Case Study of Airborne Pathogen Dispersion Patterns in Emergency Departments with Different Ventilation and Partition Conditions
Abstract
:1. Introduction
1.1. Background
1.2. Purpose of the Study
2. Methods
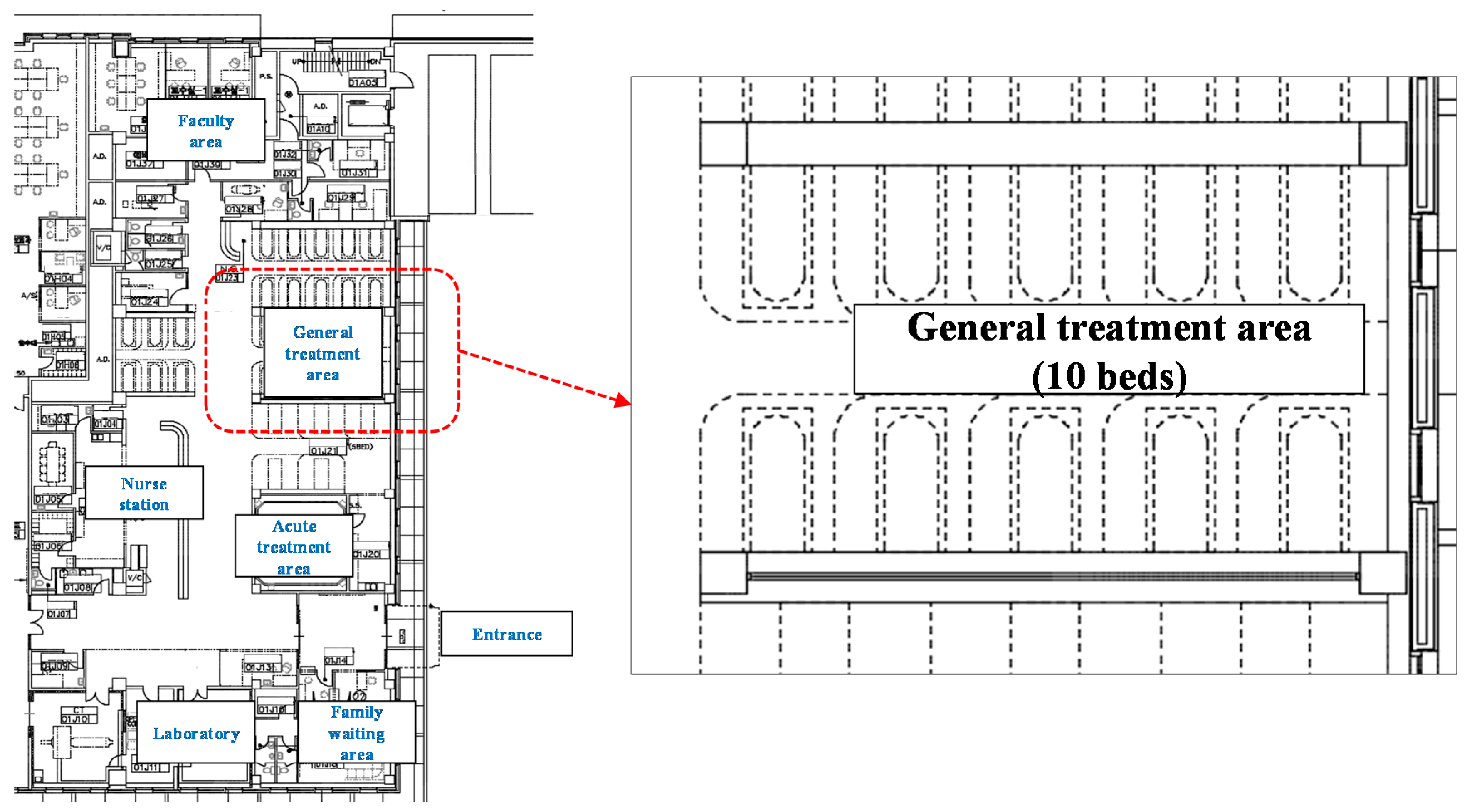
2.1. Selected Spaces for the Study
2.2. Simulation Tool
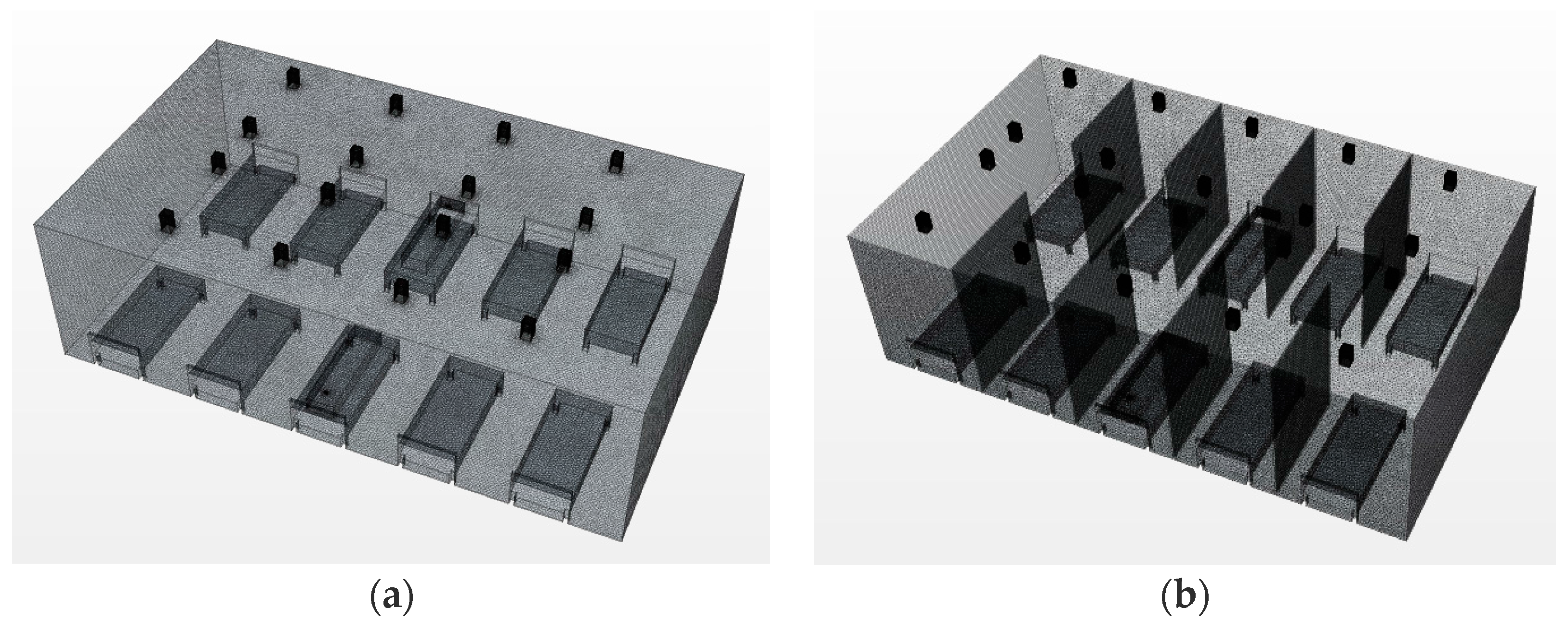
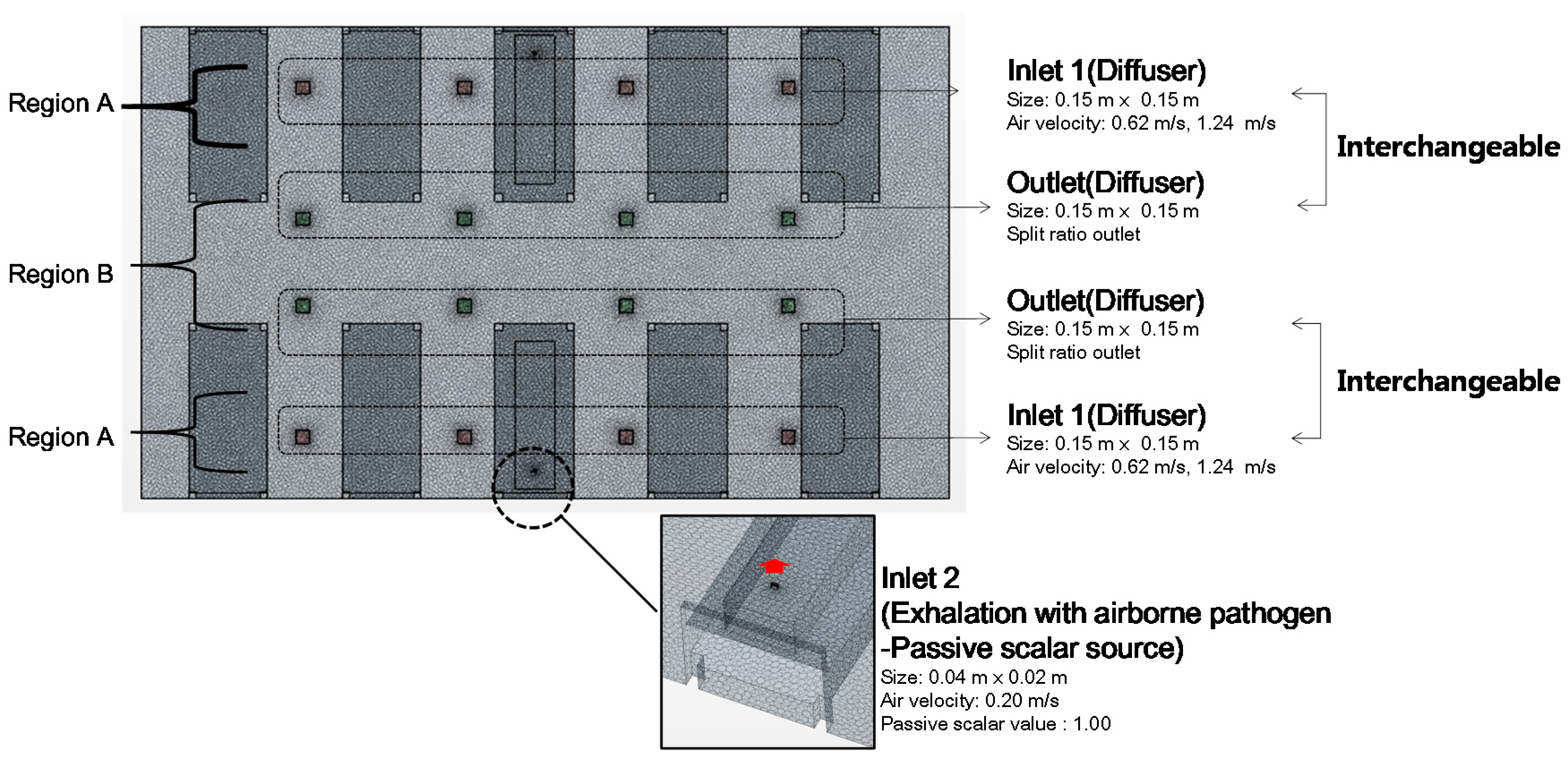
2.3. Modeling Condition
2.4. Simulation Cases
2.5. Wells–Riley Equation
- PI: Airborne infection probability of a susceptible person;
- C: Number of infection cases;
- I: Number of infectors;
- p: Breathing rate of a susceptible person; and
- q: Quantum generation rate by an infector.
2.6. Exposure Condition
3. Results
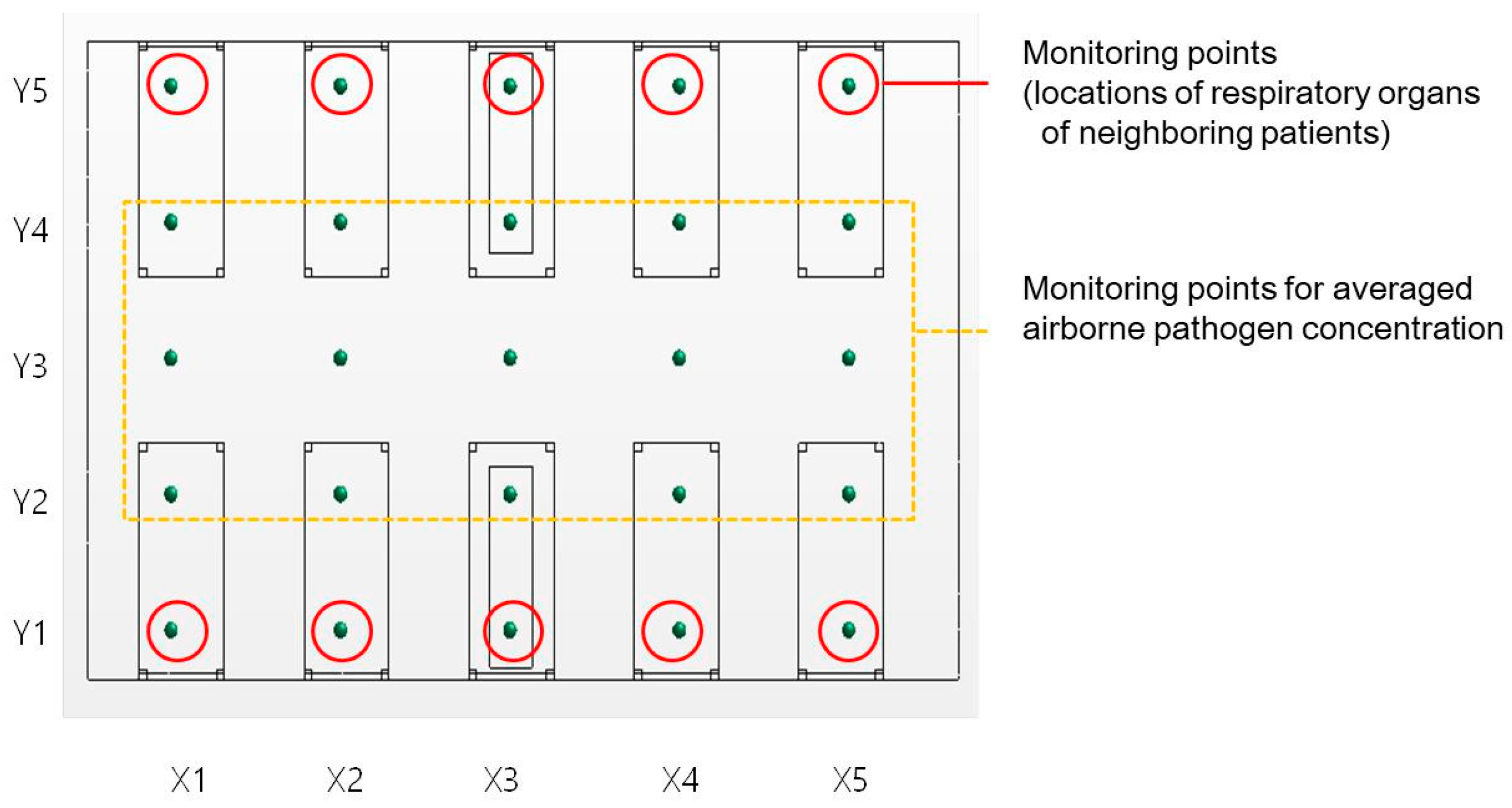
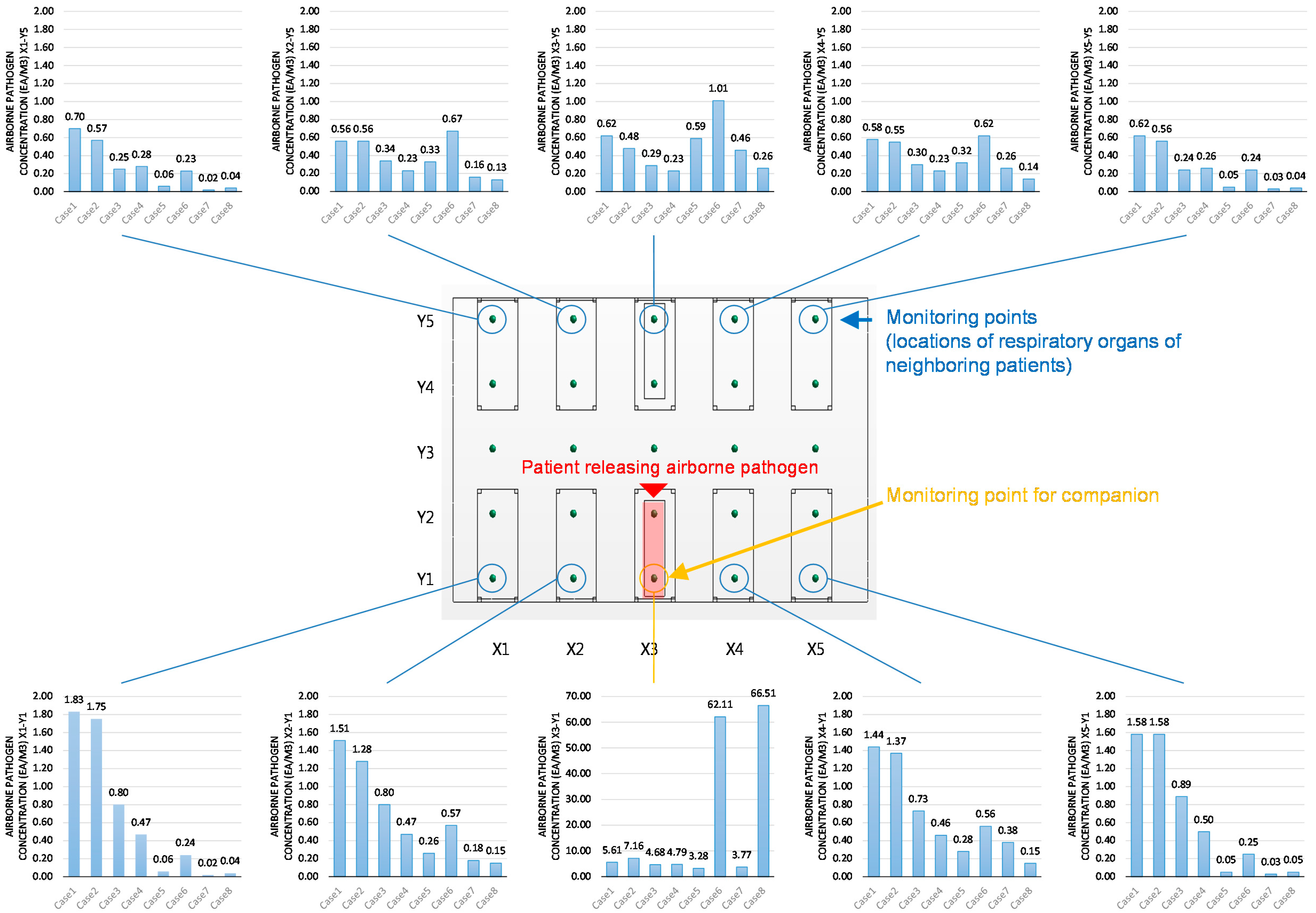
3.1. Pathogen Concentrations at Monitoring Points
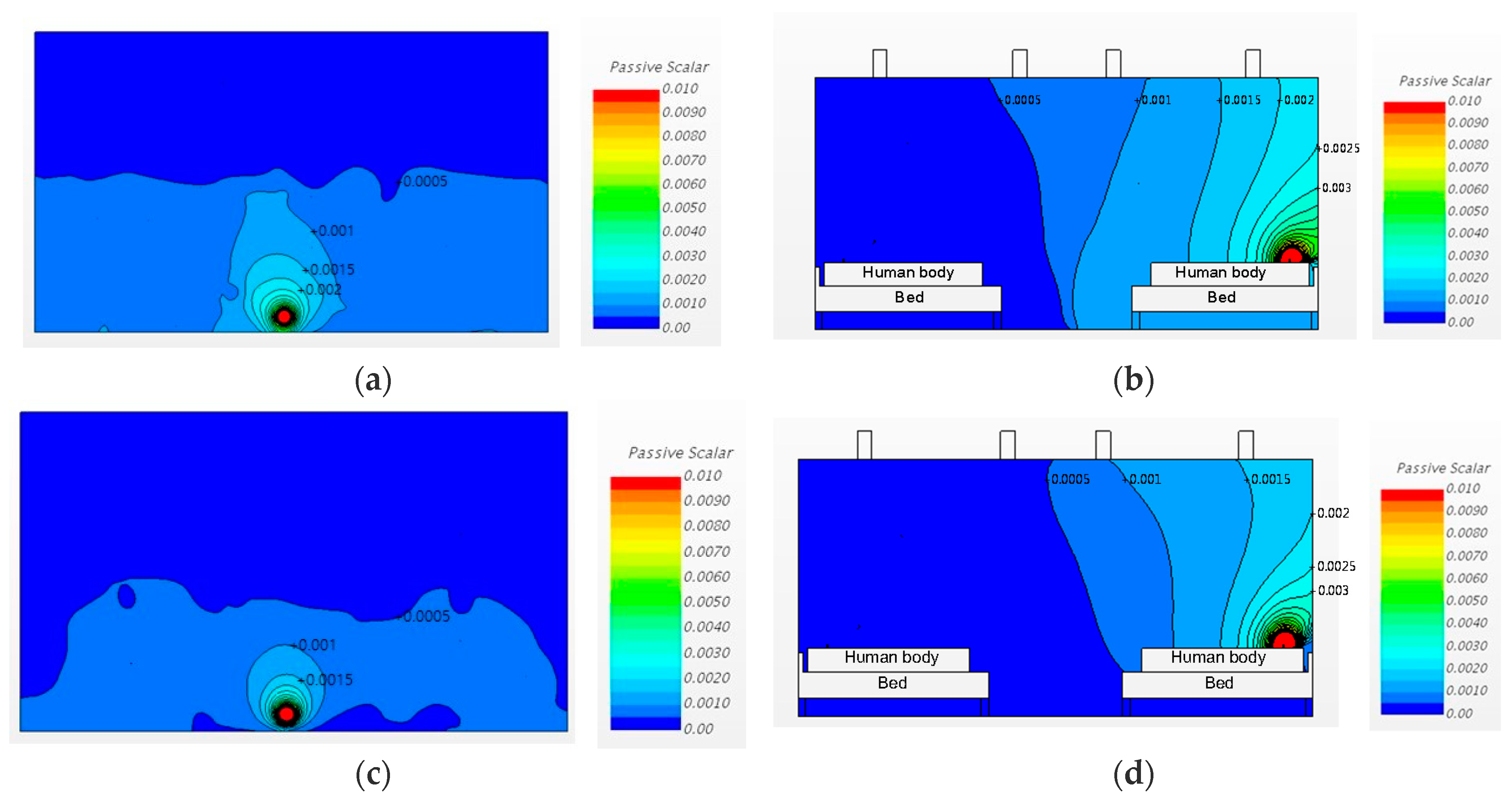
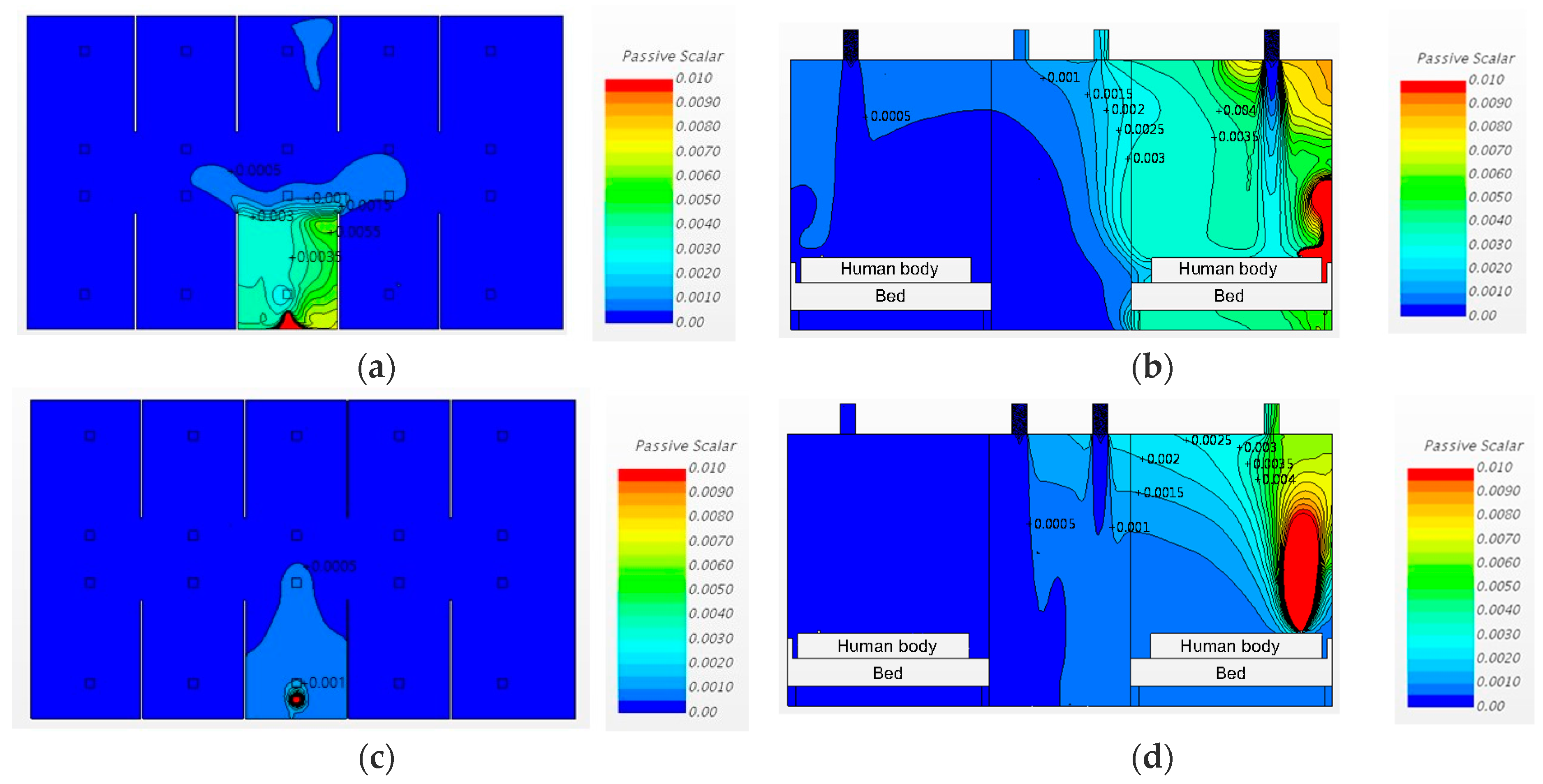
3.2. Airborne-Pathogen Concentration in the Cases without Partitions
3.2.1. Changing the Diffuser Locations
3.2.2. Changing the Diffuser Locations and Increasing the Ventilation Rate
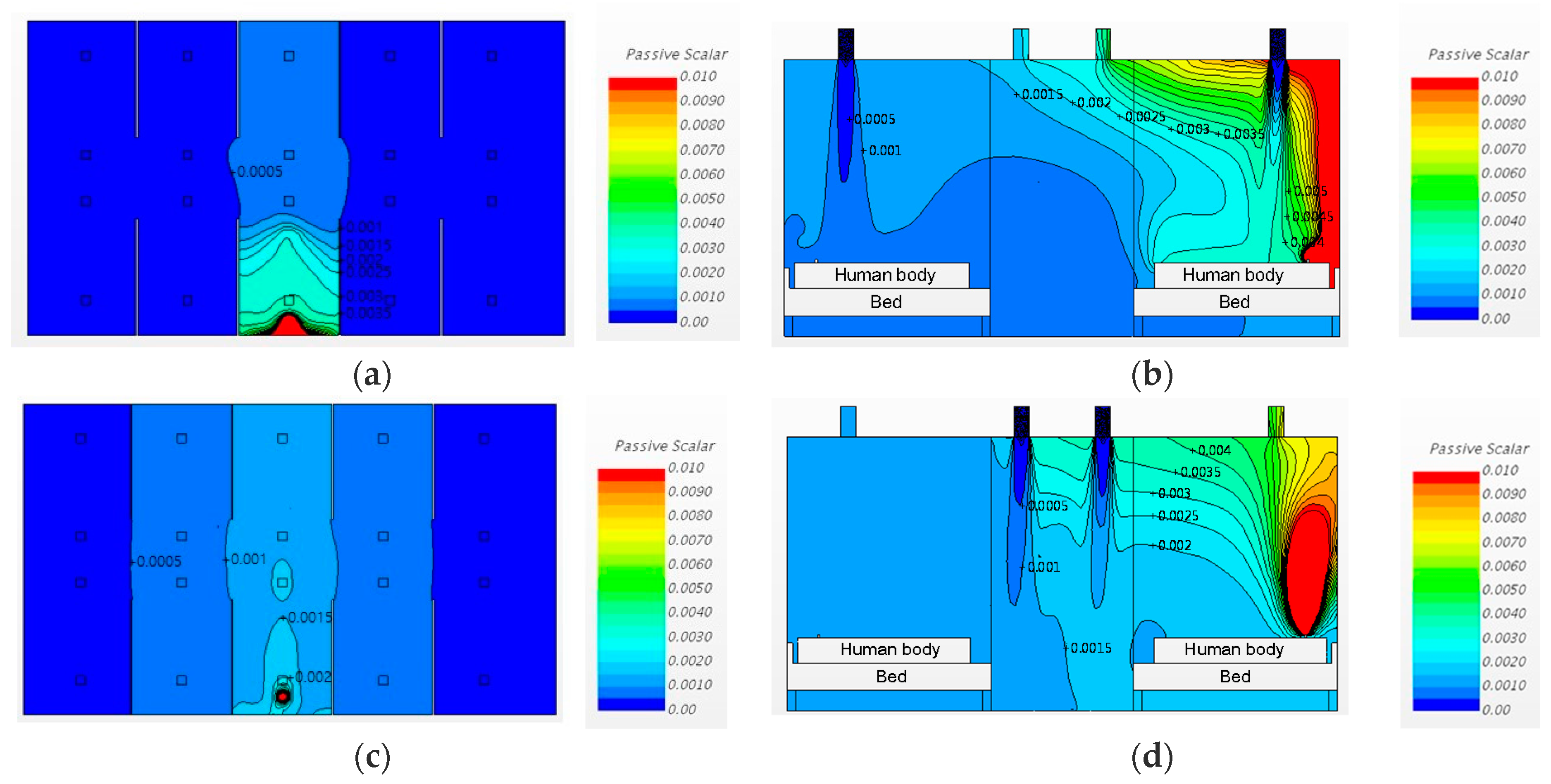
3.3. Airborne Pathogen Concentrations in Cases with Partitions
3.3.1. Changing the Diffuser Locations
3.3.2. Changing the Diffuser Locations and Increasing the Ventilation Rate
4. Discussion
5. Conclusions
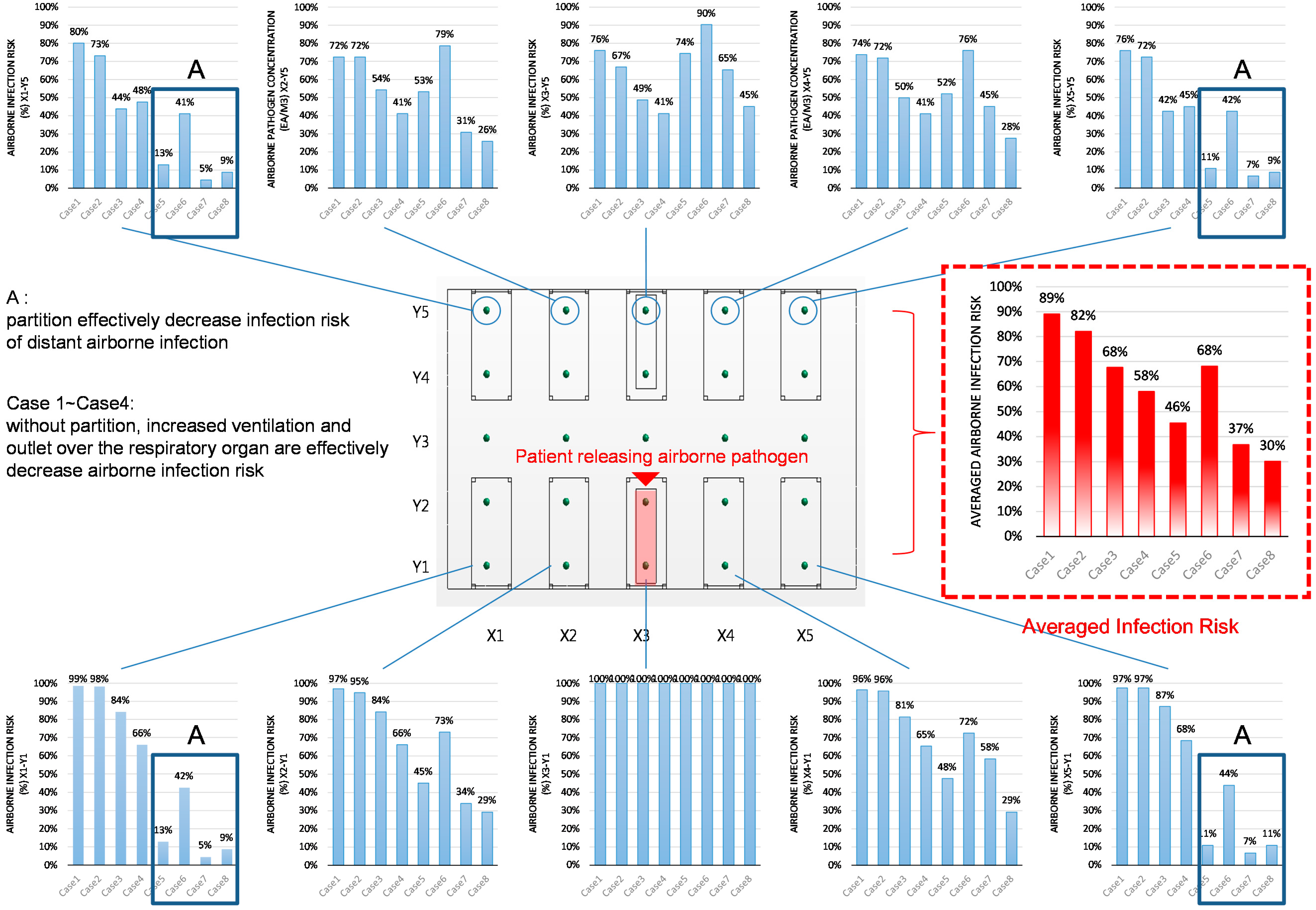
- (1).
- The most effective method for controlling airborne pathogen dispersion is increasing the ventilation rate.
- (2).
- Changing the ventilation inlet/outlet locations generally results in good prevention of airborne pathogen dispersion. However, it can also cause undesirable airborne pathogen dispersion in conditions with low ventilation rates and partitions (Case 6).
- (3).
- Installing partitions could contribute to decreasing the average airborne pathogen concentration (Cases 5, 7 and 8). However, it was also observed that the partitions could increase the pathogen concentrations in the beds opposite and adjacent to the pathogen source. Increasing the ventilation rate can enhance the effect of installing partitions.
- (4).
- In the analysis, the most effective method for pathogen control was to use all the methods studied: Increasing the ventilation rate, installing partitions, and positioning the ventilation system outlets over the patients’ respiratory organs (Region A).
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
| Location | Estimated Number of Airborne Pathogens at Each Location (Particles) | Notes | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 | Case 8 | ||
| X1-Y1 | 1.83 | 1.75 | 0.80 | 0.47 | 0.06 | 0.24 | 0.02 | 0.04 | patients nose |
| X1-Y2 | 1.64 | 1.06 | 0.71 | 0.45 | 0.08 | 0.27 | 0.02 | 0.05 | |
| X1-Y3 | 1.26 | 0.71 | 0.47 | 0.38 | 0.09 | 0.29 | 0.02 | 0.06 | |
| X1-Y4 | 0.76 | 0.64 | 0.26 | 0.28 | 0.09 | 0.26 | 0.02 | 0.05 | |
| X1-Y5 | 0.70 | 0.57 | 0.25 | 0.28 | 0.06 | 0.23 | 0.02 | 0.04 | patients nose |
| X2-Y1 | 1.51 | 1.28 | 0.80 | 0.47 | 0.26 | 0.57 | 0.18 | 0.15 | patients nose |
| X2-Y2 | 1.49 | 1.15 | 0.70 | 0.54 | 0.36 | 0.58 | 0.19 | 0.16 | |
| X2-Y3 | 1.14 | 0.67 | 0.43 | 0.40 | 0.32 | 0.85 | 0.35 | 0.20 | |
| X2-Y4 | 0.79 | 0.60 | 0.42 | 0.27 | 0.41 | 0.67 | 0.16 | 0.15 | |
| X2-Y5 | 0.56 | 0.56 | 0.34 | 0.23 | 0.33 | 0.67 | 0.16 | 0.13 | patients nose |
| X3-Y1 | 5.61 | 7.16 | 4.68 | 4.79 | 3.28 | 62.11 | 3.77 | 66.51 | pathogen source |
| X3-Y2 | 1.68 | 1.28 | 1.24 | 0.87 | 2.53 | 1.40 | 3.08 | 0.60 | |
| X3-Y3 | 1.03 | 0.56 | 0.64 | 0.30 | 0.71 | 1.38 | 0.40 | 0.43 | |
| X3-Y4 | 0.75 | 0.48 | 0.34 | 0.24 | 0.85 | 1.01 | 0.42 | 0.27 | |
| X3-Y5 | 0.62 | 0.48 | 0.29 | 0.23 | 0.59 | 1.01 | 0.46 | 0.26 | patients nose |
| X4-Y1 | 1.44 | 1.37 | 0.73 | 0.46 | 0.28 | 0.56 | 0.38 | 0.15 | patients nose |
| X4-Y2 | 1.41 | 1.00 | 0.64 | 0.54 | 0.37 | 0.58 | 0.41 | 0.18 | |
| X4-Y3 | 1.13 | 0.61 | 0.46 | 0.30 | 0.34 | 0.85 | 0.55 | 0.23 | |
| X4-Y4 | 0.74 | 0.55 | 0.37 | 0.26 | 0.40 | 0.62 | 0.27 | 0.15 | |
| X4-Y5 | 0.58 | 0.55 | 0.30 | 0.23 | 0.32 | 0.62 | 0.26 | 0.14 | patients nose |
| X5-Y1 | 1.58 | 1.58 | 0.89 | 0.50 | 0.05 | 0.25 | 0.03 | 0.05 | patients nose |
| X5-Y2 | 1.52 | 0.94 | 0.73 | 0.47 | 0.09 | 0.27 | 0.04 | 0.05 | |
| X5-Y3 | 1.19 | 0.68 | 0.47 | 0.40 | 0.09 | 0.35 | 0.05 | 0.08 | |
| X5-Y4 | 0.65 | 0.60 | 0.26 | 0.28 | 0.09 | 0.26 | 0.03 | 0.05 | |
| X5-Y5 | 0.62 | 0.56 | 0.24 | 0.26 | 0.05 | 0.24 | 0.03 | 0.04 | patients nose |
| Location | Estimated Airborne Infection Risk at Each Location (%) | Notes | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 | Case 8 | ||
| X1-Y1 | 99% | 98% | 84% | 66% | 13% | 42% | 5% | 9% | patients nose |
| X1-Y2 | 98% | 91% | 81% | 65% | 17% | 46% | 5% | 11% | |
| X1-Y3 | 95% | 81% | 66% | 58% | 19% | 49% | 5% | 13% | |
| X1-Y4 | 83% | 77% | 45% | 48% | 19% | 45% | 5% | 11% | |
| X1-Y5 | 80% | 73% | 44% | 48% | 13% | 41% | 5% | 9% | patients nose |
| X2-Y1 | 97% | 95% | 84% | 66% | 45% | 73% | 34% | 29% | patients nose |
| X2-Y2 | 97% | 93% | 80% | 71% | 56% | 74% | 35% | 31% | |
| X2-Y3 | 93% | 79% | 63% | 60% | 52% | 86% | 55% | 37% | |
| X2-Y4 | 84% | 75% | 62% | 46% | 61% | 79% | 31% | 29% | |
| X2-Y5 | 72% | 72% | 54% | 41% | 53% | 79% | 31% | 26% | patients nose |
| X3-Y1 | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 100% | pathogen source |
| X3-Y2 | 98% | 95% | 94% | 87% | 100% | 96% | 100% | 75% | |
| X3-Y3 | 91% | 72% | 77% | 50% | 81% | 96% | 60% | 63% | |
| X3-Y4 | 82% | 67% | 54% | 42% | 86% | 90% | 62% | 46% | |
| X3-Y5 | 76% | 67% | 49% | 41% | 74% | 90% | 65% | 45% | patients nose |
| X4-Y1 | 96% | 96% | 81% | 65% | 48% | 72% | 58% | 29% | patients nose |
| X4-Y2 | 96% | 90% | 77% | 71% | 57% | 74% | 61% | 34% | |
| X4-Y3 | 93% | 75% | 65% | 50% | 54% | 86% | 72% | 41% | |
| X4-Y4 | 82% | 72% | 57% | 45% | 60% | 76% | 46% | 29% | |
| X4-Y5 | 74% | 72% | 50% | 41% | 52% | 76% | 45% | 28% | patients nose |
| X5-Y1 | 97% | 97% | 87% | 68% | 11% | 44% | 7% | 11% | patients nose |
| X5-Y2 | 97% | 89% | 81% | 66% | 19% | 46% | 9% | 11% | |
| X5-Y3 | 94% | 79% | 66% | 60% | 19% | 55% | 11% | 17% | |
| X5-Y4 | 78% | 75% | 45% | 48% | 19% | 45% | 7% | 11% | |
| X5-Y5 | 76% | 72% | 42% | 45% | 11% | 42% | 7% | 9% | patients nose |
References
- Kim, S.J.; Kang, J.Y. Emergency nurses’ perception and performance of tuberculosis infection control measures. J. Korean Acad. Fundam. Nurs. 2010, 17, 351–361. [Google Scholar]
- Jo, G.R. Influencing Factors on the Compliance about Standard Precautions among ICU and ER Nurses. Master’s Thesis, Seoul National University, Seoul, Korea, 2007. Unpublished. [Google Scholar]
- Lee, A.J. Press Release for 278th Regular Session of the National Assembly of Korea. 2008. Available online: http://www.leeaejoo.net/common/filedown.php?ix=24 (accessed on 7 November 2016).
- Centers for Disease Control and Prevention. Guidelines for preventing the transmission of Mycobacterium tuberculosis in Health-Care Settings. Morb. Mortal. Wkly. Rep. 2005, 54, 1–142. [Google Scholar]
- American Institute of Architects (AIA). 1996–1997 Guidelines for Design and Construction of Hospitals and Health Care Facilities; The American Institute of Architect Press: Washington, DC, USA, 1996. [Google Scholar]
- Francis, J.; Curry National Tuberculosis Center; Institutional Consultation Services. Isolation Rooms: Design, Assessment, and Upgrade. 1999, pp. 9–12. Available online: https://books.google.ch/books/about/Isolation_Rooms.html?id=ySnPtgAACAAJ&redir_esc=y (accessed on 7 November 2016).
- Sung, M. HVAC system and contamination control in hospital relating MERS. Mag. Soc. Air-Cond. Refrig. Eng. Korea 2015, 44, 58–64. [Google Scholar]
- Atkinson, J.; Chartier, Y.; Pessoa-Silva, C.L.; Jensen, P.; Li, Y.; Seto, W.-H. Natural Ventilation for Infection Control in Health-Care Settings; World Health Organization: Geneva, Switzerland, 2009. [Google Scholar]
- Cheong, C.H.; Seo, Y.M.; Lee, S.H.; Kang, J.J. Improvement of airborne infection prevention methods in emergency room by design case study. J. Korean Soc. Living Environ. Syst. 2016, 23, 433–442. [Google Scholar] [CrossRef]
- Korea Centers for Disease Control & Prevention. Plan for Extended Installation of Negative Pressure Units at Nationally Designated Isolation Hospitals; Korea Centers for Disease Control & Prevention: Cheongju, Korea, 2015.
- Hathway, E.A.; Noakes, C.J.; Sleigh, P.A.; Fletcher, L.A. CFD simulation of airborne pathogen transport due to human activities. Build. Environ. 2011, 46, 2500–2511. [Google Scholar] [CrossRef]
- Zhu, S.; Srebric, J.; Spengler, J.D.; Demokritou, P. An advanced numerical model for the assessment of airborne transmission of influenza in bus microenvironments. Build. Environ. 2012, 47, 67–75. [Google Scholar] [CrossRef]
- Issarow, C.M.; Mulder, N.; Wood, R. Modelling the risk of airborne infectious disease using exhaled air. J. Theor. Biol. 2015, 372, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Rudnick, S.N.; Milton, D.K. Risk of indoor airborne infection transmission estimated from carbon dioxide concentration. Indoor Air 2003, 13, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Wells, W.F. Airborne Contagion and Air Hygiene; Cambridge University Press: Cambridge, MA, USA, 1955; pp. 117–122. [Google Scholar]
- Quan, H.; Li, Y.; Nielsen, P.V.; Huang, X. Spatial distribution of infection risk of SARS transmission in a hospital ward. Build. Environ. 2009, 44, 1651–1658. [Google Scholar] [CrossRef]
- Marsden, A.G. Influenza outbreak related to air travel. Med. J. Aust. 2003, 179, 172–173. [Google Scholar] [PubMed]
- Sze, G.N.; Chao, C.Y.H. Review and comparison between the Wells-Riley and dose-response approaches to risk assessment of infectious respiratory diseases. Indoor Air 2010, 20, 2–16. [Google Scholar] [CrossRef] [PubMed]
- Azimi, P.; Stephens, B. HVAC filtration for controlling infectious airborne disease transmission in indoor environments: Predicting risk reductions and operational costs. Build. Environ. 2013, 70, 150–160. [Google Scholar] [CrossRef]
- Yang, Y.K.; Kang, I.S.; Hang, J.H.; Park, J.C. CFD Simulation of the AII-Room for Preventing Spread of the Air-Borne Infection in Hospital. J. Archit. Inst. Korea Struct. Constr. 2017, 33, 61–68. [Google Scholar] [CrossRef]
- Edwards, D.A.; Man, J.C.; Brand, P.; Katstra, J.P.; Sommerer, K.; Stone, H.A.; Nardell, E.; Scheuch, G. Inhaling to mitigate exhaled bioaerosols. Proc. Natl. Acad. Sci. USA 2004, 101, 17383–17388. [Google Scholar] [CrossRef] [PubMed]
- Papineni, R.S.; Rosenthal, F.S. The size distribution of droplets in the exhaled breath of healthy human subjects. J. Aerosol Med. 1996, 10, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Fiegel, J.; Clarke, R.; Edwards, D.A. Airborne infectious disease and the suppression of pulmonary bioaerosols. Drug Discov Today 2006, 11, 51–57. [Google Scholar] [CrossRef]
- Park, S.Y.; Sin, D.S.; Lee, H.G.; Kim, H.S. Compliance with nosocomial infection control and related factors among emergency room nurses. J. Korean Acad. Fundam. Nurs. 2008, 15, 153–160. [Google Scholar]
- Yoon, J.M.; Sung, M.H. The relationship between empowerment and performance of infection control by emergency department nurses. J. Korean Acad. Fundam. Nurs. 2009, 16, 412–420. [Google Scholar]
- Simonds, A.K.; Hanak, A.; Chatwin, M.; Morrell, M.; Hall, A.; Parker, K.H.; Siggers, J.H.; Dickinson, R.J. Evaluation of droplet dispersion during non-invasive ventilation, oxygen therapy, nebulizer treatment and chest physiotherapy in clinical practice: Implications for management of pandemic influenza and other airborne infections. Health Technol. Assess. 2010, 14, 131–172. [Google Scholar] [CrossRef] [PubMed]
- Ching, W.H.; Leung, M.K.H.; Leung, D.Y.C. Reducing Risk of Airborne Transmitted Infection in Hospitals by Use of Hospital Curtains. Indoor Built Environ. 2008, 17, 252–259. [Google Scholar] [CrossRef]
- Tong, Z.; Chen, Y.; Malkawi, A.; Adamkiewicz, G.; Spengler, J.D. Quantifying the impact of traffic-related air pollution on the indoor air quality of a naturally ventilated building. Environ. Int. 2016, 89–90, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Deng, X. Assessment of Natural Ventilation Potential for Residential Buildings across Different Climate Zones in Australia. Atmosphere 2017, 8, 177. [Google Scholar] [CrossRef]
- Kolokotroni, M.; Giannitsaris, I.; Watkins, R. The effect of the London urban heat island on building summer cooling demand and night ventilation strategies. Sol. Energy 2006, 80, 383–392. [Google Scholar] [CrossRef]


| Types | Base Model & Type 1 |
|---|---|
| Physical conditions | Steady state flow: Segregated flow energy: Segregated fluid temperature turbulence: Realizable k-ε turbulence model gas: Air, ideal gas, incompressible gravity: −9.81 m/s2 Solver: Steady state |
| Meshing conditions | type: Polyhedral mesh prism layer thickness: 33% of the base (6 layers) |
| Inlet conditions 1 (diffuser) | velocity inlet (size: 0.15 m × 0.15 m) turbulence intensity: 2.5%, turbulent viscosity ratio: 10 3 ACH conditions: 0.62 m/s 6 ACH: conditions: 1.24 m/s |
| Outlet conditions (diffuser) | split ratio outlet (size: 0.15 m × 0.15 m) |
| Inlet conditions 2 (Exhalation with airborne pathogen) | velocity inlet (size: 0.04 m × 0.02 m) turbulence intensity: 1%, turbulent viscosity ratio: 10 passive scalar concentration: 1 |
| Ventilation rate | 3 ACH (base) or 6 ACH (type 1) |
| Thermal | inlet air flow: 23 °C, wall: 22 °C human body: 31 °C, exhalation: 36.5 °C |
| Convergence criteria | <10−4 |
| Models | Number of Cells (Volume Mesh) |
|---|---|
| Base model | 2,405,265 |
| Type 1 | 2,691,618 |
| Model | Case # | Partition Condition | Ventilation Rate | Diffuser Location |
|---|---|---|---|---|
| Base model | Case 1 | Without partition | 3 ACH | Inlet (Region A) Outlet (Region B) |
| Case 2 | Inlet (Region B) Outlet (Region A) | |||
| Case 3 | 6 ACH | Inlet (Region A) Outlet (Region B) | ||
| Case 4 | Inlet (Region B) Outlet (Region A) | |||
| Type 1 | Case 5 | With partition | 3 ACH | Inlet (Region A) Outlet (Region B) |
| Case 6 | Inlet (Region B) Outlet (Region A) | |||
| Case 7 | 6 ACH | Inlet (Region A) Outlet (Region B) | ||
| Case 8 | Inlet (Region B) Outlet (Region A) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheong, C.H.; Lee, S. Case Study of Airborne Pathogen Dispersion Patterns in Emergency Departments with Different Ventilation and Partition Conditions. Int. J. Environ. Res. Public Health 2018, 15, 510. https://doi.org/10.3390/ijerph15030510
Cheong CH, Lee S. Case Study of Airborne Pathogen Dispersion Patterns in Emergency Departments with Different Ventilation and Partition Conditions. International Journal of Environmental Research and Public Health. 2018; 15(3):510. https://doi.org/10.3390/ijerph15030510
Chicago/Turabian StyleCheong, Chang Heon, and Seonhye Lee. 2018. "Case Study of Airborne Pathogen Dispersion Patterns in Emergency Departments with Different Ventilation and Partition Conditions" International Journal of Environmental Research and Public Health 15, no. 3: 510. https://doi.org/10.3390/ijerph15030510




