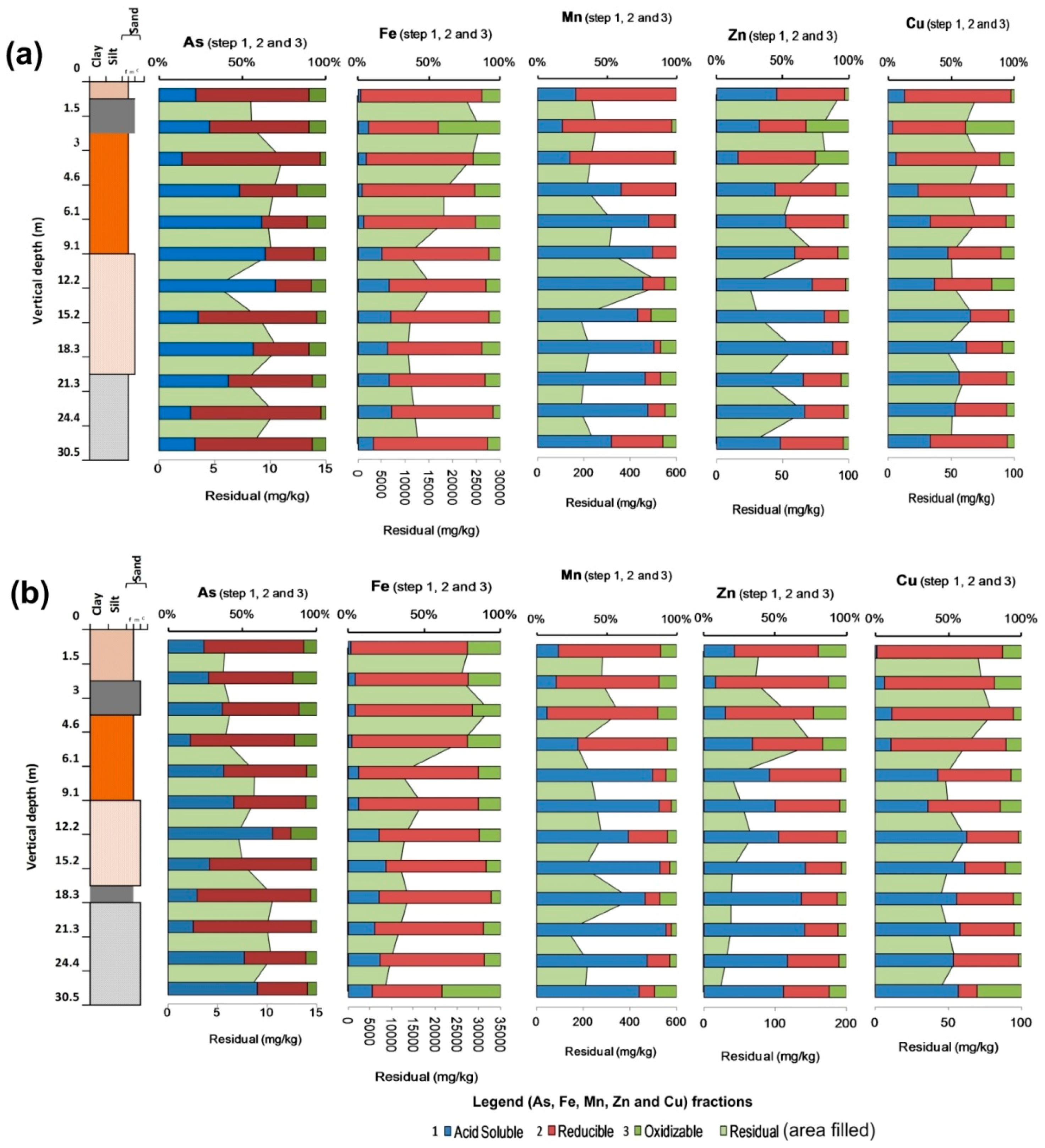
3.4. Arsenic and Other Elements (Cu, Fe, Mn, Zn) Speciation Studies in Vertical Sediments
3.5. Risk Assessment Code (RAC)
3.6. Geo-Accumulation Index (Igeo)
3.7. Enrichment Factor (EF)
3.8. Correlation Matrix
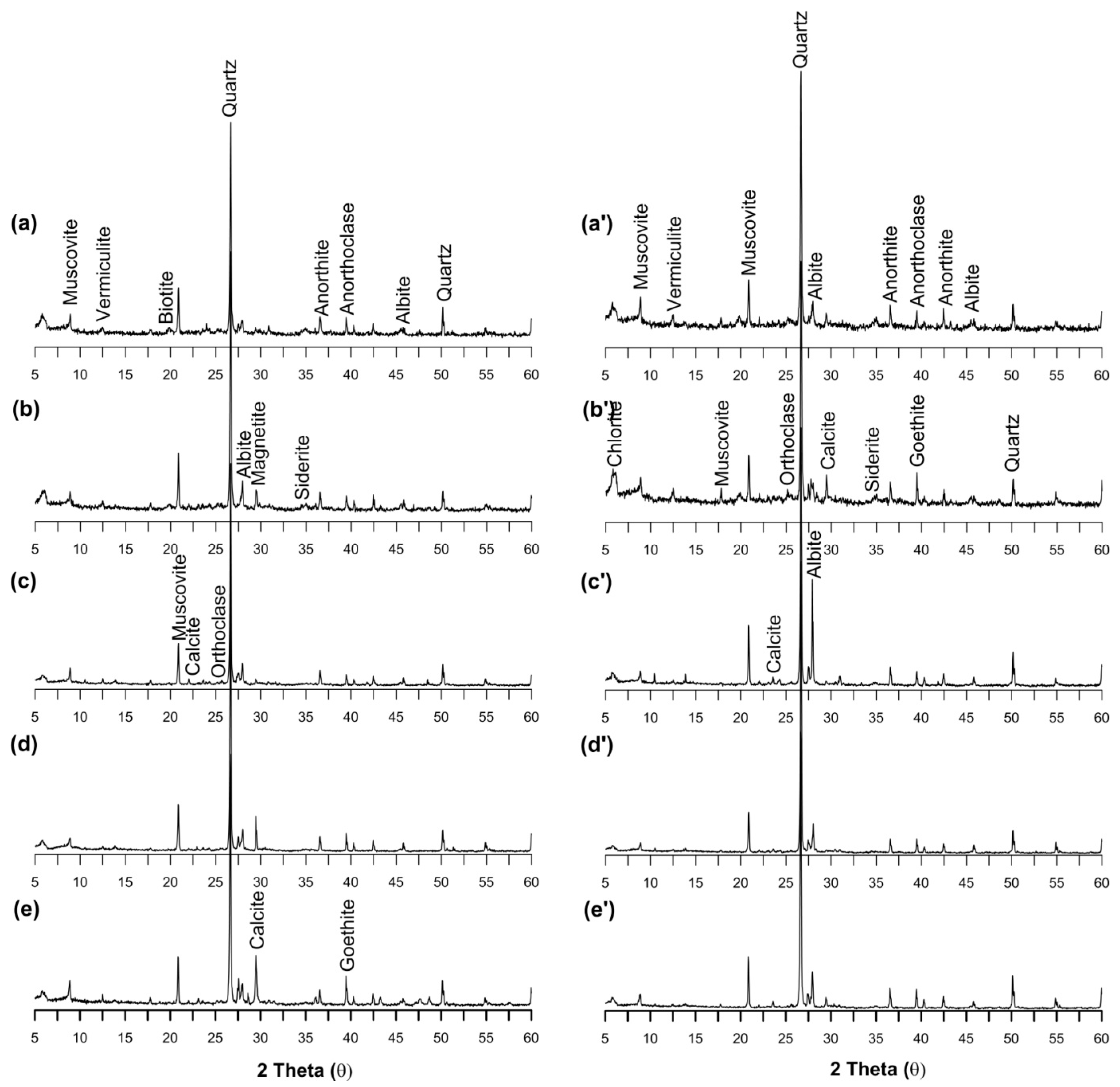
3.9. Mineralogical Evidence of As and Other Elemental Occurrence and Compositions
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bacon, J.R.; Davidson, C.M. Is there a future for sequential chemical extraction? Analyst 2008, 133, 25–46. [Google Scholar] [CrossRef] [PubMed]
- Scokart, P.; Meeus-Verdinne, K.; Borger, R.D. Speciation of heavy metals in polluted soils by sequential extraction and ICP spectrometry. Int. J. Environ. Anal. Chem. 1987, 29, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.; Page, A.; Warneke, J.; Grgurevic, E. Sequential extraction of soil heavy metals following a sludge application. J. Environ. Qual. 1984, 13, 33–38. [Google Scholar] [CrossRef]
- Tessier, A.; Campbell, P.G.; Bisson, M. Sequential extraction procedure for the speciation of particulate trace metals. Anal. Chem. 1979, 51, 844–851. [Google Scholar] [CrossRef]
- Abdel-Satar, A.M.; Goher, M.E. Heavy metals fractionation and risk assessment in surface sediments of Qarun and Wadi El-Rayan Lakes, Egypt. Environ. Monit. Assess. 2015, 187, 346. [Google Scholar] [CrossRef] [PubMed]
- Cappuyns, V.; Swennen, R.; Niclaes, M. Application of the BCR sequential extraction scheme to dredged pond sediments contaminated by Pb-Zn mining: A combined geochemical and mineralogical approach. J. Geochem. Explor. 2007, 93, 78–90. [Google Scholar] [CrossRef]
- Kumar, M.; Ramanathan, A.; Tripathi, R.; Farswan, S.; Kumar, D.; Bhattacharya, P. A study of trace element contamination using multivariate statistical techniques and health risk assessment in groundwater of Chhaprola industrial area, Gautam Buddha Nagar, Uttar Pradesh, India. Chemosphere 2017, 166, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Ramanathan, A.; Rahman, M.M.; Naidu, R. Concentrations of inorganic arsenic in groundwater, agricultural soils and subsurface sediments from the middle Gangetic plain of Bihar, India. Sci. Total Environ. 2016, 573, 1103–1114. [Google Scholar] [CrossRef] [PubMed]
- Giacomino, A.; Malandrino, M.; Abollino, O.; Velayutham, M.; Chinnathangavel, T.; Mentasti, E. An approach for arsenic in a contaminated soil: Speciation, fractionation, extraction and effluent decontamination. Environ. Pollut. 2010, 158, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Lock, K.; Janssen, C. The effect of ageing on the toxicity of zinc for the potworm Enchytraeus albidus. Environ. Pollut. 2002, 116, 289–292. [Google Scholar] [CrossRef]
- Rauret, G.; López-Sánchez, J.; Sahuquillo, A.; Rubio, R.; Davidson, C.; Ure, A.; Quevauviller, P. Improvement of the BCR three step sequential extraction procedure prior to the certification of new sediment and soil reference materials. J. Environ. Monit. 1999, 1, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Pardo, R.; Helena, B.; Cazurro, C.; Guerra, C.; Deban, L.; Guerra, C.; Vega, M. Application of two-and three-way principal component analysis to the interpretation of chemical fractionation results obtained by the use of the BCR procedure. Anal. Chim. Acta 2004, 523, 125–132. [Google Scholar] [CrossRef]
- Biswas, A.; Nath, B.; Bhattacharya, P.; Halder, D.; Kundu, A.K.; Mandal, U.; Mukherjee, A.; Chatterjee, D.; Mörth, C.-M.; Jacks, G. Hydrogeochemical contrast between brown and grey sand aquifers in shallow depth of Bengal basin: Consequences for sustainable drinking water supply. Sci. Total Environ. 2012, 431, 402–412. [Google Scholar] [CrossRef] [PubMed]
- Bundschuh, J.; Litter, M.I.; Bhattacharya, P. Targeting arsenic-safe aquifers for drinking water supplies. Environ. Geochem. Health 2010, 32, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Von Brömssen, M.; Jakariya, M.; Bhattacharya, P.; Ahmed, K.M.; Hasan, M.A.; Sracek, O.; Jonsson, L.; Lundell, L.; Jacks, G. Targeting low-arsenic aquifers in Matlab Upazila, Southeastern Bangladesh. Sci. Total Environ. 2007, 379, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Von Brömssen, M.; Larsson, S.H.; Bhattacharya, P.; Hasan, M.A.; Ahmed, K.M.; Jakariya, M.; Sikder, M.A.; Sracek, O.; Bivén, A.; Doušová, B. Geochemical characterisation of shallow aquifer sediments of Matlab Upazila, southeastern Bangladesh—Implications for targeting low-as aquifers. J. Contam. Hydrol. 2008, 99, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.; Bhattacharya, P.; Frape, S.K.; Jacks, G.; Islam, M.M.; Rahman, M.M.; von Brömssen, M.; Hasan, M.A.; Ahmed, K.M. Sediment color tool for targeting arsenic-safe aquifers for the installation of shallow drinking water tubewells. Sci. Total Environ. 2014, 493, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Robinson, C.; Von Broemssen, M.; Bhattacharya, P.; Häller, S.; Bivén, A.; Hossain, M.; Jacks, G.; Ahmed, K.M.; Hasan, M.A.; Thunvik, R. Dynamics of arsenic adsorption in the targeted arsenic-safe aquifers in Matlab, south-eastern Bangladesh: Insight from experimental studies. Appl. Geochem. 2011, 26, 624–635. [Google Scholar] [CrossRef]
- Kumar, M.; Kumar, P.; Ramanathan, A.L.; Bhattacharya, P.; Thunvik, R.; Singh, U.K.; Tsujimura, M.; Sracek, O. Arsenic enrichment in groundwater in the middle Gangetic plain of Ghazipur district in Uttar Pradesh, India. J. Geochem. Explor. 2010, 105, 83–94. [Google Scholar] [CrossRef]
- Kumar, P.; Kumar, M.; Ramanathan, AL.; Tsujimura, M. Tracing the factors responsible for arsenic enrichment in groundwater of the middle Gangetic plain, India: A source identification perspective. Environ. Geochem. Health 2010, 32, 129–146. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Ramanathan, A.; Prabha, S.; Ranjan, R.K.; Ranjan, S.; Singh, G. Metal speciation studies in the aquifer sediments of Semria Ojhapatti, Bhojpur district, Bihar. Environ. Monit. Assess. 2012, 184, 3027–3042. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Sharma, Y. Arsenic occurrence and accumulation in soil and water of eastern districts of Uttar Pradesh, India. Environ. Monit. Assess. 2013, 185, 4995–5002. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Rahman, M.M.; Ramanathan, A.; Naidu, R. Arsenic and other elements in drinking water and dietary components from the middle Gangetic plain of Bihar, India: Health risk index. Sci. Total Environ. 2016, 539, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Kumar, M.; Kumar, A.; Singh, V.B.; Kumar, S.; Ramanathan, A.; Bhattacharya, P. Arsenic distribution and mobilization: A case study of three districts of Uuttar Pradesh and Bihar (India). In Safe and Sustainable Use of Arsenic-Contaminated Aquifers in the Gangetic Plain; Springer: Berlin, Germany, 2015; pp. 111–123. [Google Scholar]
- Chauhan, V.S.; Nickson, R.T.; Chauhan, D.; Iyengar, L.; Sankararamakrishnan, N. Ground water geochemistry of Ballia district, Uttar Pradesh, India and mechanism of arsenic release. Chemosphere 2009, 75, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Saha, D.; Shukla, R.R. Genesis of arsenic-rich groundwater and the search for alternative safe aquifers in the Gangetic plain, India. Water Environ. Res. 2013, 85, 2254–2264. [Google Scholar] [CrossRef] [PubMed]
- Saha, D.; Sahu, S.; Chandra, P.C. Arsenic-safe alternate aquifers and their hydraulic characteristics in contaminated areas of middle Ganga plain, eastern India. Environ. Monit. Assess. 2010, 175, 331–348. [Google Scholar] [CrossRef] [PubMed]
- Heroy, D.C.; Kuehl, S.A.; Goodbred, S.L. Mineralogy of the Ganges and Brahmaputra rivers: Implications for river switching and late quaternary climate change. Sediment. Geol. 2003, 155, 343–359. [Google Scholar] [CrossRef]
- Acharyya, S.K.; Shah, B.A. Groundwater arsenic contamination affecting different geologic domains in India—A review: Influence of geological setting, fluvial geomorphology and quaternary stratigraphy. J. Environ. Sci. Health Part A 2007, 42, 1795–1805. [Google Scholar] [CrossRef] [PubMed]
- Ahamed, S.; Sengupta, M.K.; Mukherjee, A.; Hossain, M.A.; Das, B.; Nayak, B.; Pal, A.; Mukherjee, S.C.; Pati, S.; Dutta, R.N. Arsenic groundwater contamination and its health effects in the state of Uttar Pradesh (up) in upper and middle Ganga plain, India: A severe danger. Sci. Total Environ. 2006, 370, 310–322. [Google Scholar] [CrossRef] [PubMed]
- Chakraborti, D.; Rahman, M.M.; Das, B.; Chatterjee, A.; Das, D.; Nayak, B.; Pal, A.; Chowdhury, U.K.; Ahmed, S.; Biswas, B.K. Groundwater arsenic contamination and its health effects in India. Hydrogeol. J. 2017, 25, 1165–1187. [Google Scholar] [CrossRef]
- Shapiro, L. Rapid Analysis of Silicate, Carbonate, and Phosphate Rocks-Revised Edition; US Geological Survey: Leston, VA, USA, 1975.
- Berglund, B.E. Handbook of Holocene Palaeoecology and Palaeohydrology; John Wiley & Sons Ltd.: New York, NY, USA, 1987. [Google Scholar]
- Dean, W.E., Jr. Determination of carbonate and organic matter in calcareous sediments and sedimentary rocks by loss on ignition: Comparison with other methods. J. Sediment. Res. 1974, 44, 242–246. [Google Scholar]
- Walkley, A.; Black, I.A. An examination of the degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Perin, G.; Craboledda, L.; Lucchese, M.; Cirillo, R.; Dotta, L.; Zanette, M.; Orio, A. Heavy metal speciation in the sediments of northern Adriatic Sea. A new approach for environmental toxicity determination. Heavy Metals Environ. 1985, 2, 454–456. [Google Scholar]
- Muller, G. Schwermetalle in den sedimenten des rheins-veranderungen seit 1971. Umschau 1979, 79, 778–783. [Google Scholar]
- Xie, X.; Wang, Y.; Su, C.; Liu, H.; Duan, M.; Xie, Z. Arsenic mobilization in shallow aquifers of Datong Basin: Hydrochemical and mineralogical evidences. J. Geochem. Explor. 2008, 98, 107–115. [Google Scholar] [CrossRef]
- Turekian, K.K.; Wedepohl, K.H. Distribution of the elements in some major units of the earth’s crust. Geol. Soc. Am. Bull. 1961, 72, 175–192. [Google Scholar] [CrossRef]
- Buat-Menard, P.; Chesselet, R. Variable influence of the atmospheric flux on the trace metal chemistry of oceanic suspended matter. Earth Planet. Sci. Lett. 1979, 42, 399–411. [Google Scholar] [CrossRef]
- Sutherland, R. Bed sediment-associated trace metals in an urban stream, Oahu, Hawaii. Environ. Geol. 2000, 39, 611–627. [Google Scholar] [CrossRef]
- Lee, D.; Garland, J.; Fox, A. Atmospheric concentrations of trace elements in urban areas of the United Kingdom. Atmos. Environ. 1994, 28, 2691–2713. [Google Scholar] [CrossRef]
- Hasan, M.A.; von Brömssen, M.; Bhattacharya, P.; Ahmed, K.M.; Sikder, A.M.; Jacks, G.; Sracek, O. Geochemistry and mineralogy of shallow alluvial aquifers in Daudkandi Upazila in the Meghna flood plain, Bangladesh. Environ. Geol. 2009, 57, 499–511. [Google Scholar] [CrossRef]
- Kettler, T.; Doran, J.W.; Gilbert, T. Simplified method for soil particle-size determination to accompany soil-quality analyses. Soil Sci. Soc. Am. J. 2001, 65, 849–852. [Google Scholar] [CrossRef]
- Calmano, W.; Hong, J.; Förstner, U. Binding and mobilization of heavy metals in contaminated sediments affected by ph and redox potential. Water Sci. Technol. 1993, 28, 223–235. [Google Scholar]
- Harvey, C.F.; Swartz, C.H.; Badruzzaman, A.B.M.; Keon-Blute, N.; Yu, W.; Ali, M.A.; Jay, J.; Beckie, R.; Niedan, V.; Brabander, D. Groundwater arsenic contamination on the ganges delta: Biogeochemistry, hydrology, human perturbations, and human suffering on a large scale. C. R. Geosci. 2005, 337, 285–296. [Google Scholar] [CrossRef]
- Smedley, P.; Kinniburgh, D. A review of the source, behaviour and distribution of arsenic in natural waters. Appl. Geochem. 2002, 17, 517–568. [Google Scholar] [CrossRef]
- Patel, K.S.; Shrivas, K.; Brandt, R.; Jakubowski, N.; Corns, W.; Hoffmann, P. Arsenic contamination in water, soil, sediment and rice of central India. Environ. Geochem. Health 2005, 27, 131–145. [Google Scholar] [CrossRef] [PubMed]
- Koretsky, C.M.; Haas, J.R.; Ndenga, N.T.; Miller, D. Seasonal variations in vertical redox stratification and potential influence on trace metal speciation in minerotrophic peat sediments. Water Air Soil Pollut. 2006, 173, 373–403. [Google Scholar] [CrossRef]
- Nickson, R.; McArthur, J.; Ravenscroft, P.; Burgess, W.; Ahmed, K. Mechanism of arsenic release to groundwater, Bangladesh and West Bengal. Appl. Geochem. 2000, 15, 403–413. [Google Scholar] [CrossRef]
- Bostick, B.; Hansel, C.; La Force, M.; Fendorf, S. Seasonal fluctuations in zinc speciation within a contaminated wetland. Environ. Sci. Technol. 2001, 35, 3823–3829. [Google Scholar] [CrossRef] [PubMed]
- McLean, J.E.; Bledsoe, B.E. Behavior of metals in soils. In EPA Environmental Assessment Sourcebook; CRC Press: Boca Raton, FL, USA, 1992; pp. 19–56. [Google Scholar]
- Achterberg, E.P.; Van Den Berg, C.M.; Boussemart, M.; Davison, W. Speciation and cycling of trace metals in esthwaite water: A productive English lake with seasonal deep-water anoxia. Geochim. Cosmochim. Acta 1997, 61, 5233–5253. [Google Scholar] [CrossRef]
- Loganathan, P.; Isirimah, N.; Nwachuku, D. Phosphorus sorption by ultisols and inceptisols of the Niger delta in Southern Nigeria. Soil Sci. 1987, 144, 330–338. [Google Scholar] [CrossRef]
- Morse, J.W. The surface chemistry of calcium carbonate minerals in natural waters: An overview. Mar. Chem. 1986, 20, 91–112. [Google Scholar] [CrossRef]
- Benjamin, M.M.; Leckie, J.O. Multiple-site adsorption of Cd, Cu, Zn, and Pb on amorphous iron oxyhydroxide. J. Colloid Interface Sci. 1981, 79, 209–221. [Google Scholar] [CrossRef]
- El Bilali, L.; Rasmussen, P.; Hall, G.; Fortin, D. Role of sediment composition in trace metal distribution in lake sediments. Appl. Geochem. 2002, 17, 1171–1181. [Google Scholar] [CrossRef]
- Butcher, J.N.; Williams, C.; Graham, J.R.; Archer, R.; Tellegen, A.; Ben-Porath, Y.; Kaemmer, B. Minnesota Multiphasic Personality Inventory-Adolescent; University of Minnesota Press: Minneapolis, MN, USA, 1992. [Google Scholar]
- Balistrieri, L.S.; Murray, J.W.; Paul, B. The cycling of iron and manganese in the water column of lake Sammamish, Washington. Limnol. Oceanogr. 1992, 37, 510–528. [Google Scholar] [CrossRef]
- Rajmohan, N.; Prathapar, S.; Jayaprakash, M.; Nagarajan, R. Vertical distribution of heavy metals in soil profile in a seasonally waterlogging agriculture field in eastern Ganges Basin. Environ. Monit. Assess. 2014, 186, 5411–5427. [Google Scholar] [CrossRef] [PubMed]
- Massolo, S.; Bignasca, A.; Sarkar, S.K.; Chatterjee, M.; Bhattacharya, B.D.; Alam, A. Geochemical fractionation of trace elements in sediments of Hugli River (Ganges) and Sundarban wetland (West Bengal, India). Environ. Monit. Assess. 2012, 184, 7561–7577. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zang, S.; Xiao, H.; Wu, C. Speciation and distribution characteristics of heavy metals and pollution assessments in the sediments of Nashina Lake, Heilongjiang, China. Ecotoxicology 2014, 23, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Chakrapani, V.; Angus, J.C.; Anderson, A.B.; Wolter, S.D.; Stoner, B.R.; Sumanasekera, G.U. Charge transfer equilibria between diamond and an aqueous oxygen electrochemical redox couple. Science 2007, 318, 1424–1430. [Google Scholar] [CrossRef] [PubMed]
- Jain, C.; Malik, D.; Yadav, R. Metal fractionation study on bed sediments of lake Nainital, Uttaranchal, India. Environ. Monit. Assess. 2007, 130, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Kumar, M.; Ramanathan, A. Assessment of heavy metal contamination in the surface sediments in the mangrove ecosystem of Gulf of Kachchh, West coast of India. Environ. Earth Sci. 2015, 74, 545–556. [Google Scholar] [CrossRef]
- Winkel, L.; Berg, M.; Amini, M.; Hug, S.J.; Johnson, C.A. Predicting groundwater arsenic contamination in southeast Asia from surface parameters. Nat. Geosci. 2008, 1, 536–542. [Google Scholar] [CrossRef]
- Ahmed, K.M.; Bhattacharya, P.; Hasan, M.A.; Akhter, S.H.; Alam, S.M.; Bhuyian, M.H.; Imam, M.B.; Khan, A.A.; Sracek, O. Arsenic enrichment in groundwater of the alluvial aquifers in Bangladesh: An overview. Appl. Geochem. 2004, 19, 181–200. [Google Scholar] [CrossRef]
- Islam, F.S.; Gault, A.G.; Boothman, C.; Polya, D.A.; Charnock, J.M.; Chatterjee, D.; Lloyd, J.R. Role of metal-reducing bacteria in arsenic release from Bengal Delta sediments. Nature 2004, 430, 68–71. [Google Scholar] [CrossRef] [PubMed]
- Mumford, A.C.; Barringer, J.L.; Benzel, W.M.; Reilly, P.A.; Young, L. Microbial transformations of arsenic: Mobilization from glauconitic sediments to water. Water Res. 2012, 46, 2859–2868. [Google Scholar] [CrossRef] [PubMed]

| Depth (m) | Rigni Chhapra | Chaube Chhapra | ||||
|---|---|---|---|---|---|---|
| Sand | Silt | Clay | Sand | Silt | Clay | |
| 0.0 | 21.71 | 65.13 | 13.16 | 7.33 | 64.00 | 28.67 |
| 1.5 | 16.67 | 54.00 | 29.33 | 22.67 | 56.67 | 20.67 |
| 3.0 | 7.33 | 64.00 | 28.67 | 2.65 | 56.29 | 41.06 |
| 4.5 | 66.67 | 28.00 | 5.33 | 13.33 | 66.00 | 20.67 |
| 6.0 | 84.00 | 15.33 | 0.67 | 82.67 | 14.67 | 2.67 |
| 9.1 | 93.42 | 5.92 | 0.66 | 78.81 | 15.23 | 5.96 |
| 12.1 | 88.08 | 4.64 | 7.28 | 86.67 | 6.67 | 6.67 |
| 15.2 | 95.45 | 3.25 | 1.30 | 96.67 | 2.67 | 0.67 |
| 18.2 | 93.20 | 0.67 | 6.14 | 81.33 | 10.67 | 8.00 |
| 21.3 | 95.14 | 2.66 | 2.20 | 91.33 | 4.67 | 4.00 |
| 24.4 | 96.00 | 2.67 | 1.33 | 85.33 | 2.67 | 12.00 |
| 30.5 | 80.00 | 3.33 | 16.67 | 97.33 | 1.33 | 1.33 |
| Depth (m) | Rigni Chhapra | Chaube Chhapra | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| As | Fe | Mn | Zn | Cu | As | Fe | Mn | Zn | Cu | |
| 0.0 | 11.98 | 26,248 | 566 | 140.2 | 98.8 | 7.65 | 33,777 | 783 | 120.2 | 93.1 |
| 1.5 | 11.40 | 31,088 | 711 | 124.2 | 89.1 | 7.50 | 30,216 | 546 | 100.3 | 95.3 |
| 3.0 | 14.17 | 28,996 | 597 | 107.1 | 95.1 | 7.69 | 37,052 | 1052 | 140.9 | 107.9 |
| 4.5 | 12.76 | 22,617 | 370 | 82.0 | 81.1 | 9.50 | 31,819 | 804 | 198.0 | 82.8 |
| 6.0 | 13.10 | 21,173 | 453 | 87.3 | 90.5 | 10.11 | 14,909 | 370 | 62.1 | 60.1 |
| 9.1 | 12.47 | 13,871 | 401 | 101.6 | 69.4 | 11.45 | 20,354 | 381 | 76.5 | 64.7 |
| 12.1 | 6.94 | 17,854 | 659 | 45.0 | 67.6 | 9.21 | 16,706 | 401 | 97.1 | 79.7 |
| 15.2 | 12.92 | 14,756 | 267 | 52.2 | 89.6 | 10.38 | 13,731 | 298 | 64.3 | 68.9 |
| 18.2 | 13.02 | 13,849 | 329 | 85.7 | 66.0 | 13.21 | 17,079 | 504 | 64.6 | 61.7 |
| 21.3 | 9.39 | 14,040 | 319 | 79.2 | 72.1 | 13.05 | 14,740 | 267 | 66.7 | 68.5 |
| 24.3 | 11.88 | 15,017 | 298 | 132.2 | 68.9 | 12.22 | 12,878 | 339 | 59.8 | 68.3 |
| 35.5 | 11.41 | 17,051 | 649 | 163.8 | 69.7 | 9.94 | 10,936 | 319 | 65.1 | 61.9 |
| Variable | Fe | Mn | Zn | Cu | As | OC | Carbonate | Phosphate | Sand | Silt | Clay |
|---|---|---|---|---|---|---|---|---|---|---|---|
| (a) | |||||||||||
| Fe | 1 | ||||||||||
| Mn | 0.67 * | 1 | |||||||||
| Zn | 0.31 | 0.37 | 1 | ||||||||
| Cu | 0.76 ** | 0.25 | 0.10 | 1 | |||||||
| As | 0.23 | −0.29 | 0.27 | 0.46 | 1 | ||||||
| OC | 0.87 ** | 0.60 * | 0.56 | 0.78 ** | 0.24 | 1 | |||||
| Carbonate | 0.23 | 0.44 | 0.75 ** | 0.09 | 0.11 | 0.43 | 1 | ||||
| Phosphorous | 0.09 | −0.09 | 0.28 | 0.29 | 0.13 | 0.37 | 0.08 | 1 | |||
| Sand | −0.95 ** | −0.65 * | −0.41 | −0.73 ** | −0.27 | −0.91 ** | −0.18 | −0.15 | 1 | ||
| Silt | 0.93 ** | 0.54 | 0.35 | 0.80 ** | 0.31 | 0.91 ** | 0.09 | 0.29 | −0.98 ** | 1 | |
| Clay | 0.81 ** | 0.78 ** | 0.45 | 0.44 | 0.13 | 0.73 ** | 0.34 | −0.21 | −0.87 ** | 0.75 ** | 1 |
| (b) | |||||||||||
| Fe | 1 | ||||||||||
| Mn | 0.93 ** | 1 | |||||||||
| Zn | 0.82 ** | 0.82 ** | 1 | ||||||||
| Cu | 0.89 ** | 0.83 ** | 0.69 * | 1 | |||||||
| As | −0.71 ** | −0.63 * | −0.57 | −0.79 ** | 1 | ||||||
| OC | 0.88 ** | 0.76 ** | 0.59 * | 0.78 ** | −0.75 ** | 1 | |||||
| Carbonate | 0.88 ** | 0.88 ** | 0.72 ** | 0.86 ** | −0.55 | 0.76 ** | 1 | ||||
| Phosphate | 0.18 | −0.08 | 0.03 | 0.23 | −0.17 | 0.16 | −0.02 | 1 | |||
| Sand | −0.98 ** | −0.92 ** | −0.83 ** | −0.88 ** | 0.72 ** | −0.91 ** | −0.89 ** | −0.15 | 1 | ||
| Silt | 0.97 ** | 0.88 ** | 0.84 ** | 0.82 ** | −0.73 ** | 0.90 ** | 0.81 ** | 0.19 | −0.99 ** | 1 | |
| Clay | 0.92 ** | 0.94 ** | 0.71 * | 0.92 ** | −0.65 * | 0.82 ** | 0.96 ** | 0.04 | −0.93 ** | 0.85 ** | 1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, M.; Ramanathan, A. Vertical Geochemical Variations and Speciation Studies of As, Fe, Mn, Zn, and Cu in the Sediments of the Central Gangetic Basin: Sequential Extraction and Statistical Approach. Int. J. Environ. Res. Public Health 2018, 15, 183. https://doi.org/10.3390/ijerph15020183
Kumar M, Ramanathan A. Vertical Geochemical Variations and Speciation Studies of As, Fe, Mn, Zn, and Cu in the Sediments of the Central Gangetic Basin: Sequential Extraction and Statistical Approach. International Journal of Environmental Research and Public Health. 2018; 15(2):183. https://doi.org/10.3390/ijerph15020183
Chicago/Turabian StyleKumar, Manoj, and AL. Ramanathan. 2018. "Vertical Geochemical Variations and Speciation Studies of As, Fe, Mn, Zn, and Cu in the Sediments of the Central Gangetic Basin: Sequential Extraction and Statistical Approach" International Journal of Environmental Research and Public Health 15, no. 2: 183. https://doi.org/10.3390/ijerph15020183