Biosorption of Cadmium by Non-Toxic Extracellular Polymeric Substances (EPS) Synthesized by Bacteria from Marine Intertidal Biofilms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains
Bacillus sp. MC3B-22 Identification
2.2. EPS Production
2.3. Cadmium Biosorption Experiments
2.3.1. Equilibrium Biosorption Isotherm
2.3.2. X-ray Photoelectron Spectroscopy (XPS)
2.4. Toxicity Test
3. Results and Discussion
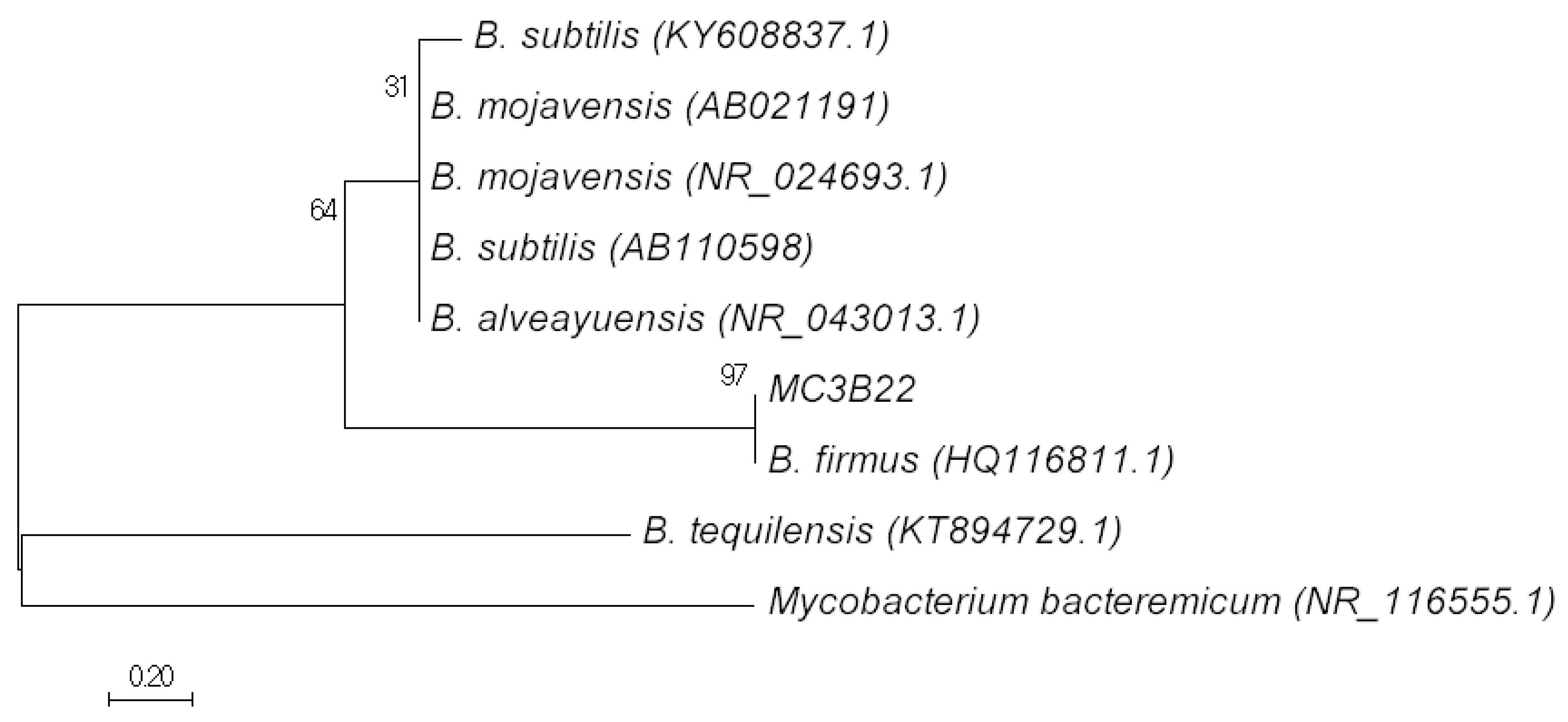
3.1. Molecular Identification of Bacillus sp. MC3B-22
3.2. Cadmium Biosorption Isotherms of Microbactan and B. firmus EPS
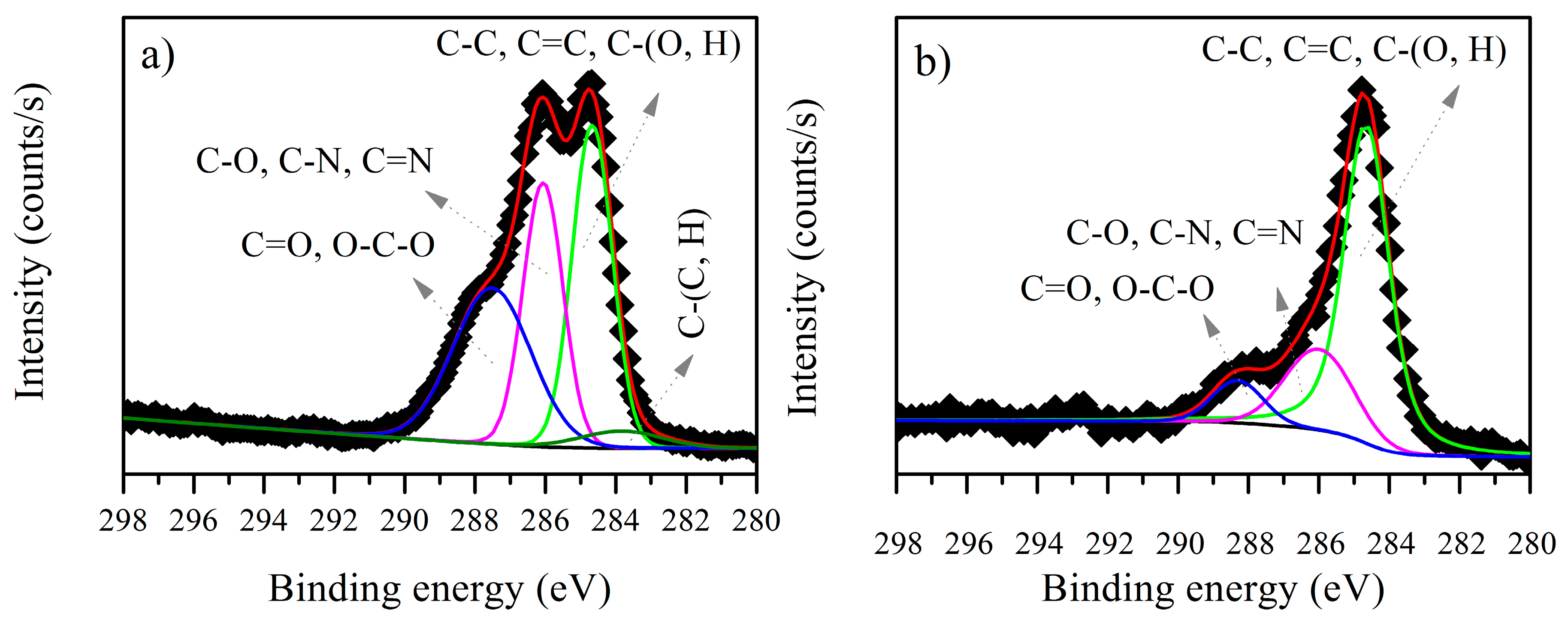
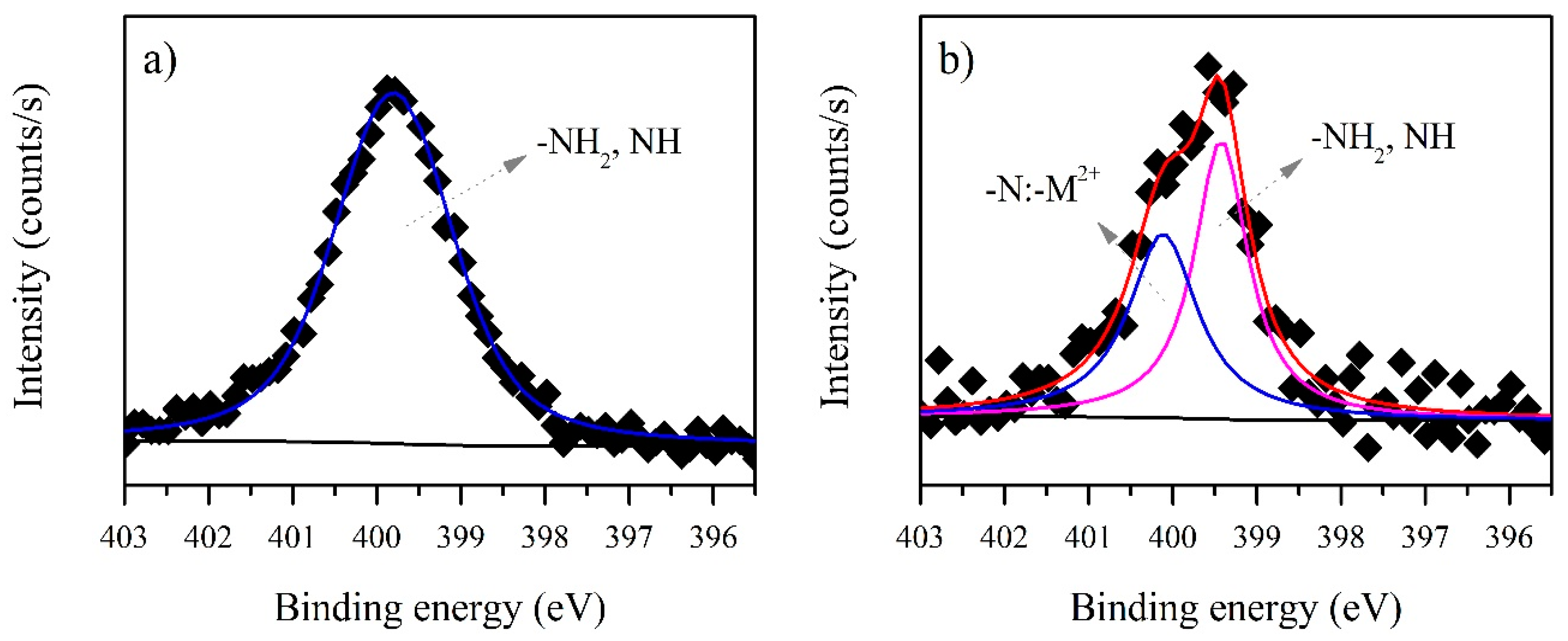
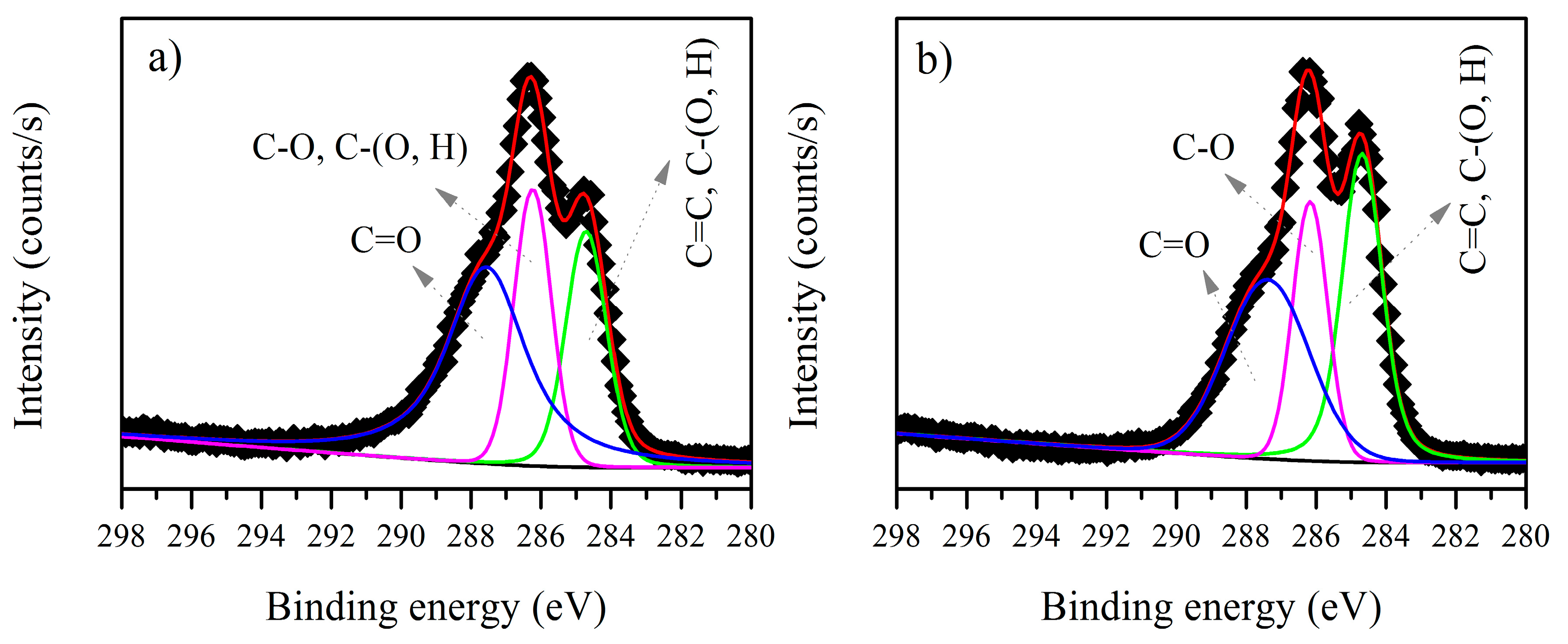
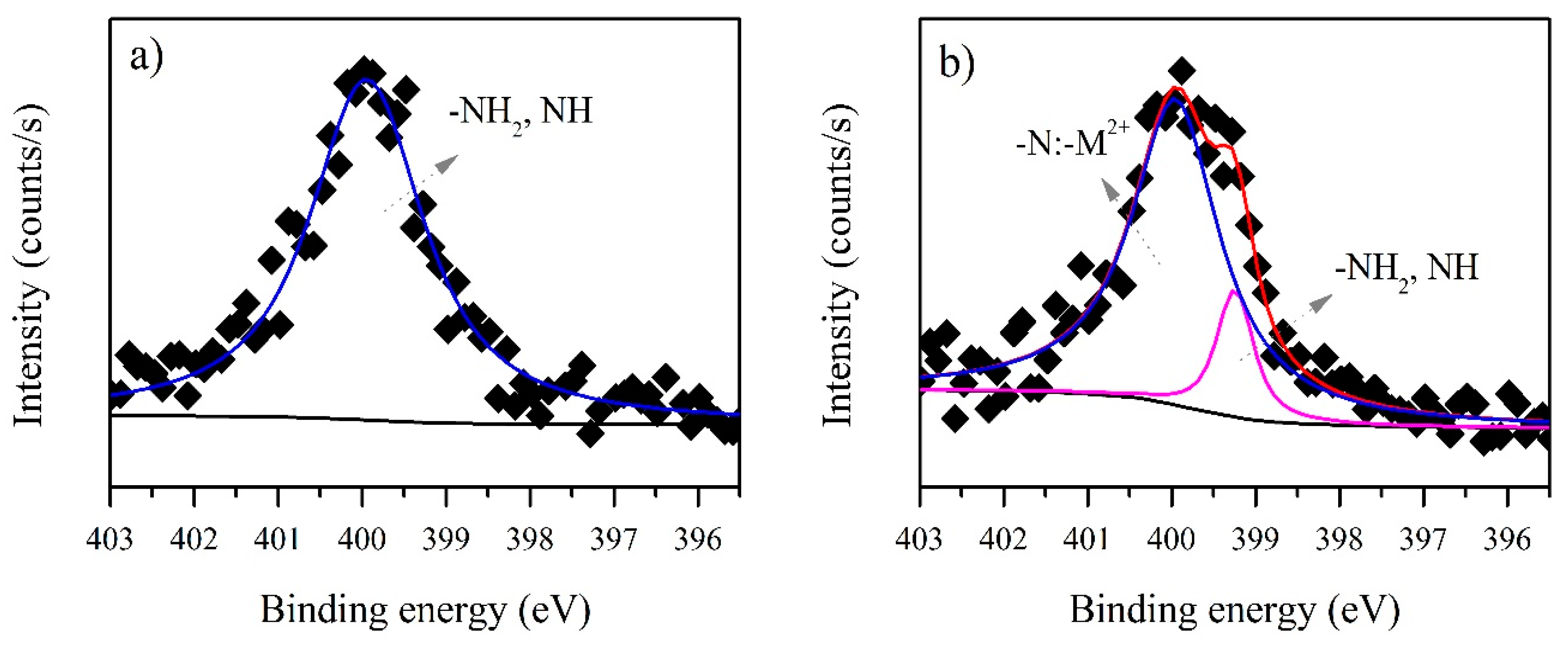
3.3. XPS Analysis before and after Biosorption
3.3.1. B. firmus EPS
3.3.2. Microbactan
3.4. Toxicity of Microbactan and B. firmus EPS
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Khairy, M.; El-Safty, S.A.; Shenashen, M.A. Environmental remediation and monitoring of cadmium. Trends Anal. Chem. 2014, 62, 56–68. [Google Scholar] [CrossRef]
- Bendell, L.I. Cadmium in shellfish: The British Columbia, Canada experience—A mini-review. Toxicol. Lett. 2010, 198, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.Y.; Yang, Y.F. Heavy metal (Pb, Co., Cd, Cr, Cu, Fe, Mn and Zn) concentrations in harvest-size white shrimp Litopenaeus vannamei tissues from aquaculture and wild source. J. Food Compos. Anal. 2011, 24, 62–65. [Google Scholar] [CrossRef]
- Pavlaki, M.D.; Araújo, M.J.; Cardoso, D.N.; Silva, A.R.; Cruz, A.; Mendo, S.; Soares, M.V.; Calado, R.; Loureiro, S. Ecotoxicity and genotoxicity of cadmium in different marine trophic levels. Environ. Pollut. 2016, 215, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.L.; Du, Y.; Zhai, M.M.; Shang, Q. Cadmium exposure and its health effects: A 19-year follow-up study of a polluted area in China. Sci. Total Environ. 2014, 470, 224–228. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.F.; Song, Y.H.; Yuan, P.; Cui, X.Y.; Qiiu, G.L. The remediation of heavy metals contaminated sediment. J. Hazard. Mater. 2009, 161, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Hashim, M.A.; Mukhopadhyay, S.; Narayan-Sahu, J.; Sengupta, B. Remediation technologies for heavy metal contaminated groundwater. J. Environ. Manag. 2011, 92, 2355–2388. [Google Scholar] [CrossRef] [PubMed]
- Fomina, M.; Gadd, G.M. Biosorption: Current perspectives on concept, definition and application. Bioresour. Technol. 2014, 160, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Kumari, D.; Pan, X.; Lee, D.J.; Achal, V. Immobilization of cadmium in soil by microbially induced carbonate precipitation with Exiguobacterium undae at low temperature. Int. Biodeterior. Biodegrad. 2014, 94, 98–102. [Google Scholar] [CrossRef]
- Bazrafshan, E.; Zarei, A.A.; Mostafapour, F.K. Biosorption of cadmium from aqueous solutions by Trichoderma fungus: Kinetic, thermodynamic, and equilibrium study. Desalin. Water Treat. 2015, 57, 14598–14608. [Google Scholar] [CrossRef]
- Wang, J.; Chen, C. Biosorbents for heavy metals removal and their future. Biotechnol. Adv. 2009, 27, 195–226. [Google Scholar] [CrossRef] [PubMed]
- Ayangbenro, A.; Babalola, O. A new strategy for heavy metal polluted environments: A review of microbial biosorbents. Int. J. Environ. Res. Public Health 2017, 14, 94. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, M.; Kumar, R.; Datta, A.; Nehra, V.; Garg, N. Bioremediation of heavy metals by microbes. In Bioremediation of Salt Affected Soils: An Indian Perspective, 1st ed.; Arora, S., Singh, A., Singh, Y., Eds.; Springer: Lucknow, India, 2017; pp. 233–255. ISBN 978-3-319-48257-6. [Google Scholar]
- Naik, M.M.; Dubey, S. Lead- and mercury-resistant marine bacteria and their application in lead and mercury bioremediation. In Marine Pollution and Microbial Remediation, 1st ed.; Naik, M., Dubey, S., Eds.; Springer: Berlin, Germany, 2017; pp. 29–40. ISBN 978-981-10-1044-6. [Google Scholar]
- Li, W.W.; Yu, H.Q. Insight into the roles of microbial extracellular polymer substances in metal biosorption. Bioresour. Technol. 2014, 160, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Deschatre, M.; Lescop, B.; Colin, C.S.; Ghilebaert, F.; Guezennec, J.; Rioual, S. Characterization of exopolysaccharides after sorption of silver ions in aqueous solution. J. Environ. Chem. Eng. 2015, 3, 210–216. [Google Scholar] [CrossRef]
- Dobrowolski, R.; Szczes, A.; Czemierska, M.; Jarosz-Wikołazka, A. Studies of cadmium(II), lead(II), nickel(II), cobalt(II) and chromium(VI) sorption on extracellular polymeric substances produced by Rhodococcus opacus and Rhodococcus rhodochrous. Bioresour. Technol. 2017, 225, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.C.; Neu, T.R.; Wozniak, D. The EPS matrix: The “house of biofilm cells”. J. Bacteriol. 2007, 189, 7945–7947. [Google Scholar] [CrossRef] [PubMed]
- Camacho-Chab, J.C.; Lango-Reynoso, F.; Castañeda-Chávez, M.R.; Galaviz-Villa, I.; Hinojosa-Garro, D.; Ortega-Morales, B.O. Extracellular polymeric substance matrices of microbial habitats associated with coastal aquaculture systems. Water 2016, 8, 369. [Google Scholar] [CrossRef]
- More, T.T.; Yadav, J.S.S.; Yan, S.; Tyagi, R.D.; Surampalli, R.Y. Extracellular polymeric substances of bacteria and their potential environmental applications. J. Environ. Manag. 2014, 144, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Morales, B.O.; Chan-Bacab, M.J.; De la Rosa-García, S.; Camacho-Chab, J.C. Valuable processes and products from marine intertidal microbial communities. Curr. Opin. Biotech. 2010, 21, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Morales, B.O.; Santiago-García, J.L.; Chan-Bacab, M.J.; Moppert, X.; Miranda-Tello, E.; Fardeau, M.L.; Guezennec, J. Characterization of extracellular polymers synthesized by tropical intertidal biofilm bacteria. J. Appl. Microbiol. 2007, 102, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Camacho-Chab, J.C.; Guézennec, J.; Chan-Bacab, M.J.; Ríos-Leal, E.; Sinquin, C.; Muñiz-Salazar, R.; Ortega-Morales, B.O. Emulsifying activity and stability of a non-toxic bioemulsifier synthesized by Microbacterium sp. MC3B-10. Int. J. Mol. Sci. 2013, 14, 18959–18972. [Google Scholar] [CrossRef] [PubMed]
- Sambrook, J.; Russell, D.W. Molecular Cloning: A Laboratory Manual, 3rd ed.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2001; ISBN 978-0-87969-577-4. [Google Scholar]
- Ausubel, F.M. Short Protocols in Molecular Biology: A Compendium of Methods from Current Protocols in Molecular Biology, 5th ed.; Wiley: New York, NY, USA, 2002; Section 2.1; ISBN 0471250929. [Google Scholar]
- Deschatre, M.; Ghillebaert, F.; Guezennec, J.; Simon-Colin, C. Sorption of copper (II) and silver (I) by four bacterial exopolysaccharides. Appl. Biochem. Biotechnol. 2013, 171, 1313–1327. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.; Yu, T.; Ai-Zhong, D.; Jin-Sheng, W. Adsorption of Cd (II), Zn (II) by extracellular polymeric substances extracted from waste activated sludge. Water Sci. Technol. 2008, 58, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Volesky, B. Biosorption and me. Water Res. 2007, 41, 4017–4029. [Google Scholar] [CrossRef] [PubMed]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Kumar, G.; Dabu, D. Effect of light, temperature and salinity on the growth of Artemia. IJESI 2015, 4, 7–14, 2319–6726. [Google Scholar]
- Marques, A.; Dinh, T.; Loakeimidis, C.; Huys, G.; Swings, J.; Verstraete, W.; Dhont, J.; Sorgeloss, P.; Bossier, P. Effects of bacteria on Artemia franciscana cultured in different gnotobiotic environments. Appl. Environ. Microbiol. 2015, 71, 4307–4317. [Google Scholar] [CrossRef] [PubMed]
- Salehizadeh, H.; Shojaosadati, S.A. Removal of metal ions from aqueous solution by polysaccharide produced from Bacillus firmus. Water Res. 2003, 37, 4231–4235. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef] [PubMed]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Nei, M.; Kumar, S. Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc. Natl. Acad. Sci. USA 2004, 101, 11030–11035. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2015, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.F.; Haydar, S.; Bhatti, A.A.; Baria, A.J. Application of artificial neural network for the prediction of biosorption capacity of immobilized Bacillus subtilis for the removal of cadmium ions from aqueous solution. Biochem. Eng. J. 2014, 84, 83–90. [Google Scholar] [CrossRef]
- Xiao, X.; Luo, S.; Zeng, G.; Wei, W.; Wan, Y.; Chen, L.; Guo, H.; Cao, Z.; Yang, L.; Chen, J.; et al. Biosorption of cadmium by endophytic fungus (EF) Microsphaeropsis sp. LSE10 isolated from cadmium hyperaccumulator Solanum nigrum L. Bioresour. Technol. 2010, 101, 1668–1674. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Lu, Y.; Luo, G. Ca(II) imprinted chitosan microspheres: An effective and green adsorbent for the removal of Cu(II), Cd(II) and Pb(II) from aqueous solutions. Chem. Eng. J. 2014, 244, 202–208. [Google Scholar] [CrossRef]
- Guo, Z.; Zhang, X.; Kang, Y.; Zhang, J. Biomass-derived carbon sorbents for Cd (II) removal: Activation and adsorption mechanisms. Sustain. Chem. Eng. 2017, 5, 4103–4109. [Google Scholar] [CrossRef]
- Lei, Z.; Yu, T.; Ai-Zhong, D.; De-Zhi, S. Spectral analysis of Cd, Zn, and Pb adsorption by extracellular polymeric substances from activated sludge. JJRST 2012, 9, 29–39. [Google Scholar]
- Zhuo, Y.; Zhang, Z.; Zhang, J.; Xia, S. New insight into adsorption characteristics and mechanisms of the biosorbents from waste activated sludge for heavy metals. J. Environ. Sci. 2016, 45, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Liu, Y.; Liu, S.; Zeng, G.; Tan, X.; Huang, B.; Tang, X.; Wang, S.; Hua, Q.; Yan, Z. Competitive adsorption of Pb(II), Cd(II) and Cu(II) onto chitosan-pyromellitic dianhydride modified biochar. J. Colloid Interface Sci. 2017, 506, 355–364. [Google Scholar] [CrossRef] [PubMed]
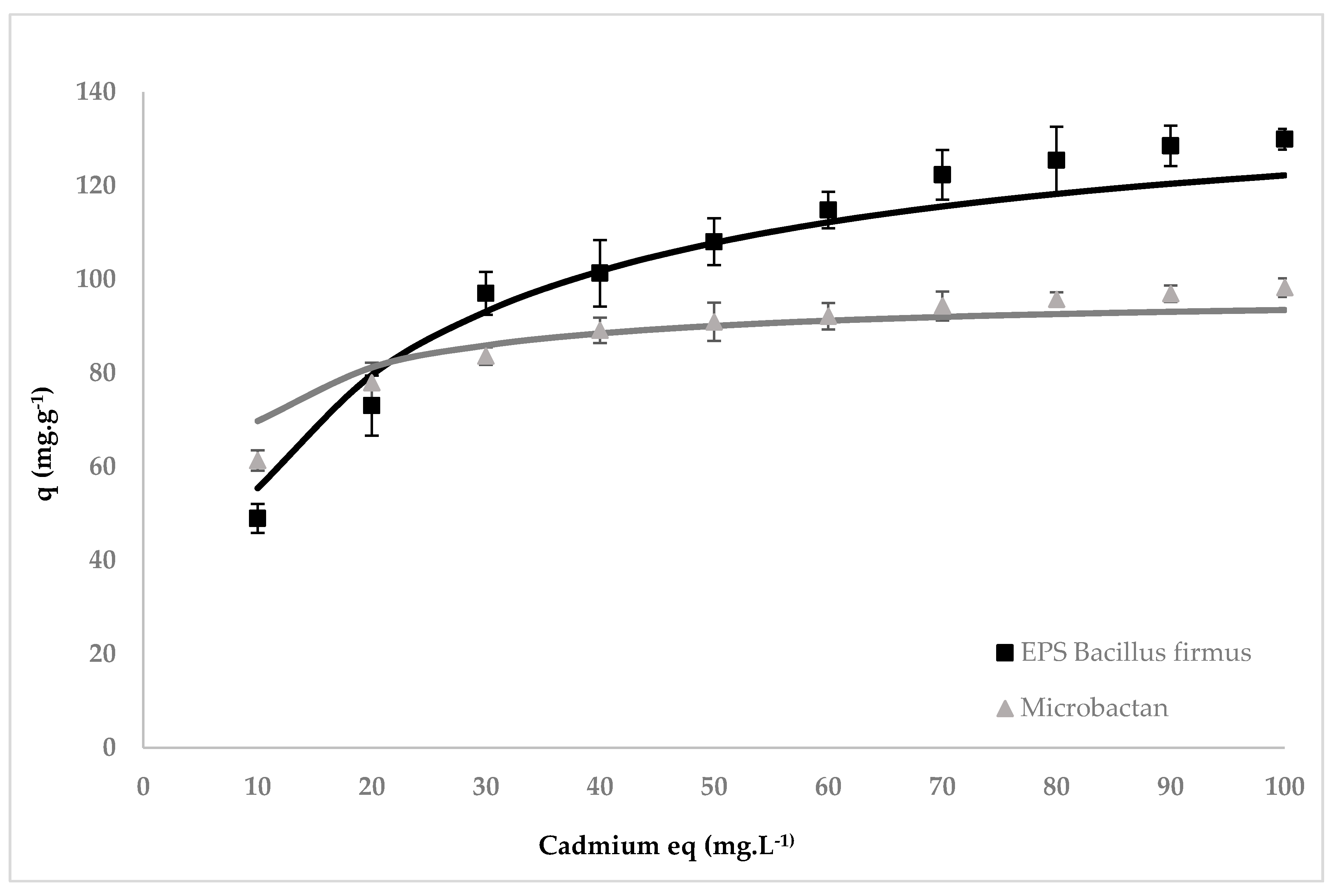
| EPS | Qmax (mg g−1) | K (L mg−1) | R2 |
|---|---|---|---|
| Microbactan | 97.12 | 0.254 | 0.954 |
| B. firmus | 141.10 | 0.064 | 0.990 |
| Element | Peak (eV) before Biosorption | Assignment | Peak (eV) after Biosorption | Area before Biosorption | Area after Biosorption |
|---|---|---|---|---|---|
| C1s | 283.82 | C–(C, H) | - | 1984.1 | - |
| C1s | 284.65 | C–C | 284.63 | 18,133 | 18,644 |
| C=C | |||||
| C–(O, H) | |||||
| C1s | 286.05 | C–O | 285.98 | 14,729 | 6053.3 |
| C–N | |||||
| C=N | |||||
| C1s | 287.50 | C=O | 288.28 | 16,978 | 2351.4 |
| O–C–O | |||||
| N1s | 399.78 | NH2 | 399.42 | 5593.1 | 970.79 |
| NH | |||||
| Interaction | - | –N:–Cd2+ | 400.11 | - | 809.53 |
| Element | Peak (eV) before Biosorption | Assignment | Peak (eV) after Biosorption | Area before Biosorption | Area after Biosorption |
|---|---|---|---|---|---|
| C1s | 284.69 | C=C | 284.67 | 12,005 | 21,890 |
| C–(O, H) | |||||
| C1s | 286.22 | C–O | 286.16 | 12,383 | 14,376 |
| C–(O, H) | |||||
| C1s | 287.55 | C=O | 287.37 | 24,212 | 23,695 |
| N1s | 399.93 | NH2 | 399.23 | 2263.6 | 361.49 |
| NH | |||||
| Interaction | - | –N:–Cd2+ | 399.95 | - | 2175.5 |
| Biopolymers | IC50 µg mL−1 |
|---|---|
| Microbactan | >1000 |
| B. firmus EPS | >1000 |
| Alginate | >1000 |
| Xanthan gum | >1000 |
| CuSO4·5H2O (Positive control) | 9.89 ± 4.69 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Camacho-Chab, J.C.; Castañeda-Chávez, M.D.R.; Chan-Bacab, M.J.; Aguila-Ramírez, R.N.; Galaviz-Villa, I.; Bartolo-Pérez, P.; Lango-Reynoso, F.; Tabasco-Novelo, C.; Gaylarde, C.; Ortega-Morales, B.O. Biosorption of Cadmium by Non-Toxic Extracellular Polymeric Substances (EPS) Synthesized by Bacteria from Marine Intertidal Biofilms. Int. J. Environ. Res. Public Health 2018, 15, 314. https://doi.org/10.3390/ijerph15020314
Camacho-Chab JC, Castañeda-Chávez MDR, Chan-Bacab MJ, Aguila-Ramírez RN, Galaviz-Villa I, Bartolo-Pérez P, Lango-Reynoso F, Tabasco-Novelo C, Gaylarde C, Ortega-Morales BO. Biosorption of Cadmium by Non-Toxic Extracellular Polymeric Substances (EPS) Synthesized by Bacteria from Marine Intertidal Biofilms. International Journal of Environmental Research and Public Health. 2018; 15(2):314. https://doi.org/10.3390/ijerph15020314
Chicago/Turabian StyleCamacho-Chab, Juan Carlos, María Del Refugio Castañeda-Chávez, Manuel Jesús Chan-Bacab, Ruth Noemí Aguila-Ramírez, Itzel Galaviz-Villa, Pascual Bartolo-Pérez, Fabiola Lango-Reynoso, Carolina Tabasco-Novelo, Christine Gaylarde, and Benjamín Otto Ortega-Morales. 2018. "Biosorption of Cadmium by Non-Toxic Extracellular Polymeric Substances (EPS) Synthesized by Bacteria from Marine Intertidal Biofilms" International Journal of Environmental Research and Public Health 15, no. 2: 314. https://doi.org/10.3390/ijerph15020314







