A Qualitative Study of Airborne Minerals and Associated Organic Compounds in Southeast of Cairo, Egypt
Abstract
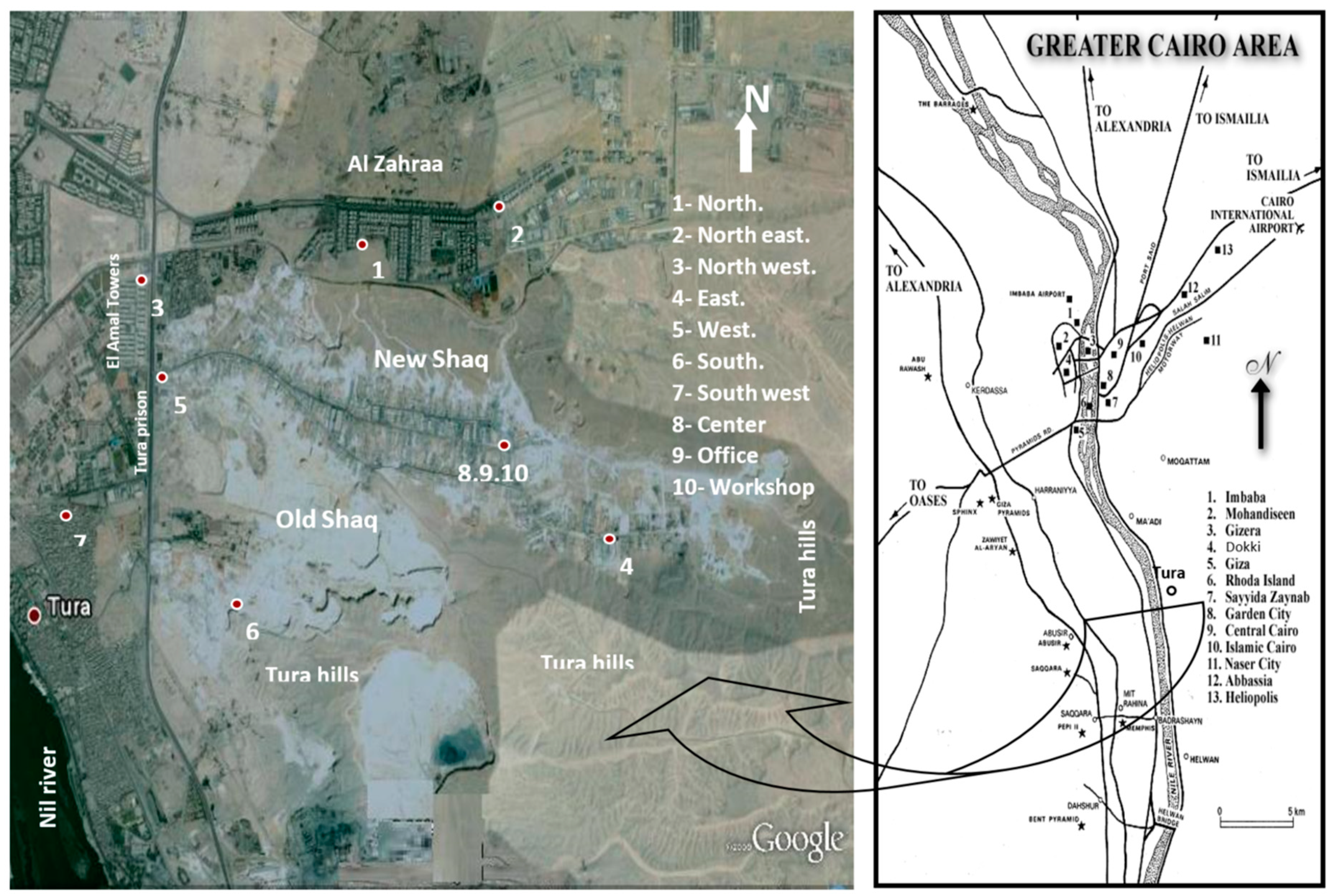
:1. Introduction
2. Materials and Methods
2.1. Polarizing Microscope
2.2. Infrared Spectroscopy
2.3. X-ray Diffraction Analysis
3. Results and Discussion
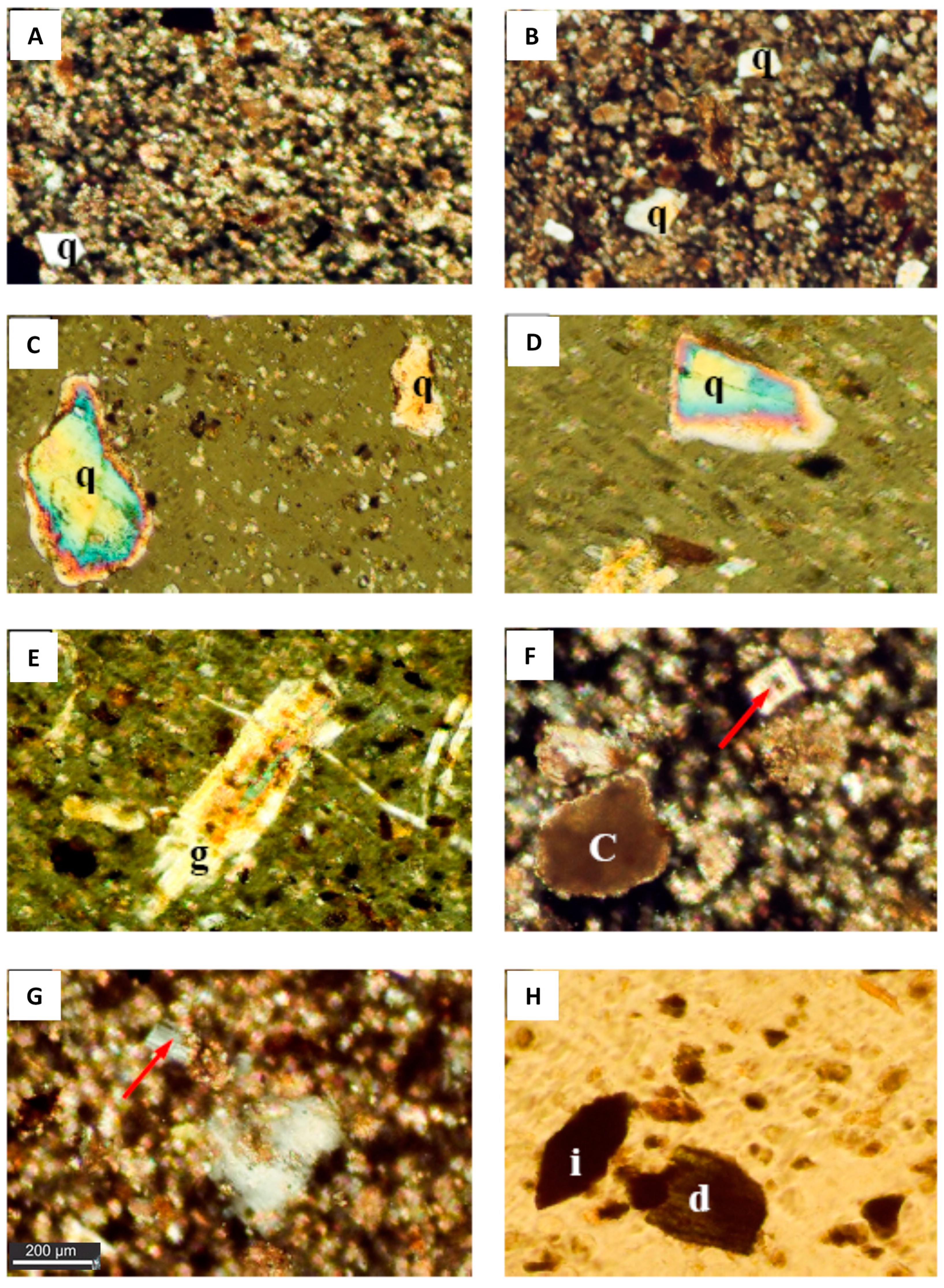
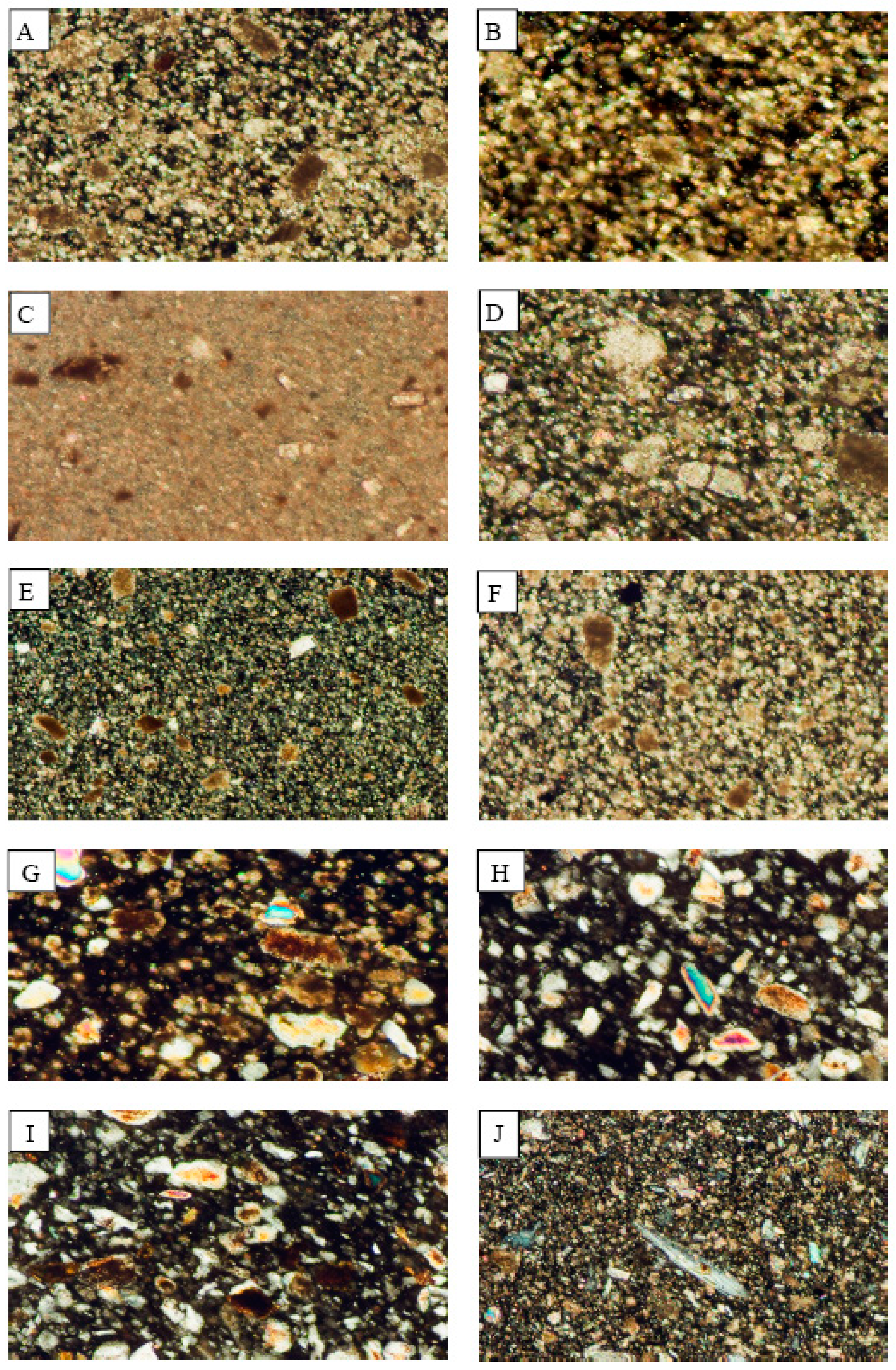
3.1. Microscopic Examination
3.1.1. Dust Fall Samples
3.1.2. Rock Samples
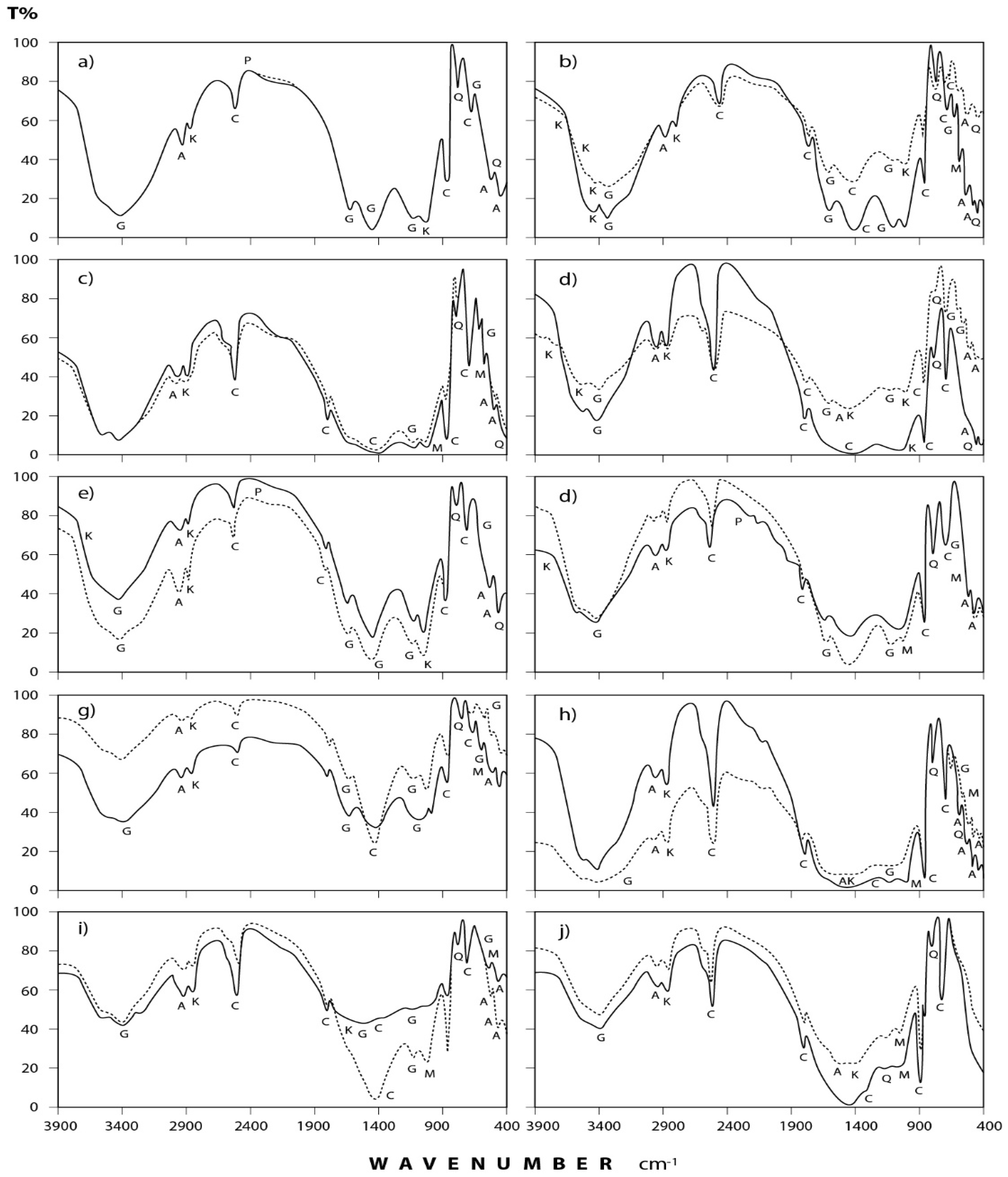
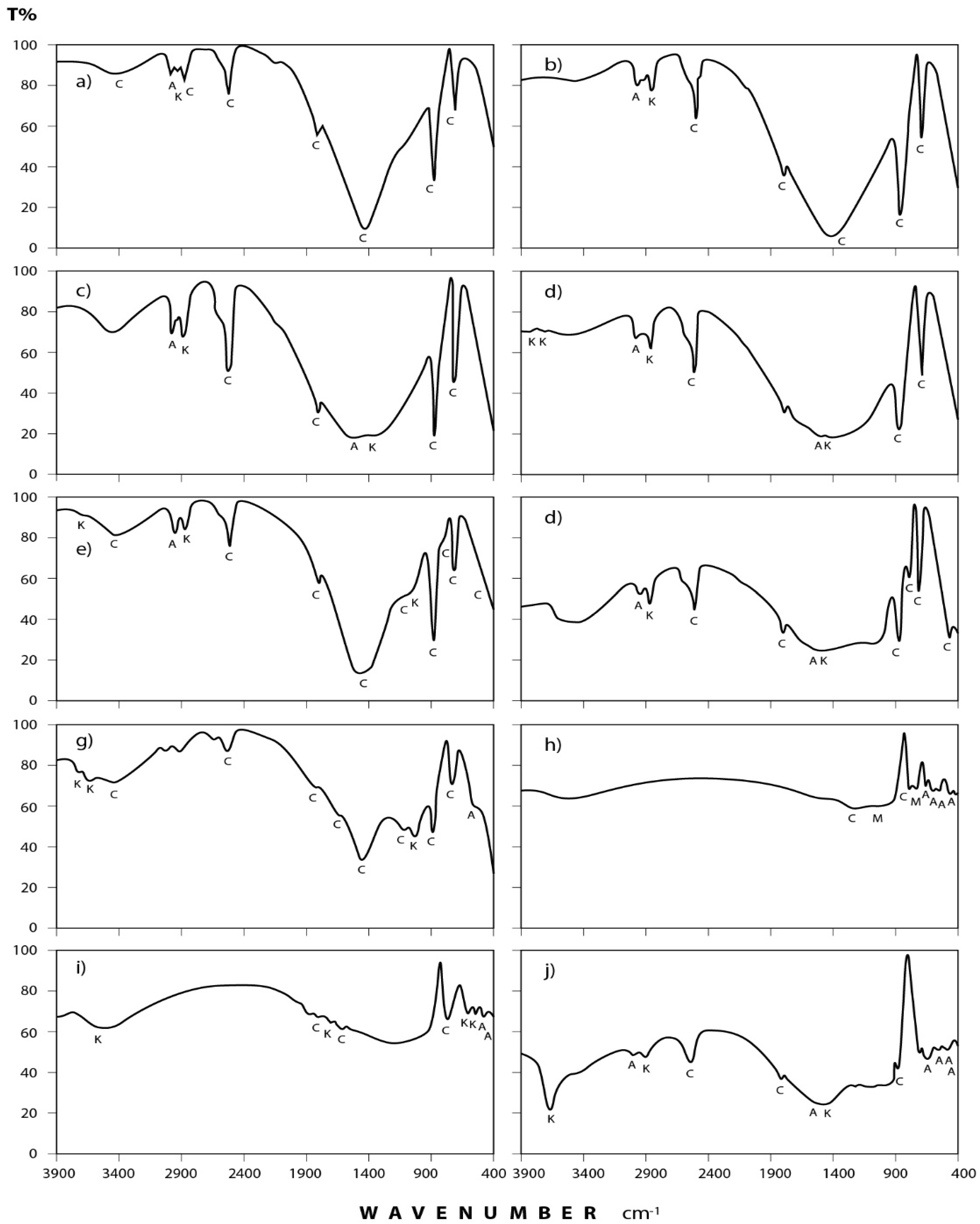
3.2. Infrared Spectra Interpretation
3.2.1. Dust Fall Samples
3.2.2. Rock Samples
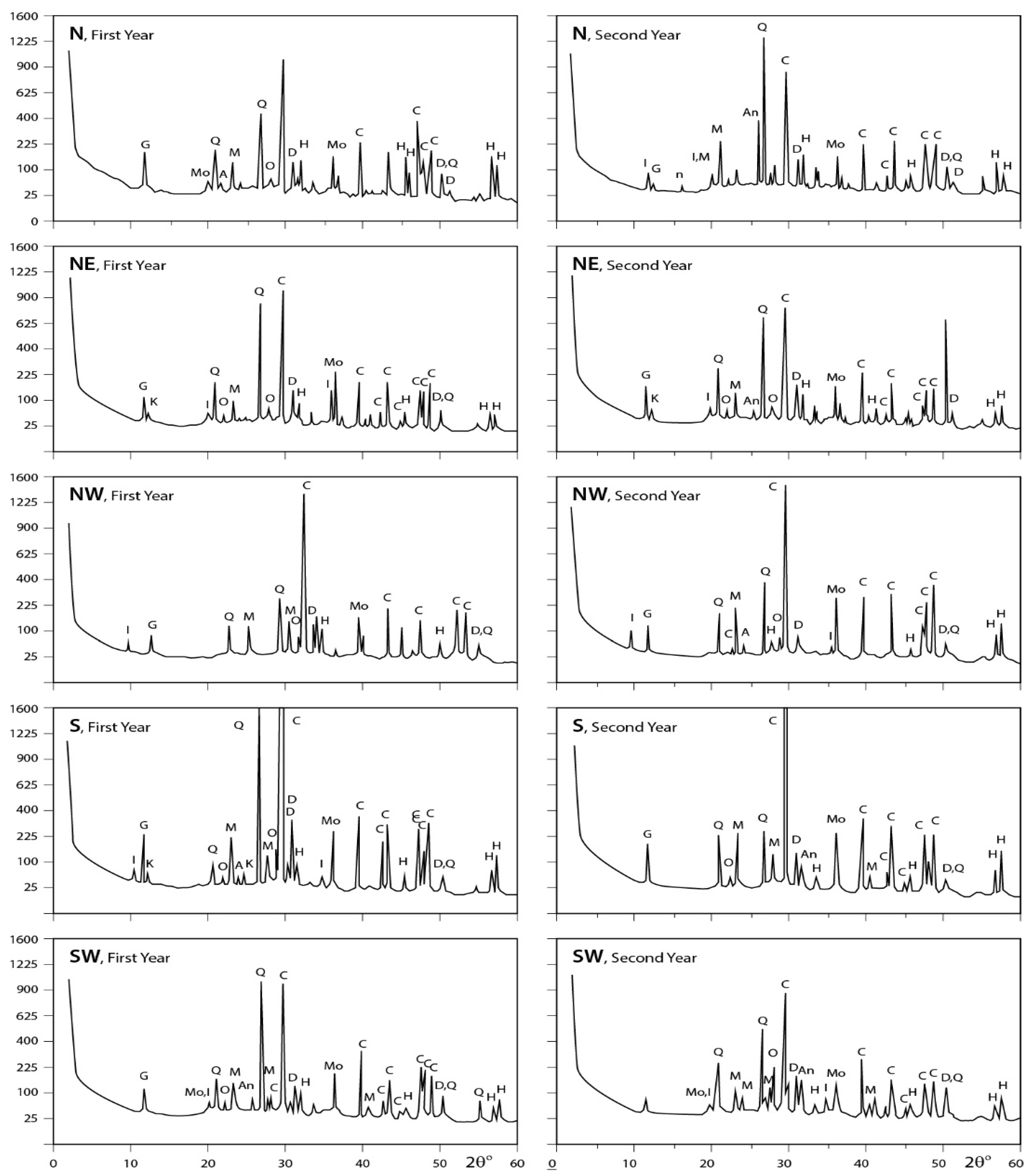
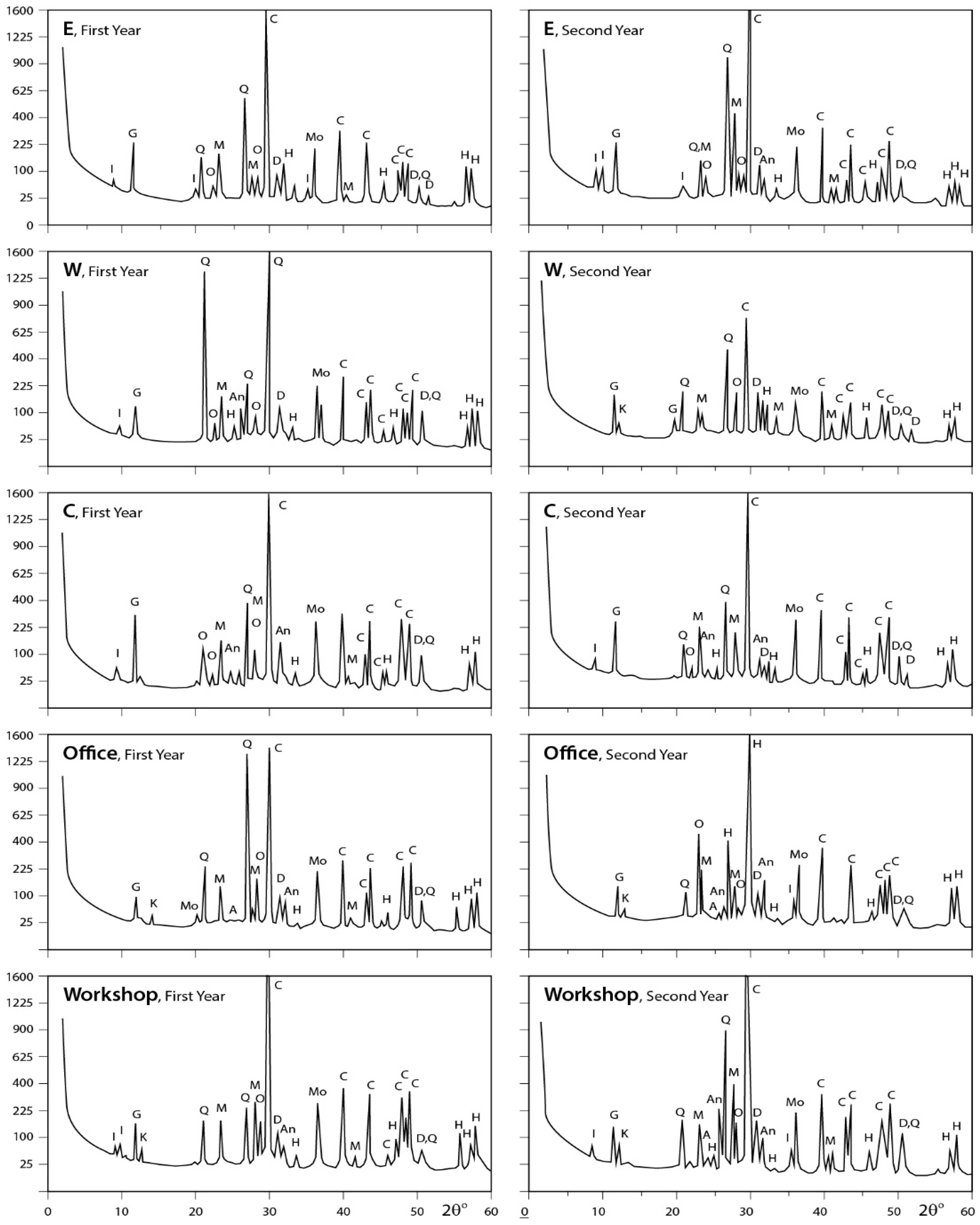
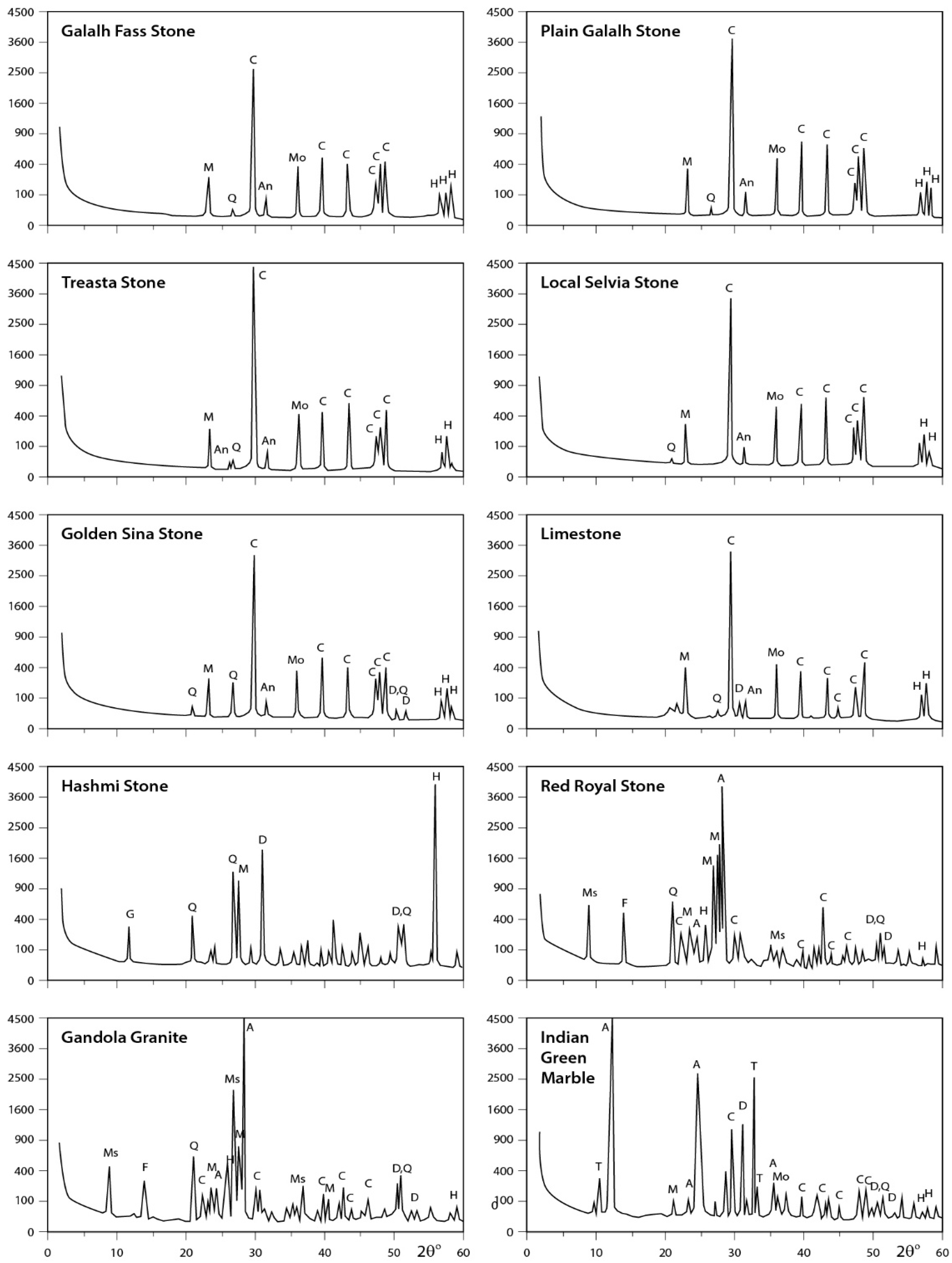
3.3. X-ray Diffraction Analysis
3.3.1. Dust Fall Samples
3.3.2. Rock Samples
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Hindy, K.T. Correlation study for dustfall composition and quarried limestone, Beni−Khalid, Middle Egypt. Environ. Pollut. 1980, 1, 269–275. [Google Scholar] [CrossRef]
- Zakey, A.S.; Abdel−Wahab, M.M.; Pettersson, J.B.C.; Gatari, M.J.; Hallquist, M. Seasonal and spatial variation of atmospheric particulate matter in a developing megacity, the Greater Cairo, Egypt. Atmosfera 2008, 21, 171–189. [Google Scholar]
- Esbert, R.M.; Diaz Pache, F.; Alonso, F.J.; Ordaz, J.; Grossi, C.M. Solid particles of atmospheric pollution found on the Hontoria limestone of Burgos Cathedral (Spain). In Proceedings of the 8th International Congress on Deterioration and Conservation of Stone, Berlin, Germany, 30 September–4 October 1996; pp. 393–399. [Google Scholar]
- Umbria, A.; Gervilla, J.; Galán, M.Y.; Valdés, R. Caracterización de Particulas. Consejeria de Medio Ambiente; Junta de Andalucia: Sevilla, Spain, 1999. [Google Scholar]
- Chabas, A.; Lefévre, R.A. Chemistry and microscopy of atmospheric particulates at Delos (Cyclades−Greece). Atmos. Environ. 2000, 34, 225–238. [Google Scholar] [CrossRef]
- Bernabé, J.M.; Carretero, M.I. Characterización mediante microscopia electrónica de barrido de particulas atmosféricas del área industrial de Huelva (SW de España). Bol. Soc. Esp. Mineral. 2003, 26, 167–177. [Google Scholar]
- Howari, F.M. Evaporation losses and dispersion of volatile organic compounds from tank farms. Environ. Monit. Assess. 2015, 187, 273. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, N.; Shimizu, A.; Matsui, I.; Nishikawa, M. A method for estimating the fraction of mineral dust in particulate matter using PM2.5−to−PM10 ratios. Particuology 2016, 28, 114–120. [Google Scholar] [CrossRef]
- Esteve, V.; Rius, J.; Ochando, L.E.; Anigó, J.M. Quantitative X-ray diffraction phase analysis of coarse airborne particulate collected cascade impactor sampling. Atmos. Environ. 1997, 31, 3963–3967. [Google Scholar] [CrossRef]
- Querol, X.; Alastuey, A.; Lopez−Soler, A.; Mantilla, E.; Plana, F. Mineralogy of atmospheric particles around a large coal−fire power station. Atmos. Environ. 1996, 30, 3557–3572. [Google Scholar] [CrossRef]
- Queralt, I.; Sanfeliu, T.; Gomez, E.; Alvarez, C. X-ray diffraction analysis of atmospheric dust using low background supports. J. Aerosol Sci. 2001, 32, 453–459. [Google Scholar] [CrossRef]
- Ekosse, G.; Van den Heever, D.J.; de Jager, L.; Totolo, O. Environmental chemistry and mineralogy of particulate air matter around Selebi Phikwe nickel–copper plant, Botswana. Min. Eng. 2004, 17, 349–353. [Google Scholar] [CrossRef]
- Ghrefat, H.; Howari, F.M. Rate of deposition and quality of airborne dust in Al Ain and Ras Al Khaimah, United Arab Emirates. Arab. J. Geosci. 2013, 6, 1033–1039. [Google Scholar] [CrossRef]
- Bernabé, J.M.; Carretero, M.I.; Galán, E. Mineralogy and origin of atmospheric particles in the industrial area of Huelva (SW Spain). Atmos. Environ. 2005, 39, 6777–6789. [Google Scholar] [CrossRef]
- Howari, F.M.; Baghdady, A.B.; Goodell, P.C. Mineralogical and geomorphologic characterization of sand dunes in the eastern part of United Arab Emirates using orbital remote sensing integrated with field investigations. Geomorphology 2007, 83, 67–81. [Google Scholar] [CrossRef]
- Buseck, P.R.; Posfai, M. Air borne minerals and related aerosol particles: effects on climate and the environment. Proc. Natl. Acad. Sci. USA 1999, 96, 3372–3379. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Prospero, J.M.; Savoie, D.L.; Millero, F.J.; Zika, R.G.; Saltzman, E.S. Photoreduction of iron(III) in marine mineral aerosol solutions. J. Geophys. Res. 1993, 98, 9039–9046. [Google Scholar] [CrossRef]
- Guthrie, G.D., Jr.; Mossman, B.T. Merging the geological and biological sciences; an integrated approach to the study of mineral−induced pulmonary diseases. In Health Effects of Mineral Dust, Reviews in Mineralogy, Mineralogical Society of America; Guthrie, G.D., Jr., Mossman, B.T., Eds.; Mineralogical Society of America: Washington, DC, USA, 1993; Volume 28, pp. 1–5. [Google Scholar]
- Bian, H.; Zender, C.S. Mineral dust and global tropospheric chemistry: relative roles of photolysis and heterogeneous uptake. J. Geophys. Res. 2003, 108, 4672. [Google Scholar] [CrossRef]
- Yehia, M.; Baghdady, A.; Howari, F.M.; Awad, S.; Gad, A. Natural radioactivity and groundwater quality assessment in the northern area of the Western Desert of Egypt. J. Hydrol. Reg. Stud. 2017, 12, 331–344. [Google Scholar] [CrossRef]
- Hindy, K.T. Types, Sources and Distribution of Dust Deposits in the Major Cities of the Nile Valley. Ph.D. Thesis, Ain Shams University, Cairo, Egypt, 1977. [Google Scholar]
- Hindy, K.T.; Baghdady, A.R. A study of airborne minerals and associated organic species in Al Ain, United Arab Emirates. Environ. Mgmt. Health 1998, 9, 160–164. [Google Scholar] [CrossRef]
- Khalaf, F.I.; Al-Kadi, A.; Saleh, S. Mineralogical composition and potential sources of dust fallout deposits in Kuwait, Northern Arabian Gulf. Sediment. Geol. 1985, 42, 255–278. [Google Scholar] [CrossRef]
- Hunt, J.M.; Wishered, P.; Bonham, L.C. Infrared absorption spectra of minerals and other inorganic compounds. J. Anal. Chem. 1950, 22, 1487–1497. [Google Scholar] [CrossRef]
- Adler, H.H.; Kerr, P.F. Infrared study of aragonite and calcite. Am. Mineral. 1962, 47, 700–717. [Google Scholar]
- Adler, H.H.; Kerr, P.F. Infrared spectra, symmetry and structural relations of some carbonate minerals. Am. Mineral. 1963, 48, 839. [Google Scholar]
- Hertzberg, G. Molecular spectra and molecular structure. In Infrared and Raman Spectra of Polyatomic Molecules, 2nd ed.; Van Nostrand: New York, NY, USA, 1945. [Google Scholar]
- Hunt, J.M.; Turner, D.C. Determination of mineral constituents of rocks by Infrared spectroscopy. J. Anal. Chem. 1953, 25, 1169–1174. [Google Scholar] [CrossRef]
- Nahin, P.G. Infrared analysis of clays and related minerals. Bull. Div. Mines Calif. 1955, 169, 112–118. [Google Scholar] [CrossRef]
- Coblentz, W.W. Investigation of Infrared Spectra; Perkin-Elmer Corporation: London, UK, 1962. [Google Scholar]
- Saksena, B.D. Analysis of the Raman and Infrared spectra of quartz. Proc. Indian Acad. Sci. 1940, 12, 93. [Google Scholar]
- Zussman, A.S. Physical Methods in Determinative Mineralogy; Academic Press: London, UK, 1967. [Google Scholar]
- Cross, A.D. An Introduction to Practical Infrared Spectroscopy; Butterworths: London, UK, 1960; pp. 57–74. [Google Scholar]
- Silverstein, R.M.; Blasser, G.C. Identification Spectrometrique des Compsés Organiques; et Cie, M., Ed.; Gauthier-Villars: Paris, France, 1968; pp. 74–95. [Google Scholar]
- Pettijon, F.J. Sedimentary Rocks, 2nd ed.; Harper & Brothers: New York, NY, USA, 1957. [Google Scholar]
- Muller, G.; Tietz, G. Der phosphor gehalt der bodensee sedimente. Neues Jb. Miner. Abh. 1966, 105, 41. [Google Scholar]
| Mineral | Formula | Spectrum Frequencies | ||
|---|---|---|---|---|
| 1−Carbonates | ||||
| Calcite | CaCO3 | 2517 | 1798 | 1435(s) |
| 873(m) | 712(m) | |||
| Dolomite | Ca Mg(CO3)2 | 1435(s) | 881(m) | 730 |
| 2−Sulphate | ||||
| Gypsum | CaSO4 | 3410(m) | 1626(m) | 1142(s) |
| 1114(s) | 1004(m) | 670(m) | ||
| 600§ | ||||
| 3−Oxides | ||||
| Magnetite | Fe3O4 | 575(b,w) | ||
| Hematite | Fe2O3 | 550§ | 475 | |
| Ilmenite | FeTiO3 | 700(w) | 540(s,b) | 455 |
| 4−Silicon oxides | ||||
| Quartz | SiO2 | 1163(s) | 1078(s) | 798§(m) |
| Amorphous silica | SiO2 | 779§(m) | 695(m) | 455 |
| 1700 | ||||
| 5−Silicates | ||||
| A−Clay minerals | ||||
| Kaolinite | Al2Si2O5(OH)4 | 3705 | 3673(w) | 3663(m) |
| 3613 | 3547 | 1667(w) | ||
| 1105(s) | 1031§(s) | 1006(s) | ||
| 935§(m) | 909§(s) | 545 | ||
| (& others) | ||||
| Montmorillonite | (Al,Mg)2Si4O10(OH)2.nH2O | 3571(m) | 1626(m) | 1117(m |
| 1042(s) | 909(m) | (& others). | ||
| Illite | K2−3 Al11Si12O35−36 | 1639(w) | 1117(m) | 1031(s) |
| 909§(m) | (& others) | |||
| B−Feldspars | ||||
| Albite | NaAlSi3O8 | 1134(s) | 1083(s) | 1028 |
| 1015(s) | 986 | 758(m) | ||
| 645§(m) | 529§ | 462 | ||
| 427 | ||||
| Orthoclase | KAlSi3O8 | 641(b) | ||
| Microcline | KAlSi3O8 | 1128(s) | 1089(s) | 1037 |
| 1009(s) | 935 | 792 | ||
| 768(m) | 726(m) | 648§(w) | ||
| 606 | 585 | 533§ | ||
| 427 | ||||
| C−Mica | ||||
| Biotite | K(Mg,Fe)3(AlSi3O10)(OH)2 | 1067 | 988 | 770 |
| 745 | 660 | 630 | ||
| 449 | ||||
| Organic Compound | Formula | Spectrum Frequency |
|---|---|---|
| 1−Alkanes | CnH2n+2 | 2982 |
| 2925 ± 10(s) asym. Stretch | ||
| 2850 ± 10(s) sym. Stretch | ||
| 1465 ± 20(m) asym. Bending | ||
| 2−Phosphines | R2PH | 2440–2350(m) P–H stretchsharp absorption band. |
| 1450–1435(m) P–R stretch, where R = aryl. | ||
| 1320–1280(m) P–R stretch,where R = CH3 only. | ||
| 3−Silicon hydrides | Si–H | 2280–2080(s) Si–H stretch |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hindy, K.T.; Baghdady, A.R.; M. Howari, F.; Abdelmaksoud, A.S. A Qualitative Study of Airborne Minerals and Associated Organic Compounds in Southeast of Cairo, Egypt. Int. J. Environ. Res. Public Health 2018, 15, 568. https://doi.org/10.3390/ijerph15040568
Hindy KT, Baghdady AR, M. Howari F, Abdelmaksoud AS. A Qualitative Study of Airborne Minerals and Associated Organic Compounds in Southeast of Cairo, Egypt. International Journal of Environmental Research and Public Health. 2018; 15(4):568. https://doi.org/10.3390/ijerph15040568
Chicago/Turabian StyleHindy, Kamal T., Ashraf R. Baghdady, Fares M. Howari, and Ahmed S. Abdelmaksoud. 2018. "A Qualitative Study of Airborne Minerals and Associated Organic Compounds in Southeast of Cairo, Egypt" International Journal of Environmental Research and Public Health 15, no. 4: 568. https://doi.org/10.3390/ijerph15040568





