Maternal Residential Proximity to Major Roadways and Pediatric Embryonal Tumors in Offspring
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Exposure Assessment
2.3. Covariates
2.4. Statistical Analysis
3. Results
3.1. Characteristics of Cases and Controls
3.2. Continuous Distance to Nearest Major Roadway
3.3. Within 500 m of a Major Roadway
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gatta, G.; Ferrari, A.; Stiller, C.A.; Pastore, G.; Bisogno, G.; Trama, A.; Capocaccia, R.; Group, R.W. Embryonal cancers in Europe. Eur. J. Cancer 2012, 48, 1425–1433. [Google Scholar] [CrossRef] [PubMed]
- Tulla, M.; Berthold, F.; Graf, N.; Rutkowski, S.; von Schweinitz, D.; Spix, C.; Kaatsch, P. Incidence, Trends, and Survival of Children With Embryonal Tumors. Pediatrics 2015, 136, e623–e632. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.M.; Padgett, L.S.; Leisenring, W.M.; Stratton, K.K.; Bishop, K.; Krull, K.R.; Alfano, C.M.; Gibson, T.M.; de Moor, J.S.; Hartigan, D.B.; et al. Survivors of childhood cancer in the United States: Prevalence and burden of morbidity. Cancer Epidemiol. Prev. Biomark. 2015, 24, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Chu, A.; Heck, J.E.; Ribeiro, K.B.; Brennan, P.; Boffetta, P.; Buffler, P.; Hung, R.J. Wilms’ tumour: A systematic review of risk factors and meta-analysis. Paediatr. Perinat. Epidemiol. 2010, 24, 449–469. [Google Scholar] [CrossRef] [PubMed]
- Lohmann, D. Retinoblastoma. Adv. Exp. Med. Biol. 2010, 685, 220–227. [Google Scholar] [PubMed]
- McLaughlin, C.C.; Baptiste, M.S.; Schymura, M.J.; Zdeb, M.S.; Nasca, P.C. Perinatal risk factors for neuroblastoma. Cancer Causes Control 2009, 20, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Spector, L.G.; Johnson, K.J.; Soler, J.T.; Puumala, S.E. Perinatal risk factors for hepatoblastoma. Br. J. Cancer 2008, 98, 1570–1573. [Google Scholar] [CrossRef] [PubMed]
- Anderson, L.M.; Diwan, B.A.; Fear, N.T.; Roman, E. Critical windows of exposure for children’s health: Cancer in human epidemiological studies and neoplasms in experimental animal models. Environ. Health Perspect. 2000, 108 (Suppl. 3), 573–594. [Google Scholar] [CrossRef] [PubMed]
- Health Effects Institute. Traffic-Related Air Pollution: A Critical Review of the Literature on Emissions, Exposure and Health Effects; 2010 Special Report #17; Health Effects Institute: Boston, MA, USA, 2010. [Google Scholar]
- International Agency for Research on Cancer. Diesel and gasoline engine exhausts and some nitroarenes. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; International Agency for Research on Cancer: Lyon, France, 1989; Volume 46, pp. 1–458. [Google Scholar]
- Population Reference Bureau. 2017 World Population Data Sheet. Available online: http://www.prb.org/pdf17/2017_World_Population.pdf (accessed on 21 January 2018).
- Danysh, H.E.; Zhang, K.; Mitchell, L.E.; Scheurer, M.E.; Lupo, P.J. Maternal residential proximity to major roadways at delivery and childhood central nervous system tumors. Environ. Res. 2016, 146, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Spycher, B.D.; Feller, M.; Roosli, M.; Ammann, R.A.; Diezi, M.; Egger, M.; Kuehni, C.E. Childhood cancer and residential exposure to highways: A nationwide cohort study. Eur. J. Epidemiol. 2015, 30, 1263–1275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steliarova-Foucher, E.; Stiller, C.; Lacour, B.; Kaatsch, P. International Classification of Childhood Cancer, third edition. Cancer 2005, 103, 1457–1467. [Google Scholar] [CrossRef] [PubMed]
- Texas Department of State Health Services. Texas Cancer Registry: Data Linkages. Available online: https://www.dshs.texas.gov/tcr/data/linkages.aspx (accessed on 21 January 2018).
- Texas Natural Resources Information System. Maps & Data: Transportation StratMap, Version 2 (2006). Available online: http://tnris.org/data-download/#/statewide (accessed on 1 December 2014).
- U.S. Census Bureau. TIGER/Line Files: Technical Documentation; U.S. Census Bureau: Suitland, MD, USA, 2007; pp. 89–91.
- Bunin, G.R.; Meadows, A.T.; Emanuel, B.S.; Buckley, J.D.; Woods, W.G.; Hammond, G.D. Pre- and postconception factors associated with sporadic heritable and nonheritable retinoblastoma. Cancer Res. 1989, 49, 5730–5735. [Google Scholar] [PubMed]
- Dadvand, P.; Ostro, B.; Figueras, F.; Foraster, M.; Basagana, X.; Valentin, A.; Martinez, D.; Beelen, R.; Cirach, M.; Hoek, G.; et al. Residential proximity to major roads and term low birth weight: The roles of air pollution, heat, noise, and road-adjacent trees. Epidemiology 2014, 25, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.J.; Puumala, S.E.; Soler, J.T.; Spector, L.G. Perinatal characteristics and risk of neuroblastoma. Int. J. Cancer 2008, 123, 1166–1172. [Google Scholar] [CrossRef] [PubMed]
- Yorifuji, T.; Naruse, H.; Kashima, S.; Takao, S.; Murakoshi, T.; Doi, H.; Kawachi, I. Residential proximity to major roads and adverse birth outcomes: A hospital-based study. Environ. Health 2013, 12, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hertz-Picciotto, I.; Jusko, T.A.; Willman, E.J.; Baker, R.J.; Keller, J.A.; Teplin, S.W.; Charles, M.J. A cohort study of in utero polychlorinated biphenyl (PCB) exposures in relation to secondary sex ratio. Environ. Health 2008, 7, 37. [Google Scholar] [CrossRef] [PubMed]
- Matz, C.J.; Stieb, D.M.; Davis, K.; Egyed, M.; Rose, A.; Chou, B.; Brion, O. Effects of age, season, gender and urban-rural status on time-activity: Canadian Human Activity Pattern Survey 2 (CHAPS 2). Int. J. Environ. Res. Public Health 2014, 11, 2108–2124. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, P.; Itriago, E.; Rodriguez-Galindo, C.; Ribeiro, K. Racial and Ethnic Disparities in the Incidence of Pediatric Extracranial Embryonal Tumors. J. Natl. Cancer Inst. 2017, 109. [Google Scholar] [CrossRef] [PubMed]
- Heck, J.E.; Wu, J.; Lombardi, C.; Qiu, J.; Meyers, T.J.; Wilhelm, M.; Cockburn, M.; Ritz, B. Childhood cancer and traffic-related air pollution exposure in pregnancy and early life. Environ. Health Perspect. 2013, 121, 1385–1391. [Google Scholar] [CrossRef] [PubMed]
- Azary, S.; Ganguly, A.; Bunin, G.R.; Lombardi, C.; Park, A.S.; Ritz, B.; Heck, J.E. Sporadic Retinoblastoma and Parental Smoking and Alcohol Consumption before and after Conception: A Report from the Children’s Oncology Group. PLoS ONE 2016, 11, e0151728. [Google Scholar] [CrossRef] [PubMed]
- Bunin, G.R. Nongenetic causes of childhood cancers: Evidence from international variation, time trends, and risk factor studies. Toxicol. Appl. Pharmacol. 2004, 199, 91–103. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, C.C. Childhood Cancer. In Fundamentals of Cancer Epidemiology, 2nd ed.; Nasca, P.C., Pastides, H., Eds.; Jones and Bartlett Publishers: Sudbury, MA, USA, 2008. [Google Scholar]
- Herbstman, J.B.; Tang, D.; Zhu, D.; Qu, L.; Sjodin, A.; Li, Z.; Camann, D.; Perera, F.P. Prenatal exposure to polycyclic aromatic hydrocarbons, benzo[a]pyrene-DNA adducts, and genomic DNA methylation in cord blood. Environ. Health Perspect. 2012, 120, 733–738. [Google Scholar] [CrossRef] [PubMed]
- Perera, F.; Tang, D.; Whyatt, R.; Lederman, S.A.; Jedrychowski, W. DNA damage from polycyclic aromatic hydrocarbons measured by benzo [a] pyrene-DNA adducts in mothers and newborns from Northern Manhattan, the World Trade Center Area, Poland, and China. Cancer Epidemiol. Prev. Biomark. 2005, 14, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Shimada, T.; Fujii-Kuriyama, Y. Metabolic activation of polycyclic aromatic hydrocarbons to carcinogens by cytochromes P450 1A1 and 1B1. Cancer Sci. 2004, 95, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Moller, P.; Jacobsen, N.R.; Folkmann, J.K.; Danielsen, P.H.; Mikkelsen, L.; Hemmingsen, J.G.; Vesterdal, L.K.; Forchhammer, L.; Wallin, H.; Loft, S. Role of oxidative damage in toxicity of particulates. Free Radic. Res. 2010, 44, 1–46. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.S. The signaling mechanism of ROS in tumor progression. Cancer Metastasis Rev. 2006, 25, 695–705. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, N.L.; Woodhouse, S.; Stieb, D.M.; Brook, J.R. Ambient nitrogen dioxide and distance from a major highway. Sci. Total Environ. 2003, 312, 43–46. [Google Scholar] [CrossRef]
- Danysh, H.E.; Mitchell, L.E.; Zhang, K.; Scheurer, M.E.; Lupo, P.J. Differences in environmental exposure assignment due to residential mobility among children with a central nervous system tumor: Texas, 1995–2009. J. Expo. Sci. Environ. Epidemiol. 2017, 27, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Lupo, P.J.; Symanski, E.; Chan, W.; Mitchell, L.E.; Waller, D.K.; Canfield, M.A.; Langlois, P.H. Differences in exposure assignment between conception and delivery: The impact of maternal mobility. Paediatr. Perinat. Epidemiol. 2010, 24, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Heck, J.E.; Park, A.S.; Qiu, J.; Cockburn, M.; Ritz, B. An exploratory study of ambient air toxics exposure in pregnancy and the risk of neuroblastoma in offspring. Environ. Res. 2013, 127, 1–6. [Google Scholar] [CrossRef] [PubMed]
- U.S. Environmental Protection Agency Toxics Release Inventory (TRI) Explorer (2012 Dataset) [Internet Database]. Available online: http://www.epa.gov/triexplorer (accessed on 15 February 2015).
| Characteristic, n (%) | All Embryonal Tumors | Neuroblastoma | Wilms Tumor | Retinoblastoma | Hepatoblastoma | Controls | |
|---|---|---|---|---|---|---|---|
| Unilateral | Bilateral | ||||||
| Total subjects | 571 (100) | 252 (44.1) | 143 (25.0) | 89 (15.6) | 32 (5.6) | 55 (9.6) | 2855 (100) |
| Infant | |||||||
| Male sex | 314 (55.0) | 154 (61.1) | 65 (45.4) | 46 (51.6) | 19 (59.4) | 30 (54.5) | 1436 (50.3) |
| Gestational age | |||||||
| 37–42 weeks | 424 (74.3) | 187 (74.2) | 117 (81.8) | 64 (71.9) | 24 (75.0) | 32 (58.2) | 2320 (81.3) |
| <37 weeks | 104 (18.2) | 45 (17.9) | NS 3 | 14 (15.7) | NS 3 | NS 3 | 350 (12.3) |
| >42 weeks | 43 (7.5) | 20 (7.9) | NS 3 | 11 (12.4) | NS 3 | NS 3 | 185 (6.5) |
| Birth weight | |||||||
| 2500–3999 g | 464 (81.3) | 210 (83.1) | 122 (85.0) | 73 (82.0) | 28 (87.5) | 31 (56.4) | 2430 (85.1) |
| <2500 g | 71 (12.4) | 30 (12.2) | 9 (6.4) | 10 (11.2) | NS 3 | 18 (32.7) | 252 (8.8) |
| ≥4000 g | 36 (6.3) | 12 (4.7) | 12 (8.6) | 6 (6.8) | NS 3 | 6 (10.9) | 173 (6.1) |
| Season of birth 1 | |||||||
| Summer | 124 (22.8) | 60 (23.8) | 30 (20.9) | 17 (19.1) | 9 (28.1) | 16 (29.1) | 732 (25.6) |
| Fall | 144 (26.6) | 66 (26.2) | 34 (23.8) | 29 (32.6) | 9 (28.1) | 13 (23.6) | 721 (25.3) |
| Winter | 143 (26.4) | 64 (25.4) | 35 (24.5) | 25 (28.1) | 8 (25.0) | 18 (32.7) | 673 (23.6) |
| Spring | 131 (24.2) | 62 (24.6) | 44 (30.8) | 18 (22.2) | 6 (18.8) | 8 (14.6) | 729 (25.5) |
| Age at diagnosis | |||||||
| ≤1 year | 393 (68.8) | 200 (79.4) | 65 (45.5) | 61 (68.5) | NS 3 | 36 (65.5) | |
| >1 year | 178 (31.2) | 52 (20.6) | 78 (54.5) | 28 (31.5) | NS 3 | 19 (34.5) | |
| Maternal 2 | |||||||
| Race/ethnicity | |||||||
| NH White | 258 (45.2) | 132 (52.4) | 66 (44.8) | 33 (37.1) | 8 (25.0) | 21 (38.2) | 1031 (36.1) |
| NH Black | 70 (12.3) | 26 (10.3) | NS 3 | NS 3 | NS 3 | NS 3 | 353 (12.4) |
| Hispanic | 225 (39.4) | 82 (32.5) | 54 (38.5) | 39 (43.8) | 18 (56.3) | 31 (56.4) | 1348 (47.2) |
| Other | 18 (3.2) | 12 (4.7) | NS 3 | NS 3 | NS 3 | NS 3 | 123 (4.3) |
| Age 4 | |||||||
| <20 years | 74 (12.9) | 34 (13.5) | 16 (11.2) | 11 (12.4) | 8 (25.0) | NS 3 | 414 (14.5) |
| 20 to <25 years | 128 (22.4) | 54 (21.4) | 31 (21.7) | 20 (22.5) | 6 (18.8) | NS 3 | 793 (27.8) |
| 25 to <30 years | 132 (23.1) | 55 (21.8) | 36 (25.2) | 23 (25.8) | 8 (25.0) | 10 (18.2) | 730 (25.6) |
| 30 to <35 years | 152 (26.6) | 68 (27.0) | 36 (25.2) | 25 (28.1) | NS 3 | 16 (29.1) | 574 (20.1) |
| ≥35 years | 85 (14.9) | 41 (16.3) | 24 (16.7) | 10 (11.2) | NS 3 | 7 (12.7) | 344 (12.1) |
| Completed education 4 | |||||||
| >HS | 293 (51.5) | 136 (54.0) | 83 (58.0) | 40 (45.5) | 11 (41.9) | 21 (38.2) | 1231 (43.3) |
| Completed HS | 137 (24.1) | 58 (23.0) | 32 (22.4) | 23 (26.1) | 7 (22.6) | 17 (30.9) | 790 (27.8) |
| <HS | 139 (24.4) | 58 (23.0) | 28 (19.6) | 25 (28.4) | 11 (35.5) | 17 (30.9) | 822 (28.9) |
| Neighborhood | |||||||
| Area-level poverty | |||||||
| Low poverty | 363 (63.6) | 175 (69.4) | 96 (67.1) | 49 (55.1) | 18 (56.3) | 25 (45.5) | 1680 (58.9) |
| High poverty | 208 (36.4) | 77 (30.6) | 47 (32.9) | 40 (44.9) | 14 (43.7) | 30 (54.5) | 1174 (41.1) |
| Urban status | |||||||
| Rural | 54 (9.5) | 22 (8.8) | 17 (11.9) | NS 3 | NS 3 | NS 3 | 288 (10.1) |
| Urban | 517 (90.5) | 228 (91.2) | 126 (88.1) | NS 3 | NS 3 | NS 3 | 2566 (89.9) |
| Tumor Type | Mean (SD) | Odds Ratio (95% CI) | |||||
|---|---|---|---|---|---|---|---|
| Cases | Controls | Unadjusted | p-Value | Adjusted 1 | p-Value | ||
| All embryonal tumors | 0.43 (0.59) | 0.46 (0.66) | 1.06 (0.92, 1.22) | 0.443 | 1.13 (0.97, 1.32) | 0.121 | |
| Neuroblastoma | 0.47 (0.59) | 0.46 (0.66) | 0.96 (0.80, 1.16) | 0.672 | 1.06 (0.86, 1.30) | 0.591 | |
| Wilms tumor | 0.45 (0.64) | 0.46 (0.66) | 1.00 (0.77, 1.30) | 0.988 | 1.07 (0.81, 1.43) | 0.633 | |
| Retinoblastoma | |||||||
| Unilateral | 0.28 (0.31) | 0.46 (0.66) | 2.52 (1.28, 4.97) | 0.008 | 2.57 (1.28, 5.15) | 0.008 | |
| Bilateral | 0.41 (0.33) | 0.46 (0.66) | 1.10 (0.60, 2.03) | 0.750 | 1.04 (0.57, 1.90) | 0.909 | |
| Hepatoblastoma | 0.46 (0.80) | 0.46 (0.66) | 1.00 (0.68, 1.49) | 0.982 | 1.06 (0.72, 1.56) | 0.785 | |
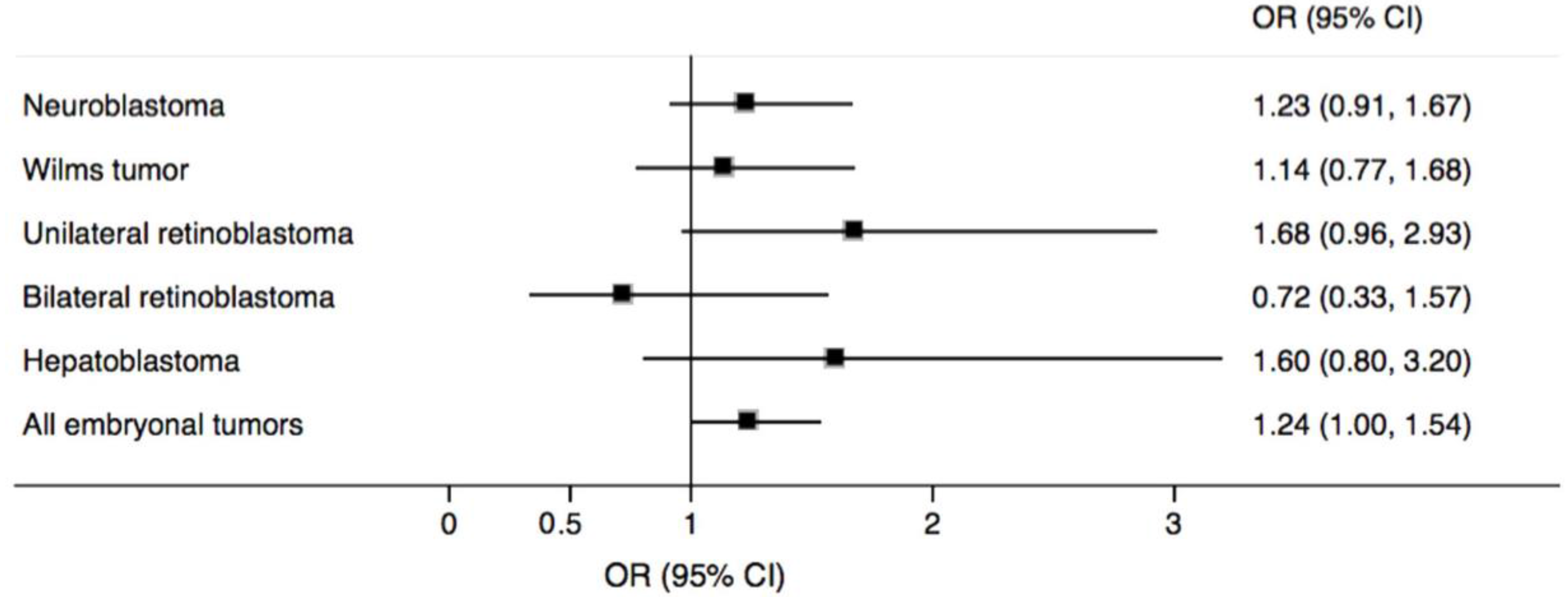
| Proximity to Major Roadway | n (%) | Odds Ratio (95% CI) | |||||
|---|---|---|---|---|---|---|---|
| Cases | Controls | Unadjusted | p-Value | Adjusted 1 | p-Value | ||
| All embryonal tumors | |||||||
| >500 m (ref.) | 142 (24.9) | 772 (27.0) | 1.00 | 1.00 | |||
| ≤500 m | 429 (75.1) | 2083 (73.0) | 1.12 (0.91, 1.38) | 0.284 | 1.24 (1.00, 1.54) | 0.048 | |
| Neuroblastoma | |||||||
| >500 m (ref.) | 67 (26.6) | 772 (27.0) | 1.00 | 1.00 | |||
| ≤500 m | 185 (73.4) | 2083 (73.0) | 1.03 (0.77, 1.38) | 0.841 | 1.23 (0.91, 1.67) | 0.175 | |
| Wilms tumor | |||||||
| >500 m (ref.) | 38 (26.7) | 772 (27.0) | 1.00 | 1.00 | |||
| ≤500 m | 105 (73.4) | 2083 (73.0) | 1.02 (0.70, 1.49) | 0.928 | 1.14 (0.77, 1.68) | 0.517 | |
| Retinoblastoma | |||||||
| Unilateral | |||||||
| >500 m (ref.) | 16 (18.0) | 772 (27.0) | 1.00 | 1.00 | |||
| ≤500 m | 73 (82.0) | 2083 (73.0) | 1.68 (0.97, 2.91) | 0.063 | 1.68 (0.96, 2.93) | 0.071 | |
| Bilateral | |||||||
| >500 m (ref.) | 10 (31.3) | 772 (27.0) | 1.00 | 1.00 | |||
| ≤500 m | 22 (68.7) | 2083 (73.0) | 0.79 (0.37, 1.68) | 0.539 | 0.72 (0.33, 1.57) | 0.416 | |
| Hepatoblastoma | |||||||
| >500 m (ref.) | 11 (20.0) | 772 (27.0) | 1.00 | 1.00 | |||
| ≤500 m | 44 (80.0) | 2083 (73.0) | 1.49 (0.76, 2.90) | 0.243 | 1.60 (0.80, 3.20) | 0.181 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, S.V.; Lupo, P.J.; Pompeii, L.A.; Danysh, H.E. Maternal Residential Proximity to Major Roadways and Pediatric Embryonal Tumors in Offspring. Int. J. Environ. Res. Public Health 2018, 15, 505. https://doi.org/10.3390/ijerph15030505
Kumar SV, Lupo PJ, Pompeii LA, Danysh HE. Maternal Residential Proximity to Major Roadways and Pediatric Embryonal Tumors in Offspring. International Journal of Environmental Research and Public Health. 2018; 15(3):505. https://doi.org/10.3390/ijerph15030505
Chicago/Turabian StyleKumar, Shwetha V., Philip J. Lupo, Lisa A. Pompeii, and Heather E. Danysh. 2018. "Maternal Residential Proximity to Major Roadways and Pediatric Embryonal Tumors in Offspring" International Journal of Environmental Research and Public Health 15, no. 3: 505. https://doi.org/10.3390/ijerph15030505




