Identification of Pancreatic Injury in Patients with Elevated Amylase or Lipase Level Using a Decision Tree Classifier: A Cross-Sectional Retrospective Analysis in a Level I Trauma Center
Abstract
:1. Background
2. Methods
2.1. Study Population
2.2. Decision Tree Classifier
2.3. Multivariate Logistic Regression
2.4. Performance of the Decision Tree Classifier
2.5. Statistical Analysis
3. Results
3.1. Characteristics and Outcomes of Patients with Elevated Amylase or Lipase Levels
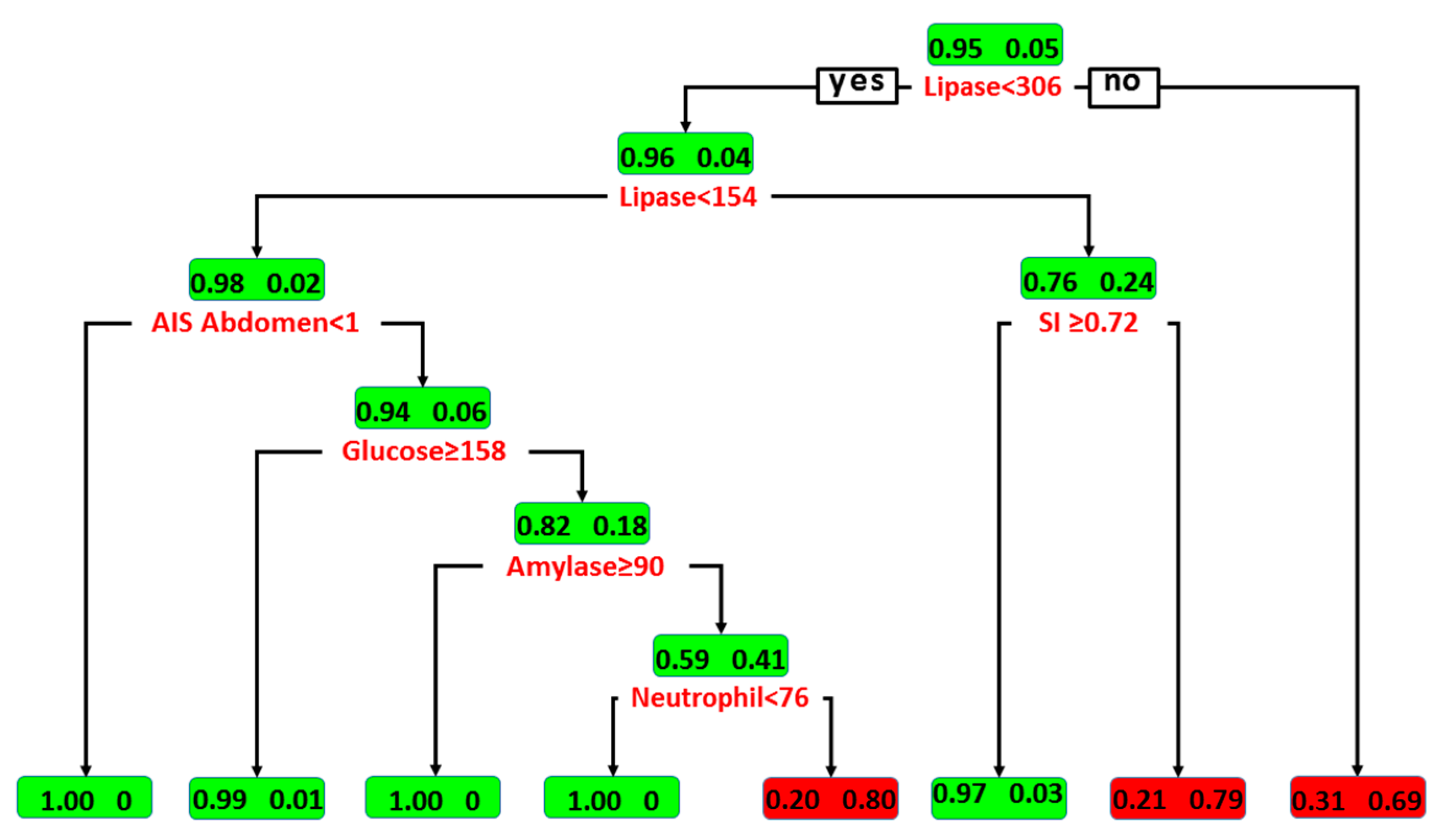
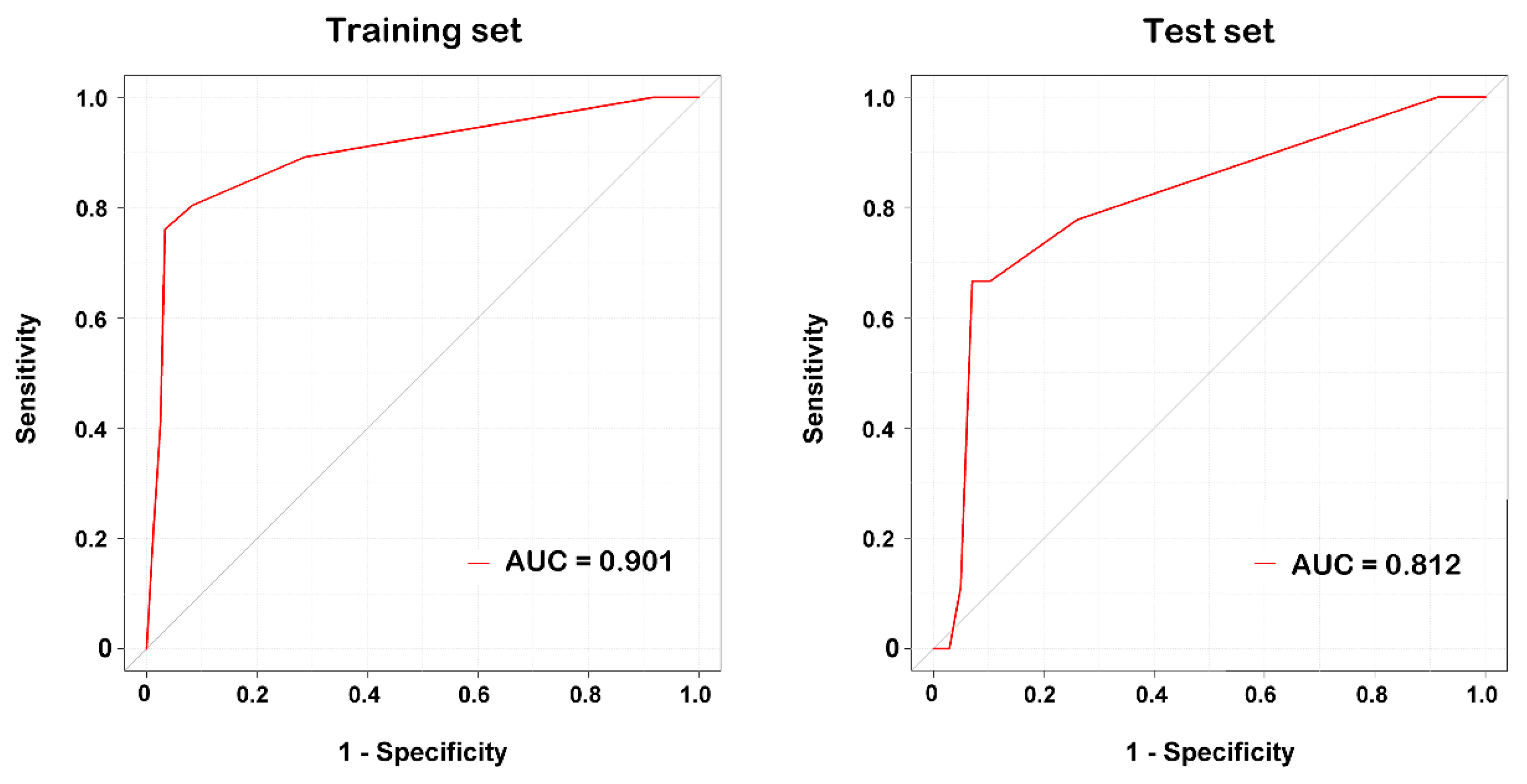
3.2. Classification by Decision Tree Algorithm
3.3. Classification by Multivariate LR
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jurkovich, G.J.; Carrico, C.J. Pancreatic trauma. Surg. Clin. N. Am. 1990, 70, 575–593. [Google Scholar] [CrossRef]
- Wisner, D.H.; Wold, R.L.; Frey, C.F. Diagnosis and treatment of pancreatic injuries. An analysis of management principles. Arch. Surg. 1990, 125, 1109–1113. [Google Scholar] [CrossRef] [PubMed]
- Patton, J.H., Jr.; Lyden, S.P.; Croce, M.A.; Pritchard, F.E.; Minard, G.; Kudsk, K.A.; Fabian, T.C. Pancreatic trauma: A simplified management guideline. J. Trauma 1997, 43, 234–239, discussion 239–241. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.C.; Chen, R.J.; Fang, J.F.; Hsu, Y.P.; Kao, Y.C.; Kao, J.L. Management of blunt major pancreatic injury. J. Trauma 2004, 56, 774–778. [Google Scholar] [CrossRef] [PubMed]
- Smego, D.R.; Richardson, J.D.; Flint, L.M. Determinants of outcome in pancreatic trauma. J. Trauma 1985, 25, 771–776. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, R.L., Jr.; Koniaris, L.G. Detecting blunt pancreatic injuries. J. Gastrointest. Surg. 2002, 6, 587–598. [Google Scholar] [CrossRef]
- Stawicki, S.P.; Schwab, C.W. Pancreatic trauma: Demographics, diagnosis, and management. Am. Surg. 2008, 74, 1133–1145. [Google Scholar] [PubMed]
- Tietz, N.W. Support of the diagnosis of pancreatitis by enzyme tests—Old problems, new techniques. Clin. Chim. Acta 1997, 257, 85–98. [Google Scholar] [CrossRef]
- Schmid-Schonbein, G.W.; Hugli, T.E. A new hypothesis for microvascular inflammation in shock and multiorgan failure: Self-digestion by pancreatic enzymes. Microcirculation 2005, 12, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Pieper-Bigelow, C.; Strocchi, A.; Levitt, M.D. Where does serum amylase come from and where does it go? Gastroenterol. Clin. N. Am. 1990, 19, 793–810. [Google Scholar]
- Gumaste, V.V.; Roditis, N.; Mehta, D.; Dave, P.B. Serum lipase levels in nonpancreatic abdominal pain versus acute pancreatitis. Am. J. Gastroenterol. 1993, 88, 2051–2055. [Google Scholar] [PubMed]
- Mahajan, A.; Kadavigere, R.; Sripathi, S.; Rodrigues, G.S.; Rao, V.R.; Koteshwar, P. Utility of serum pancreatic enzyme levels in diagnosing blunt trauma to the pancreas: A prospective study with systematic review. Injury 2014, 45, 1384–1393. [Google Scholar] [CrossRef] [PubMed]
- Boulanger, B.R.; Milzman, D.P.; Rosati, C.; Rodriguez, A. The clinical significance of acute hyperamylasemia after blunt trauma. Can. J. Surg. 1993, 36, 63–69. [Google Scholar] [PubMed]
- Buechter, K.J.; Arnold, M.; Steele, B.; Martin, L.; Byers, P.; Gomez, G.; Zeppa, R.; Augenstein, J. The use of serum amylase and lipase in evaluating and managing blunt abdominal trauma. Am. Surg. 1990, 56, 204–208. [Google Scholar] [PubMed]
- Keller, M.S.; Coln, C.E.; Trimble, J.A.; Green, M.C.; Weber, T.R. The utility of routine trauma laboratories in pediatric trauma resuscitations. Am. J. Surg. 2004, 188, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Moretz, J.A., 3rd; Campbell, D.P.; Parker, D.E.; Williams, G.R. Significance of serum amylase level in evaluating pancreatic trauma. Am. J. Surg. 1975, 130, 739–741. [Google Scholar] [CrossRef]
- Frank, B.; Gottlieb, K. Amylase normal, lipase elevated: Is it pancreatitis? A case series and review of the literature. Am. J. Gastroenterol. 1999, 94, 463–469. [Google Scholar] [PubMed]
- Skude, G.; Rothman, U. Amylase isoenzymes in serum after maxillo-facial surgery. Scand. J. Plast. Reconstr. Surg. 1973, 7, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Sakorafas, G.H.; Tsiotos, G.G.; Sarr, M.G. Ischemia/Reperfusion-Induced pancreatitis. Dig. Surg. 2000, 17, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Malinoski, D.J.; Hadjizacharia, P.; Salim, A.; Kim, H.; Dolich, M.O.; Cinat, M.; Barrios, C.; Lekawa, M.E.; Hoyt, D.B. Elevated serum pancreatic enzyme levels after hemorrhagic shock predict organ failure and death. J. Trauma 2009, 67, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Sagar, S.; Subramanian, A.; Albert, V.; Pandey, R.M.; Kapoor, N. Evaluation of amylase and lipase levels in blunt trauma abdomen patients. J. Emerg. Trauma Shock 2012, 5, 135–142. [Google Scholar] [PubMed]
- Liu, K.J.; Atten, M.J.; Lichtor, T.; Cho, M.J.; Hawkins, D.; Panizales, E.; Busker, J.; Stone, J.; Donahue, P.E. Serum amylase and lipase elevation is associated with intracranial events. Am. Surg. 2001, 67, 215–219, discussion 219–220. [Google Scholar] [PubMed]
- Bouwman, D.L.; Altshuler, J.; Weaver, D.W. Hyperamylasemia: A result of intracranial bleeding. Surgery 1983, 94, 318–323. [Google Scholar] [PubMed]
- Chen, C.C. Clinical implication of increased pancreatic enzymes in ICU patients. J. Chin. Med. Assoc. 2010, 73, 129–130. [Google Scholar] [CrossRef]
- Lee, C.C.; Chung, W.Y.; Shih, Y.H. Elevated amylase and lipase levels in the neurosurgery intensive care unit. J. Chin. Med. Assoc. 2010, 73, 8–14. [Google Scholar] [CrossRef]
- Pezzilli, R.; Morselli-Labate, A.M.; Romboli, E.; Dibenedetti, F.; Massa, M.; Migliori, M.; Barakat, B.; Merlini, G.; Corinaldesi, R.; Melzi d’Eril, G.V. Pancreatic involvement during the early phase of shock. JOP J. Pancreas 2002, 3, 139–143. [Google Scholar]
- Farion, K.; Michalowski, W.; Wilk, S.; O’Sullivan, D.; Matwin, S. A tree-based decision model to support prediction of the severity of asthma exacerbations in children. J. Med. Syst. 2010, 34, 551–562. [Google Scholar] [CrossRef] [PubMed]
- Zintzaras, E.; Bai, M.; Douligeris, C.; Kowald, A.; Kanavaros, P. A tree-based decision rule for identifying profile groups of cases without predefined classes: Application in diffuse large B-cell lymphomas. Comput. Biol. Med. 2007, 37, 637–641. [Google Scholar] [CrossRef] [PubMed]
- Kasbekar, P.U.; Goel, P.; Jadhav, S.P. A Decision Tree Analysis of Diabetic Foot Amputation Risk in Indian Patients. Front. Endocrinol. 2017, 8, 25. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.H.; Hsu, S.Y.; Hsieh, H.Y.; Chen, Y.C. Differences between the sexes in motorcycle-related injuries and fatalities at a Taiwanese level I trauma center. Biomed. J. 2017, 40, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.H.; Liu, H.T.; Hsu, S.Y.; Hsieh, H.Y.; Chen, Y.C. Motorcycle-related hospitalizations of the elderly. Biomed. J. 2017, 40, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.Y.; Wang, H.W. Analysis of traffic injury severity: An application of non-parametric classification tree techniques. Accid. Anal. Prev. 2006, 38, 1019–1027. [Google Scholar] [CrossRef] [PubMed]
- Ripley, B. Tree: Classification and Regression Trees. R Package Version 1.0-34. 2013. Available online: http://CRAN.R-project.org/package=tree (accessed on 14 November 2017).
- Guilbault, R.W.R.; Ohlsson, M.A.; Afonso, A.M.; Ebell, M.H. External Validation of Two Classification and Regression Tree Models to Predict the Outcome of Inpatient Cardiopulmonary Resuscitation. J. Viral Hepat. 2017, 32, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Shi, K.Q.; Zhou, Y.Y.; Yan, H.D.; Li, H.; Wu, F.L.; Xie, Y.Y.; Braddock, M.; Lin, X.Y.; Zheng, M.H. Classification and regression tree analysis of acute-on-chronic hepatitis B liver failure: Seeing the forest for the trees. J. Viral Hepat. 2017, 24, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, R.K.; Balasubramani, G.K.; Nowalk, M.P.; Eng, H.; Urbanski, L.; Jackson, M.L.; Jackson, L.A.; McLean, H.Q.; Belongia, E.A.; Monto, A.S.; et al. Classification and Regression Tree (CART) analysis to predict influenza in primary care patients. BMC Infect. Dis. 2016, 16, 503. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Maemura, K.; Sawada, Y.; Yoshioka, T.; Sugimoto, T. Hyperamylasemia in critically injured patients. J. Trauma 1980, 20, 951–955. [Google Scholar] [CrossRef] [PubMed]
- Matsuno, W.C.; Huang, C.J.; Garcia, N.M.; Roy, L.C.; Davis, J. Amylase and lipase measurements in paediatric patients with traumatic pancreatic injuries. Injury 2009, 40, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Allgower, M.; Burri, C. [“Shock index”]. Dtsch. Med. Wochenschr. 1967, 92, 1947–1950. [Google Scholar] [PubMed]
- Mitra, B.; Fitzgerald, M.; Chan, J. The utility of a shock index >/= 1 as an indication for pre-hospital oxygen carrier administration in major trauma. Injury 2014, 45, 61–65. [Google Scholar] [CrossRef] [PubMed]
- DeMuro, J.P.; Simmons, S.; Jax, J.; Gianelli, S.M. Application of the Shock Index to the prediction of need for hemostasis intervention. Am. J. Emerg. Med. 2013, 31, 1260–1263. [Google Scholar] [CrossRef] [PubMed]
- Rau, C.S.; Wu, S.C.; Kuo, S.C.; Pao-Jen, K.; Shiun-Yuan, H.; Chen, Y.C.; Hsieh, H.Y.; Hsieh, C.H.; Liu, H.T. Prediction of Massive Transfusion in Trauma Patients with Shock Index, Modified Shock Index, and Age Shock Index. Int. J. Environ. Res. Public Health 2016, 13, 683. [Google Scholar] [CrossRef] [PubMed]
- Mutschler, M.; Nienaber, U.; Munzberg, M.; Wolfl, C.; Schoechl, H.; Paffrath, T.; Bouillon, B.; Maegele, M. The Shock Index revisited—A fast guide to transfusion requirement? A retrospective analysis on 21,853 patients derived from the TraumaRegister DGU. Crit. Care 2013, 17, R172. [Google Scholar] [CrossRef] [PubMed]
- Birkhahn, R.H.; Gaeta, T.J.; Terry, D.; Bove, J.J.; Tloczkowski, J. Shock index in diagnosing early acute hypovolemia. Am. J. Emerg. Med. 2005, 23, 323–326. [Google Scholar] [CrossRef] [PubMed]
- Rassameehiran, S.; Teerakanok, J.; Suchartlikitwong, S.; Nugent, K. Utility of the Shock Index for Risk Stratification in Patients with Acute Upper Gastrointestinal Bleeding. South. Med. J. 2017, 110, 738–743. [Google Scholar] [CrossRef] [PubMed]
- Vandromme, M.J.; Griffin, R.L.; Kerby, J.D.; McGwin, G., Jr.; Rue, L.W., 3rd; Weinberg, J.A. Identifying risk for massive transfusion in the relatively normotensive patient: Utility of the prehospital shock index. J. Trauma 2011, 70, 384–388, discussion 388–390. [Google Scholar] [CrossRef] [PubMed]
- Nadler, E.P.; Gardner, M.; Schall, L.C.; Lynch, J.M.; Ford, H.R. Management of blunt pancreatic injury in children. J. Trauma 1999, 47, 1098–1103. [Google Scholar] [CrossRef] [PubMed]
- Ciccone, M.M.; Aquilino, A.; Cortese, F.; Scicchitano, P.; Sassara, M.; Mola, E.; Rollo, R.; Caldarola, P.; Giorgino, F.; Pomo, V.; et al. Feasibility and effectiveness of a disease and care management model in the primary health care system for patients with heart failure and diabetes (Project Leonardo). Vascular Health Risk Manag. 2010, 6, 297–305. [Google Scholar] [CrossRef]
| Variables | Total (n = 911) | Pancreatic Injury | p-Value | ||
|---|---|---|---|---|---|
| No (n = 865) | Yes (n = 46) | ||||
| Sex | Female | 325 (35.7%) | 303 (35%) | 22 (48%) | 0.084 |
| Male | 586 (64.3%) | 562 (65%) | 24 (52%) | ||
| CVA | No | 896 (98.4%) | 850 (98.3%) | 46 (100%) | >0.999 |
| Yes | 15 (1.6%) | 15 (1.7%) | 0 (0%) | ||
| CAD | No | 885 (97.1%) | 839 (96.9%) | 46 (100%) | 0.637 |
| Yes | 26 (2.9%) | 26 (3.1%) | 0 (0%) | ||
| HTN | No | 729 (80.0%) | 685 (79.2%) | 44 (47.8%) | 0.004 |
| Yes | 182 (20%) | 180 (20.8%) | 2 (2.2%) | ||
| CHF | No | 905 (99.3%) | 859 (99.3%) | 46 (100%) | >0.999 |
| Yes | 6 (0.7%) | 6 (0.7%) | 0 (0%) | ||
| ESRD | No | 880 (96.6%) | 834 (96.4%) | 46 (100%) | 0.398 |
| Yes | 31 (3.4%) | 31 (3.6%) | 0 (0%) | ||
| DM | No | 793 (87%) | 747 (86.4%) | 46 (100%) | 0.003 |
| Yes | 118 (13%) | 118 (13.6%) | 0 (0%) | ||
| TBI | No | 584 (64.1%) | 544 (62.9%) | 40 (87%) | 0.001 |
| Yes | 327 (35.9%) | 321 (37.1%) | 6 (13%) | ||
| Mandible fracture | No | 846 (92.9%) | 800 (92.5%) | 46 (100%) | 0.069 |
| Yes | 65 (7.1%) | 65 (7.5%) | 0 (0%) | ||
| Maxilla fracture | No | 792 (86.9%) | 746 (86.2%) | 46 (100%) | 0.003 |
| Yes | 119 (13.1%) | 119 (13.8%) | 0 (0%) | ||
| PPU | No | 907 (99.6%) | 861 (99.5%) | 46 (100%) | >0.999 |
| Yes | 4 (0.4%) | 4 (0.5%) | 0 (0%) | ||
| Ileus | No | 908 (99.7%) | 862 (99.7%) | 46 (100%) | >0.999 |
| Yes | 3 (0.3%) | 3 (0.3%) | 0 (0%) | ||
| Torsion of ovarian cyst | No | 910 (99.9%) | 864 (99.9%) | 46 (100%) | >0.999 |
| Yes | 1 (0.1%) | 1 (0.1%) | 0 (0%) | ||
| AIS (Head) | 0 | 382 (41.9%) | 346 (40%) | 36 (78.3%) | <0.001 |
| 1 | 114 (12.5%) | 110 (12.7%) | 4 (8.7%) | ||
| 2 | 49 (5.4%) | 47 (5.4%) | 2 (4.3%) | ||
| 3 | 159 (17.5%) | 155 (17.9%) | 4 (8.7%) | ||
| 4 | 140 (15.4%) | 140 (16.2%) | 0 (0%) | ||
| 5 | 60 (6.6%) | 60 (6.9%) | 0 (0%) | ||
| 6 | 7 (0.8%) | 7 (0.8%) | 0 (0%) | ||
| AIS (Face) | 0 | 655 (71.9%) | 611 (70.6%) | 44 (95.7%) | 0.002 |
| 1 | 62 (6.8%) | 60 (6.9%) | 2 (4.3%) | ||
| 2 | 182 (20%) | 182 (21%) | 0 (0%) | ||
| 3 | 12 (1.3%) | 12 (1.4%) | 0 (0%) | ||
| AIS (Thorax) | 0 | 555 (60.9%) | 515 (59.5%) | 40 (87%) | 0.009 |
| 1 | 37 (4.1%) | 35 (4%) | 2 (4.3%) | ||
| 2 | 71 (7.8%) | 71 (8.2%) | 0 (0%) | ||
| 3 | 146 (16%) | 144 (16.6%) | 2 (4.3%) | ||
| 4 | 93 (10.2%) | 91 (10.5%) | 2 (4.3%) | ||
| 5 | 9 (1%) | 9 (1%) | 0 (0%) | ||
| AIS (Abdomen) | 0 | 547 (60%) | 545 (63%) | 2 (4.3%) | <0.001 |
| 1 | 23 (2.5%) | 23 (2.7%) | 0 (0%) | ||
| 2 | 165 (18.1%) | 149 (17.2%) | 16 (34.8%) | ||
| 3 | 101 (11.1%) | 89 (10.3%) | 12 (26.1%) | ||
| 4 | 60 (6.6%) | 46 (5.3%) | 14 (30.4%) | ||
| 5 | 15 (1.6%) | 13 (1.5%) | 2 (4.3%) | ||
| AIS (Extremity) | 0 | 413 (45.3%) | 381 (44%) | 32 (69.6%) | 0.008 |
| 1 | 55 (6%) | 53 (6.1%) | 2 (4.3%) | ||
| 2 | 235 (25.8%) | 229 (26.5%) | 6 (13%) | ||
| 3 | 190 (20.9%) | 186 (21.5%) | 4 (8.7%) | ||
| 4 | 14 (1.5%) | 12 (1.4%) | 2 (4.3%) | ||
| 5 | 4 (0.4%) | 4 (0.5%) | 0 (0%) | ||
| AIS (External) | 0 | 770 (84.5%) | 732 (84.6%) | 38 (82.6%) | 0.974 |
| 1 | 126 (13.8%) | 118 (13.6%) | 8 (17.4%) | ||
| 2 | 6 (0.7%) | 6 (0.7%) | 0 (0%) | ||
| 3 | 4 (0.4%) | 4 (0.5%) | 0 (0%) | ||
| 4 | 1 (0.1%) | 1 (0.1%) | 0 (0%) | ||
| 5 | 1 (0.1%) | 1 (0.1%) | 0 (0%) | ||
| 6 | 3 (0.3%) | 3 (0.3%) | 0 (0%) | ||
| Variables | Total (n = 911) Median (IQR) | Pancreatic Injury | p-Value | |
|---|---|---|---|---|
| No (n = 865) Median (IQR) | Yes (n = 46) Median (IQR) | |||
| Age (years) | 45 (26–61) | 46 (26–61) | 39 (18–59) | 0.039 |
| SBP (mmHg) | 133 (108–155) | 133 (108–156) | 121 (105–144) | 0.032 |
| RR (times/min) | 20 (18–20) | 20 (18–20) | 20 (18–20) | 0.714 |
| Temperature (°C) | 36.4 (36–36.9) | 36.4 (36–36.9) | 36.4 (36.1–36.8) | 0.908 |
| GCS | 15 (9–15) | 15 (9–15) | 15 (14–15) | 0.001 |
| ISS | 16 (9–24) | 16 (9–24) | 10 (8–18) | 0.022 |
| RBC (106/µL) | 4.3 (3.8–4.7) | 4.3 (3.8–4.7) | 4.0 (3.6–4.6) | 0.115 |
| WBC (103/µL) | 13.5 (9.5–18.8) | 13.5 (9.5–19) | 12.6 (9–14.8) | 0.092 |
| Neutrophil (%) | 77.1 (65–85.8) | 76.2 (64.4–85.5) | 85.2 (78.8–89) | <0.001 |
| Hb (g/dL) | 12.7 (11.2–14.3) | 12.7 (11.2–14.3) | 12.3 (10.8–14.5) | 0.300 |
| Hct (%) | 37.9 (33.6–41.9) | 38.0 (33.7–42) | 37.2 (32.7–41.2) | 0.318 |
| Platelets (103/µL) | 206 (161–251) | 206 (161–253) | 202 (164–230) | 0.734 |
| Glucose (mg/dL) | 177 (136–179) | 177 (136–179) | 150 (149–168) | 0.023 |
| Na (mEq/L) | 139 (137–141) | 139 (137–141) | 140 (137–141) | 0.213 |
| K (mEq/L) | 3.6 (3.2–4.0) | 3.6 (3.2–4.0) | 3.7 (3.5–3.9) | 0.121 |
| BUN (mg/dL) | 14 (10–18) | 14 (10–18) | 12.3 (8–14) | 0.007 |
| Cr (mg/dL) | 0.9 (0.8–1.2) | 1.0 (0.8–1.2) | 0.8 (0.7–0.9) | <0.001 |
| AST (U/L) | 86 (41–184) | 83 (41–189.5) | 100 (61–179) | 0.179 |
| ALT (U/L) | 49 (26–120) | 49 (26–121) | 65 (40–96) | 0.190 |
| Total bilirubin (mg/dL) | 0.9 (0.7–0.9) | 0.9 (0.6–0.9) | 0.9 (0.8–1.0) | 0.090 |
| Amylase (U/L) | 136 (84–183) | 136 (84–179) | 148 (87–554) | 0.005 |
| Lipase (U/L) | 61 (45–89) | 60 (43–84) | 176 (97–375) | <0.001 |
| INR | 1.1 (1.0–1.2) | 1.1 (1.0–1.1) | 1.1 (1.0–1.2) | 0.044 |
| SI | 0.7 (0.6–0.9) | 0.7 (0.6–1.0) | 0.7 (0.6–0.9) | 0.589 |
| Variables | Total (n) | Amylase | Lipase | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Median (IQR) | Max | Min | ≥138 U/L (n) | Median (IQR) | Max | Min | ≥52 U/L (n) | ||
| Traumatic brain injury | 327 | 142 (87–182) | 1960 | 12 | 176 | 56 (38–73) | 767 | 12 | 210 |
| Mandible fracture | 65 | 160 (107–239) | 843 | 47 | 43 | 55 (28–78) | 542 | 2.1 | 37 |
| Maxilla fracture | 119 | 155 (105–227) | 956 | 40 | 75 | 54 (33–75) | 542 | 15 | 70 |
| Perforated peptic ulcer | 4 | 202 (130–254) | 267 | 55 | 3 | 75 (49–93) | 93 | 27 | 3 |
| Torsion of ovarian cyst | 1 | - | 84 | 84 | 0 | - | 72 | 72 | 1 |
| Ileus | 3 | 103 (89–118) | 132 | 74 | 0 | 89 (81–132) | 174 | 73 | 3 |
| Independent Variables | Coefficient | Independent Variables | Coefficient |
|---|---|---|---|
| WBC | 0.6911 | lipase | −0.2176 |
| neutrophil | 0.1522 | AIS of face | −0.3066 |
| Cr | 0.1061 | AIS of thorax | −0.5152 |
| AST | 0.0094 | AIS of abdomen | −1.848 |
| ALT | 0.0073 | AIS of extremity | −2.1096 |
| Na | −0.0164 | DM | −19.4426 |
| Intercept | −47.4648 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rau, C.-S.; Wu, S.-C.; Chien, P.-C.; Kuo, P.-J.; Chen, Y.-C.; Hsieh, H.-Y.; Hsieh, C.-H.; Liu, H.-T. Identification of Pancreatic Injury in Patients with Elevated Amylase or Lipase Level Using a Decision Tree Classifier: A Cross-Sectional Retrospective Analysis in a Level I Trauma Center. Int. J. Environ. Res. Public Health 2018, 15, 277. https://doi.org/10.3390/ijerph15020277
Rau C-S, Wu S-C, Chien P-C, Kuo P-J, Chen Y-C, Hsieh H-Y, Hsieh C-H, Liu H-T. Identification of Pancreatic Injury in Patients with Elevated Amylase or Lipase Level Using a Decision Tree Classifier: A Cross-Sectional Retrospective Analysis in a Level I Trauma Center. International Journal of Environmental Research and Public Health. 2018; 15(2):277. https://doi.org/10.3390/ijerph15020277
Chicago/Turabian StyleRau, Cheng-Shyuan, Shao-Chun Wu, Peng-Chen Chien, Pao-Jen Kuo, Yi-Chun Chen, Hsiao-Yun Hsieh, Ching-Hua Hsieh, and Hang-Tsung Liu. 2018. "Identification of Pancreatic Injury in Patients with Elevated Amylase or Lipase Level Using a Decision Tree Classifier: A Cross-Sectional Retrospective Analysis in a Level I Trauma Center" International Journal of Environmental Research and Public Health 15, no. 2: 277. https://doi.org/10.3390/ijerph15020277






