Association between Inflammatory Bowel Disease and Cholelithiasis: A Nationwide Population-Based Cohort Study
Abstract
:1. Introduction
2. Methods
2.1. Data Source
2.2. Sampled Participants
2.3. Statistical Analysis
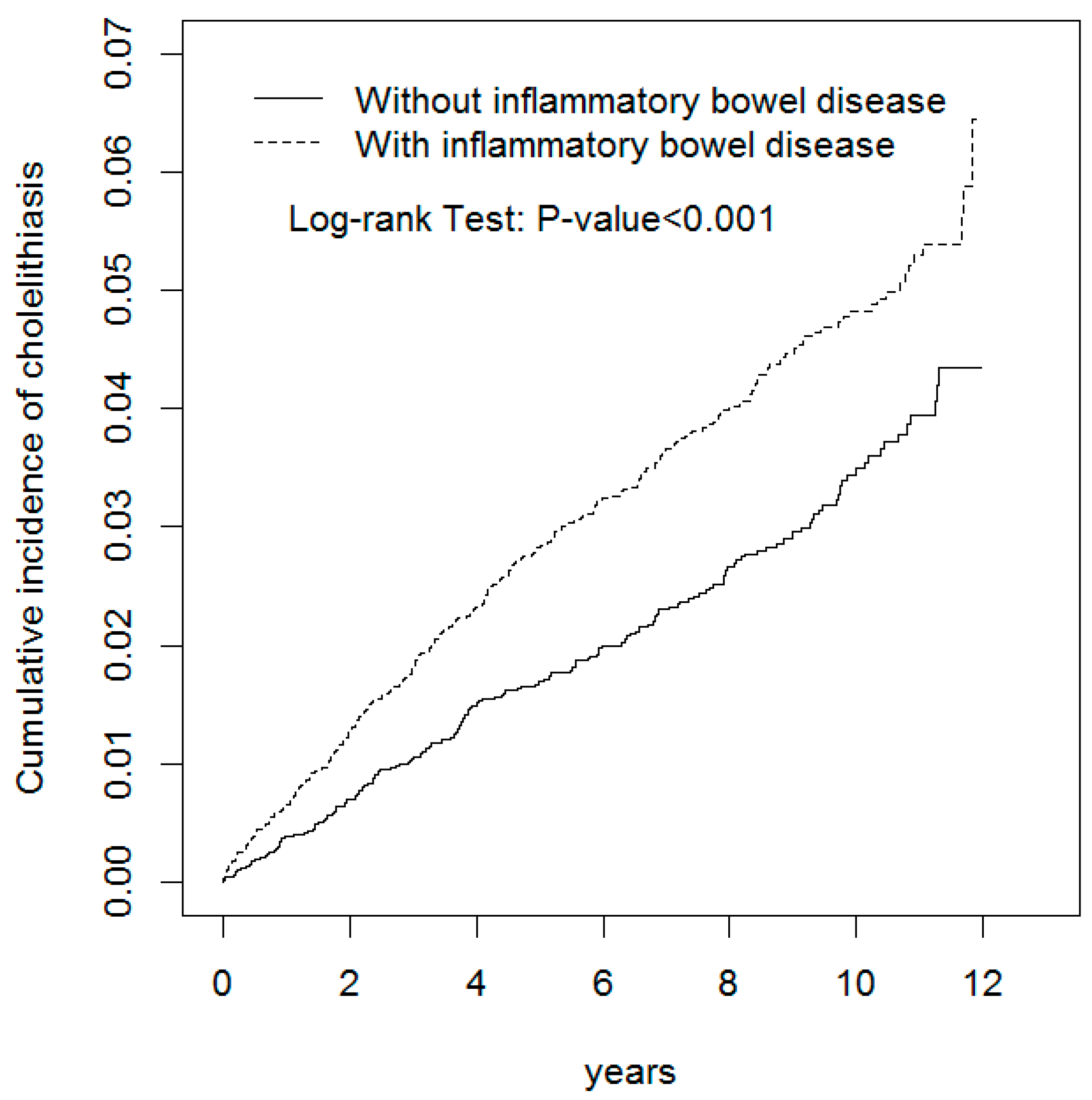
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| IBD | inflammatory bowel disease |
| CD | Crohn’s disease |
| UC | ulcerative colitis |
| aHR | adjusted hazard ratio |
| CI | confidence interval |
| LHID2000 | Longitudinal Health Insurance Research Database 2000 |
| GSD | gallbladder stone disease |
| CBD | common bile duct |
| IHSs | intrahepatic stones |
| NHI | National Health Insurance |
| ICD-9-CM | International Classification of Diseases, Ninth Revision, Clinical Modification |
| COPD | chronic obstructive pulmonary disease |
| CAD | coronary artery disease |
| HRs | hazard ratios |
| SDs | standard deviations |
| NHIRD | National Health Insurance Research Database |
References
- Shaffer, E.A. Gallstone disease: Epidemiology of gallbladder stone disease. Best Pract. Res. Clin. Gastroenterol. 2006, 20, 981–996. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Lu, C.L.; Huang, Y.S.; Tam, T.N.; Chao, Y.; Chang, F.Y.; Lee, S.D. Age is one of the risk factors in developing gallstone disease in Taiwan. Age Ageing 1998, 27, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Huang, M.H.; Yang, J.C.; Nien, C.K.; Etheredge, G.D.; Yang, C.C.; Yeh, Y.H.; Wu, H.S.; Chou, D.A.; Yueh, S.K. Prevalence and risk factors of gallstone disease in an adult population of Taiwan: An epidemiological survey. J. Gastroenterol. Hepatol. 2006, 21, 1737–1743. [Google Scholar] [CrossRef] [PubMed]
- Stinton, L.M.; Myers, R.P.; Shaffer, E.A. Epidemiology of gallstones. Gastroenterol. Clin. N. Am. 2010, 39, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.C.; Bernstein, C.N.; Vatn, M.H.; Lakatos, P.L.; Loftus, E.V., Jr.; Tysk, C.; O’Morain, C.; Moum, B.; Colombel, J.F. Epidemiology and Natural History Task Force of the International Organization of Inflammatory Bowel Disease (IOIBD). Geographical variability and environmental risk factors in inflammatory bowel disease. Gut 2013, 62, 630–649. [Google Scholar] [CrossRef] [PubMed]
- Gizard, E.; Ford, A.C.; Bronowicki, J.P.; Peyrin-Biroulet, L. Systematic review: The epidemiology of the hepatobiliary manifestations in patients with inflammatory bowel disease. Aliment. Pharmacol. Ther. 2014, 40, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Whorwell, P.J.; Hawkins, R.; Dewbury, K.; Wright, R. Ultrasound survey of gallstones and other hepatobiliary disorders in patients with Crohn’s disease. Dig. Dis. Sci. 1984, 29, 930–933. [Google Scholar] [CrossRef] [PubMed]
- Vitek, L.; Carey, M.C. Enterohepatic cycling of bilirubin as a cause of ‘black’ pigment gallstones in adult life. Eur. J. Clin. Investig. 2003, 33, 799–810. [Google Scholar] [CrossRef]
- Mibu, R.; Makino, I.; Chijiiwa, K. Gallstones and their composition in patients with ileoanal anastomosis. J. Gastroenterol. 1995, 30, 413–415. [Google Scholar] [CrossRef] [PubMed]
- Ha, J.H.; Park, Y.S.; Seon, C.S.; Son, B.K.; Ahn, S.B.; Jo, Y.K.; Kim, S.H.; Jo, Y.J.; Kim, J.H.; Han, J.H.; et al. Increased risk of asymptomatic gallstones in patients with ulcerative colitis. Intestig. Res. 2015, 13, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Said, K.; Glaumann, H.; Bergquist, A. Gallbladder disease in patients with primary sclerosing cholangitis. J. Hepatol. 2008, 48, 598–605. [Google Scholar] [CrossRef] [PubMed]
- Parente, F.; Pastore, L.; Bargiggia, S.; Cucino, C.; Greco, S.; Molteni, M.; Ardizzone, S.; Porro, G.B.; Sampietro, G.M.; Giorgi, R.; et al. Incidence and risk factors for gallstones in patients with inlammatory bowel disease: A large case-control study. Hepatology 2007, 45, 1267–1274. [Google Scholar] [CrossRef] [PubMed]
- Navaneethan, U.; Shen, B. Hepatopancreatobiliary manifestations and complications associated with inflammatory bowel disease. Inflamm. Bowel Dis. 2010, 16, 1598–1619. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.M.; Xu, C.F.; Shan, G.D.; Chen, H.T.; Xu, G.Q. Is gallstone disease associated with inflammatory bowel diseases? A meta-analysis. J. Dig. Dis. 2015, 16, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Lorusso, D.; Leo, S.; Mossa, A.; Misciagna, G.; Guerra, V. Cholelithiasis in inflammatory bowel disease. A case-control study. Dis Colon Rectum 1990, 33, 791–794. [Google Scholar] [CrossRef] [PubMed]
- Database NHIR. Taiwan. Available online: http://nhird.nhri.org.tw/en/index.html (accessed on 14 March 2018).
- Chen, H.Y.; Lin, C.L.; Kao, C.H. Does Migraine Increase the Risk of Glaucoma: A Population-Based Cohort Study. Medicine 2016, 95, e3670. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Lin, C.H.; Kao, C.H. Association between gallbladder stone disease and prostate cancer: A nationwide population-based study. Oncotarget 2016. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.C. Epidemiology of inflammatory bowel disease: Focus on Asia. Best Pract. Res. Clin. Gastroenterol. 2014, 28, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Leong, R.W.; Lau, J.Y.; Sung, J.J. The epidemiology and phenotype of Crohn’s disease in the Chinese population. Inflamm. Bowel Dis. 2004, 10, 646–651. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.C.; Tang, W.; Ching, J.Y.; Wong, M.; Chow, C.M.; Hui, A.J.; Wong, T.C.; Leung, V.K.; Tsang, S.W.; Yu, H.H.; et al. Incidence and phenotype of inflammatory bowel disease based on results from the Asia-pacific Crohn’s and colitis epidemiology study. Gastroenterology 2013, 145, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Duboc, H.; Rajca, S.; Rainteau, D.; Benarous, D.; Maubert, M.A.; Quervain, E.; Thomas, G.; Barbu, V.; Humbert, L.; Despras, G.; et al. Connecting dysbiosis, bile-acid dysmetabolism and gut inflammation in inflammatory bowel diseases. Gut 2013, 62, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Maurer, K.J.; Carey, C.; Rao, V.P.; Ge, Z.; Rogers, A.B.; Oura, T.J.; Carey, M.C.; Fox, J.G. T-cell function is critical for murine cholesterol gallstone formation. Gastroenterology 2007, 133, 1304–1315. [Google Scholar] [CrossRef] [PubMed]
- Kratzer, W.; Haenle, M.M.; Mason, R.A.; von Tirpitz, C.; Kaechele, V. Prevalence of cholelithiasis in patients with chronic inflammatory bowel disease. World J. Gastroenterol. 2005, 11, 6170–6175. [Google Scholar] [CrossRef] [PubMed]
- Fraquelli, M.; Losco, A.; Visentin, S.; Cesana, B.M.; Pometta, R.; Colli, A.; Conte, D. Gallstones disease and related risk factors in patients with Crohn disease. Arch. Intern. Med. 2001, 161, 2201–2204. [Google Scholar] [CrossRef] [PubMed]
| Variable | Inflammatory Bowel Disease | p-Value | |
|---|---|---|---|
| No | Yes | ||
| N = 8186 | N = 8186 | ||
| Age, year | 0.99 | ||
| ≤49 | 4691 (57.3) | 4820 (58.9) | |
| 50–64 | 1868 (22.8) | 1848 (22.6) | |
| 65+ | 1627 (19.9) | 1518 (18.5) | |
| Mean ± SD † | 47.9 (17.2) | 47.7 (16.8) | 0.32 |
| Sex | 0.77 | ||
| Female | 4323 (52.8) | 4304 (52.6) | |
| Male | 3863 (47.2) | 3882 (47.4) | |
| Comorbidity | |||
| Hyperlipidemia | 1457 (17.8) | 1451 (17.7) | 0.90 |
| Diabetes | 559 (6.83) | 563 (6.88) | 0.90 |
| Liver cirrhosis | 95 (1.16) | 81 (0.99) | 0.29 |
| Alcohol-related illness | 257 (3.14) | 265 (3.24) | 0.72 |
| Hypertension | 2324 (28.4) | 2283 (27.9) | 0.48 |
| COPD | 912 (11.1) | 924 (11.3) | 0.77 |
| Obesity | 96 (1.17) | 95 (1.16) | 0.94 |
| Stroke | 231 (2.82) | 227 (2.77) | 0.85 |
| CAD | 1291 (15.8) | 1260 (15.4) | 0.50 |
| Hepatitis B virus | 247 (3.02) | 243 (2.97) | 0.85 |
| Hepatitis C virus | 101 (1.23) | 109 (1.33) | 0.58 |
| Variable | Event | PY | Rate # | Crude HR & (95% CI) | Adjusted HR † (95% CI) |
|---|---|---|---|---|---|
| Inflammatory bowel disease | |||||
| No | 204 | 58,413 | 3.49 | 1.00 | 1.00 |
| Yes | 305 | 58,535 | 5.21 | 1.49 (1.25, 1.78) *** | 1.51 (1.27, 1.81) *** |
| Age, year | |||||
| ≤49 | 169 | 71,822 | 2.35 | 1.00 | 1.00 |
| 50–64 | 155 | 26,558 | 5.84 | 2.47 (1.99, 3.08) *** | 1.99 (1.57, 2.52) *** |
| 65+ | 185 | 18,568 | 9.96 | 4.21 (3.42, 5.19) *** | 2.80 (2.15, 3.66) *** |
| Sex | |||||
| Female | 295 | 62,540 | 4.72 | 1.20 (1.01, 1.43) * | 1.21 (1.01, 1.44) * |
| Male | 214 | 54,408 | 3.93 | 1.00 | 1.00 |
| Comorbidity | |||||
| Hyperlipidemia | |||||
| No | 362 | 98,034 | 3.69 | 1.00 | 1.00 |
| Yes | 147 | 18,913 | 7.77 | 2.09 (1.73, 2.54) *** | 1.13 (0.91, 1.40) |
| Diabetes | |||||
| No | 443 | 110,523 | 4.01 | 1.00 | 1.00 |
| Yes | 66 | 6424 | 10.3 | 2.54 (1.96, 3.29) *** | 1.29 (0.97, 1.70) |
| Liver cirrhosis | |||||
| No | 499 | 116,153 | 4.30 | 1.00 | 1.00 |
| Yes | 10 | 795 | 12.6 | 2.87 (1.53, 5.37) ** | 1.53 (0.79, 2.96) |
| Alcohol-related illness | |||||
| No | 491 | 114,124 | 4.30 | 1.00 | 1.00 |
| Yes | 18 | 2824 | 6.37 | 1.45 (0.91, 2.33) | - |
| Hypertension | |||||
| No | 266 | 87,340 | 3.05 | 1.00 | 1.00 |
| Yes | 243 | 29,608 | 8.21 | 2.68 (2.25, 3.19) *** | 1.28 (1.01, 1.61) * |
| COPD | |||||
| No | 403 | 106,079 | 3.80 | 1.00 | 1.00 |
| Yes | 106 | 10,869 | 9.75 | 2.68 (2.25, 3.19) *** | 1.43 (1.13, 1.81) ** |
| Obesity | |||||
| No | 504 | 115,912 | 4.35 | 1.00 | 1.00 |
| Yes | 5 | 1036 | 4.83 | 1.09 (0.45, 2.63) | - |
| Stroke | |||||
| No | 484 | 114,699 | 4.22 | 1.00 | 1.00 |
| Yes | 25 | 2248 | 11.1 | 2.58 (1.72, 3.86) *** | 1.13 (0.75, 1.72) |
| CAD | |||||
| No | 363 | 101,005 | 3.59 | 1.00 | 1.00 |
| Yes | 146 | 15943 | 9.16 | 2.54 (2.09, 3.07) *** | 1.13 (0.90, 1.42) |
| HBV | |||||
| No | 496 | 114,123 | 4.35 | 1.00 | 1.00 |
| Yes | 13 | 2825 | 4.60 | 1.05 (0.60, 1.82) | - |
| HCV | |||||
| No | 497 | 115,813 | 4.29 | 1.00 | 1.00 |
| Yes | 12 | 1135 | 10.6 | 2.44 (1.38, 4.33) ** | 1.54 (0.85, 2.82) |
| Variables | Inflammatory Bowel Disease | Crude HR & (95% CI) | Adjusted HR † (95% CI) | |||||
|---|---|---|---|---|---|---|---|---|
| No | Yes | |||||||
| Event | PY | Rate # | Event | PY | Rate # | |||
| Age, years | ||||||||
| ≤49 | 66 | 35,488 | 1.86 | 103 | 36,335 | 2.83 | 1.52 (1.12, 2.07) ** | 1.47 (1.08, 2.00) * |
| 50–64 | 56 | 13,365 | 4.19 | 99 | 13,193 | 7.50 | 1.79 (1.29, 2.48) *** | 1.80 (1.30, 2.50) *** |
| 65+ | 82 | 9561 | 8.58 | 103 | 9008 | 11.4 | 1.33 (1.00, 1.78) | 1.33 (1.00, 1.78) |
| Sex | ||||||||
| Female | 120 | 31,232 | 3.84 | 175 | 31,308 | 5.59 | 1.45 (1.15, 1.83) ** | 1.46 (1.16, 1.84) ** |
| Male | 84 | 27,181 | 3.09 | 130 | 27,227 | 4.77 | 1.54 (1.17, 2.03) ** | 1.60 (1.22, 2.11) *** |
| Comorbidity | ||||||||
| No | 65 | 36,320 | 1.79 | 108 | 34,726 | 3.11 | 1.73 (1.27, 2.36) *** | 1.73 (1.28, 2.36) *** |
| Yes | 139 | 22,092 | 6.29 | 197 | 23,808 | 8.27 | 1.32 (1.06, 1.64) * | 1.39 (1.12, 1.72) ** |
| Variables (ICD-9 Code) | Event | Rate # | Crude HR & (95% CI) | Adjusted HR † (95% CI) |
|---|---|---|---|---|
| Cholelithiasis | ||||
| Non-IBD cohort (N = 8186) | 204 | 3.49 | 1 (Reference) | 1 (Reference) |
| IBD | ||||
| Crohn’s disease (N = 872) | 42 | 7.13 | 2.03 (1.46, 2.83) *** | 1.87 (1.34, 2.61) *** |
| Ulcerative colitis (N = 7314) | 263 | 5.00 | 1.43 (1.19, 1.72) *** | 1.47 (1.22, 1.76) *** |
| Gallbladder stones | ||||
| Non-IBD cohort (N = 8186) | 182 | 3.12 | 1 (Reference) | 1 (Reference) |
| IBD | ||||
| Crohn’s disease (N = 872) | 35 | 5.94 | 1.90 (1.32, 2.73) *** | 1.76 (1.23, 2.53) ** |
| Ulcerative colitis (N = 7314) | 230 | 4.37 | 1.40 (1.15, 1.70) *** | 1.44 (1.19, 1.75) *** |
| Non-gallbladder stones | ||||
| Non-IBD cohort (N = 8186) | 22 | 0.38 | 1 (Reference) | 1 (Reference) |
| IBD | ||||
| Crohn’s disease (N = 872) | 7 | 1.19 | 3.14 (1.34, 7.35) ** | 2.78 (1.18, 6.51) * |
| Ulcerative colitis (N = 7314) | 33 | 0.63 | 1.66 (0.97, 2.84) | 1.70 (0.99, 2.91) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.-H.; Lin, C.-L.; Kao, C.-H. Association between Inflammatory Bowel Disease and Cholelithiasis: A Nationwide Population-Based Cohort Study. Int. J. Environ. Res. Public Health 2018, 15, 513. https://doi.org/10.3390/ijerph15030513
Chen C-H, Lin C-L, Kao C-H. Association between Inflammatory Bowel Disease and Cholelithiasis: A Nationwide Population-Based Cohort Study. International Journal of Environmental Research and Public Health. 2018; 15(3):513. https://doi.org/10.3390/ijerph15030513
Chicago/Turabian StyleChen, Chien-Hua, Cheng-Li Lin, and Chia-Hung Kao. 2018. "Association between Inflammatory Bowel Disease and Cholelithiasis: A Nationwide Population-Based Cohort Study" International Journal of Environmental Research and Public Health 15, no. 3: 513. https://doi.org/10.3390/ijerph15030513





