Horizontal and Vertical Distributions of Chromium in a Chromate Production District of South Central China
Abstract
:1. Introduction
2. Materials and Methods
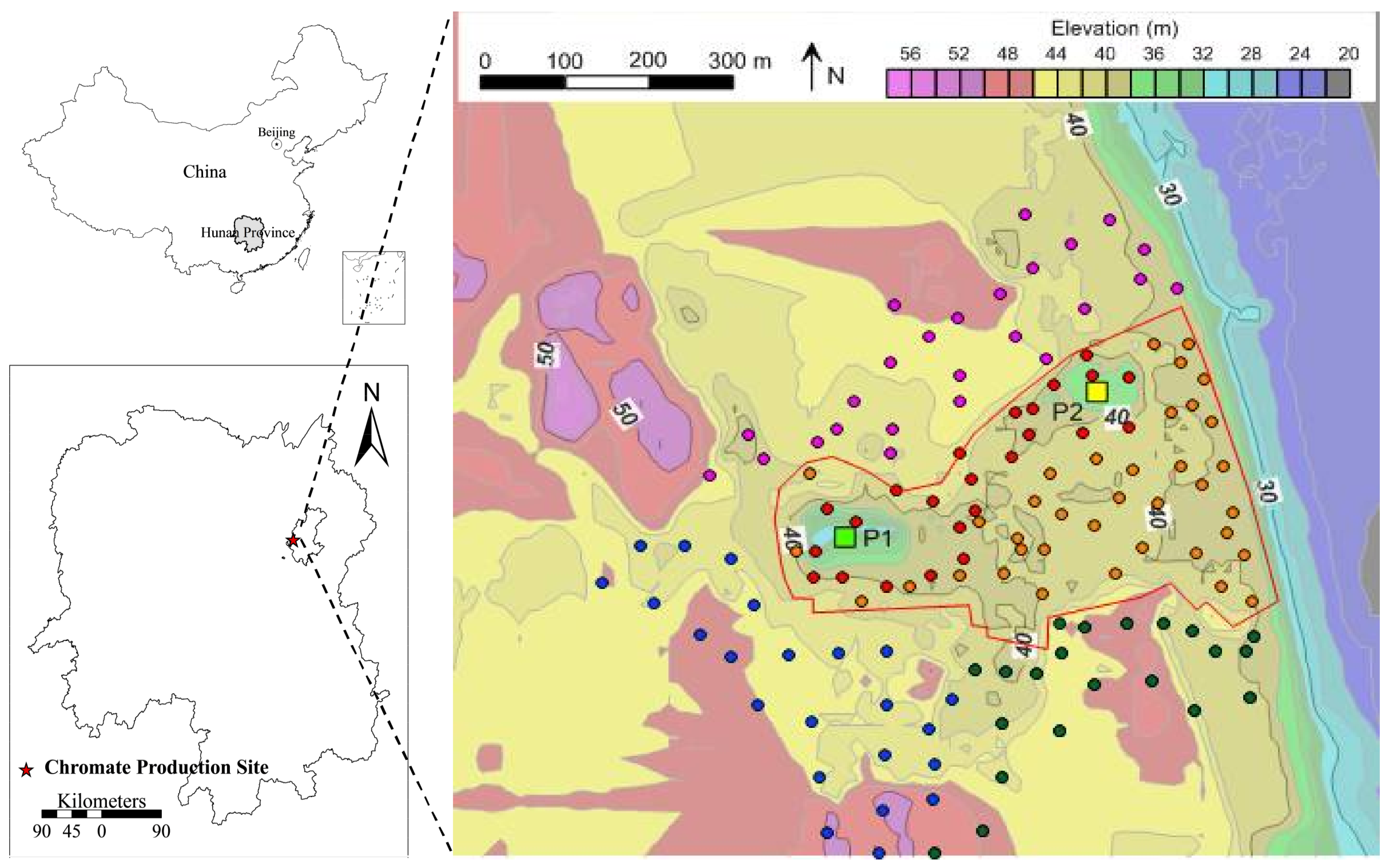
2.1. Study Area
2.2. Soil Sampling and Analysis
2.3. Calculations of Distance, LRCr, and PCr6+ and the Elevations of Sampling Points
2.4. Longitudinal Distribution of Cr Content in Soils
2.5. Statistical Analysis
3. Results
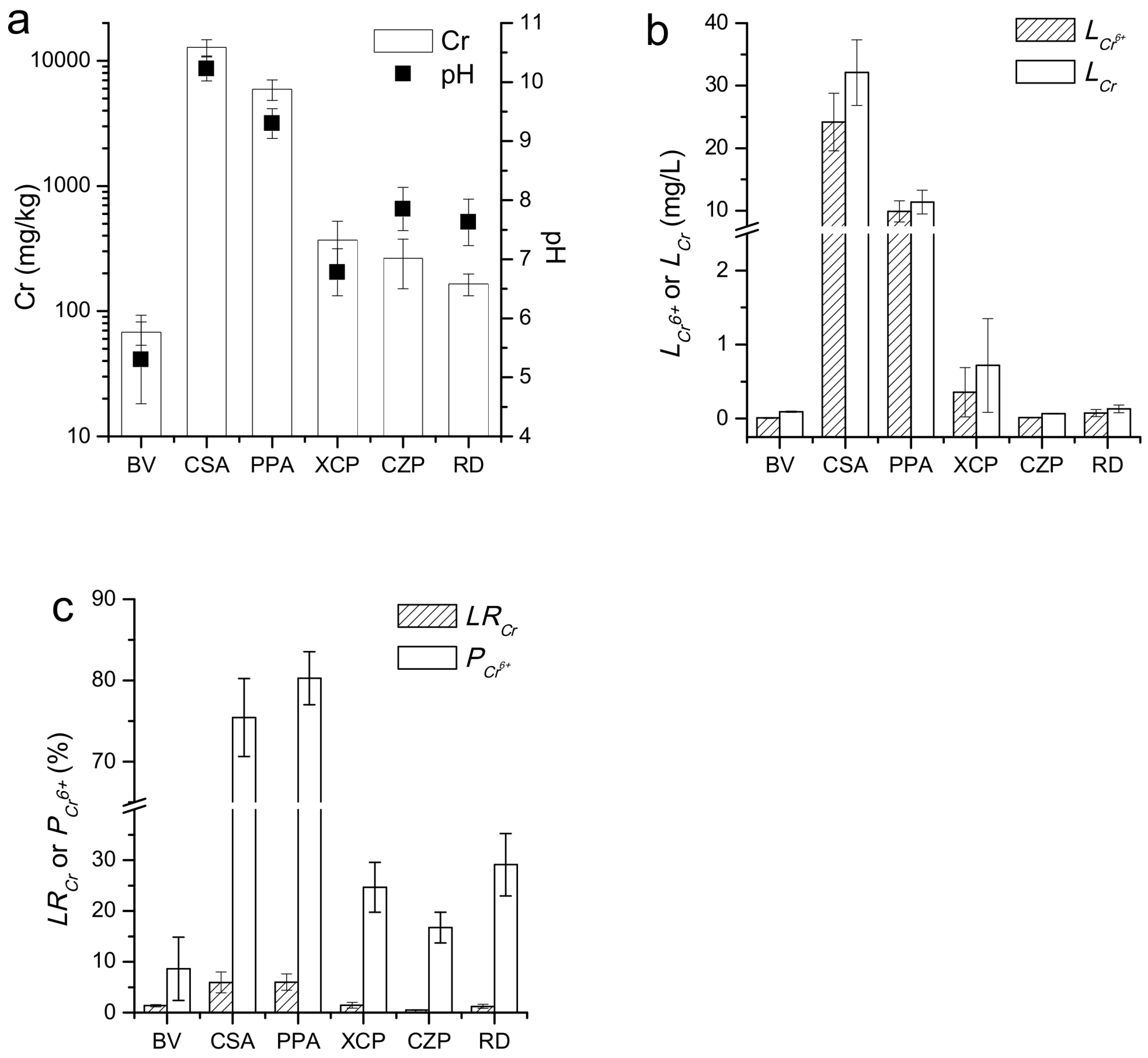
3.1. General Characteristics of Surface Soils
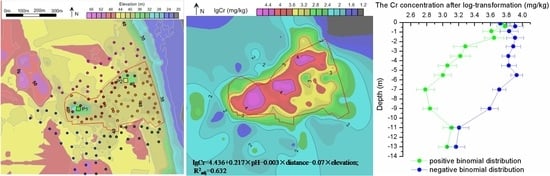
3.2. Distribution of Cr Content in Surface Soils
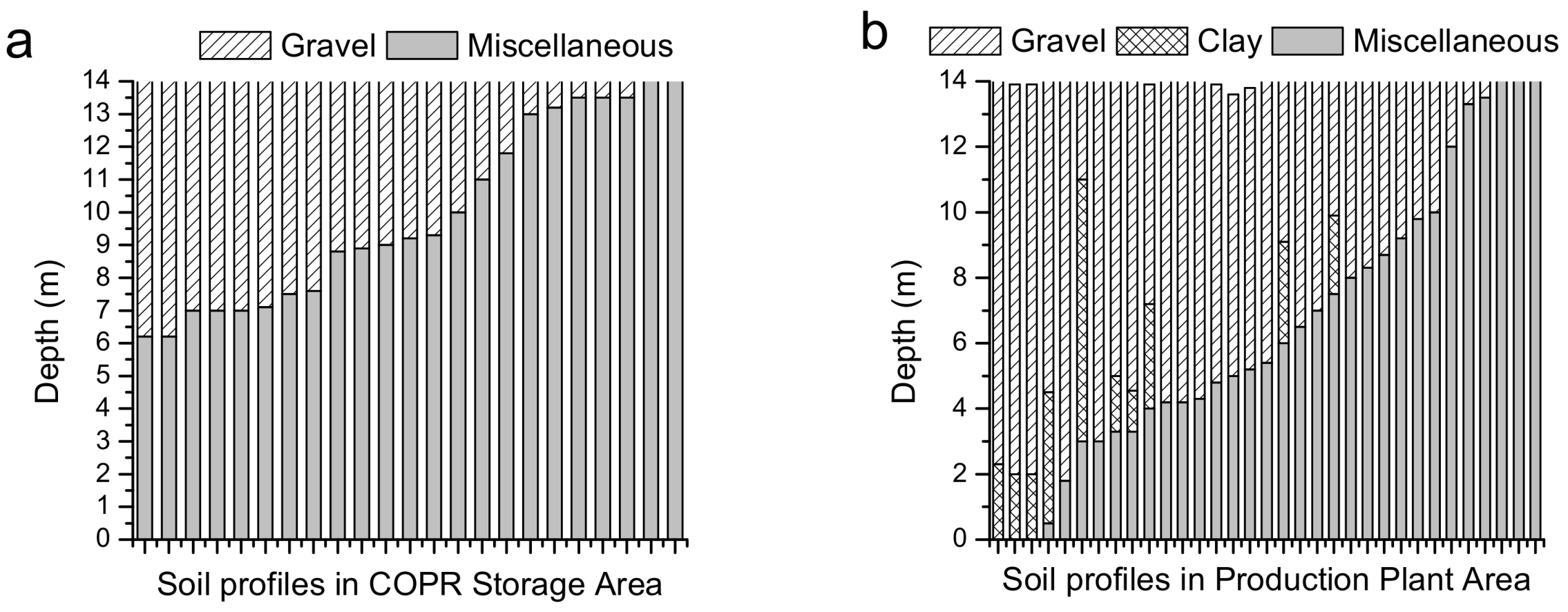
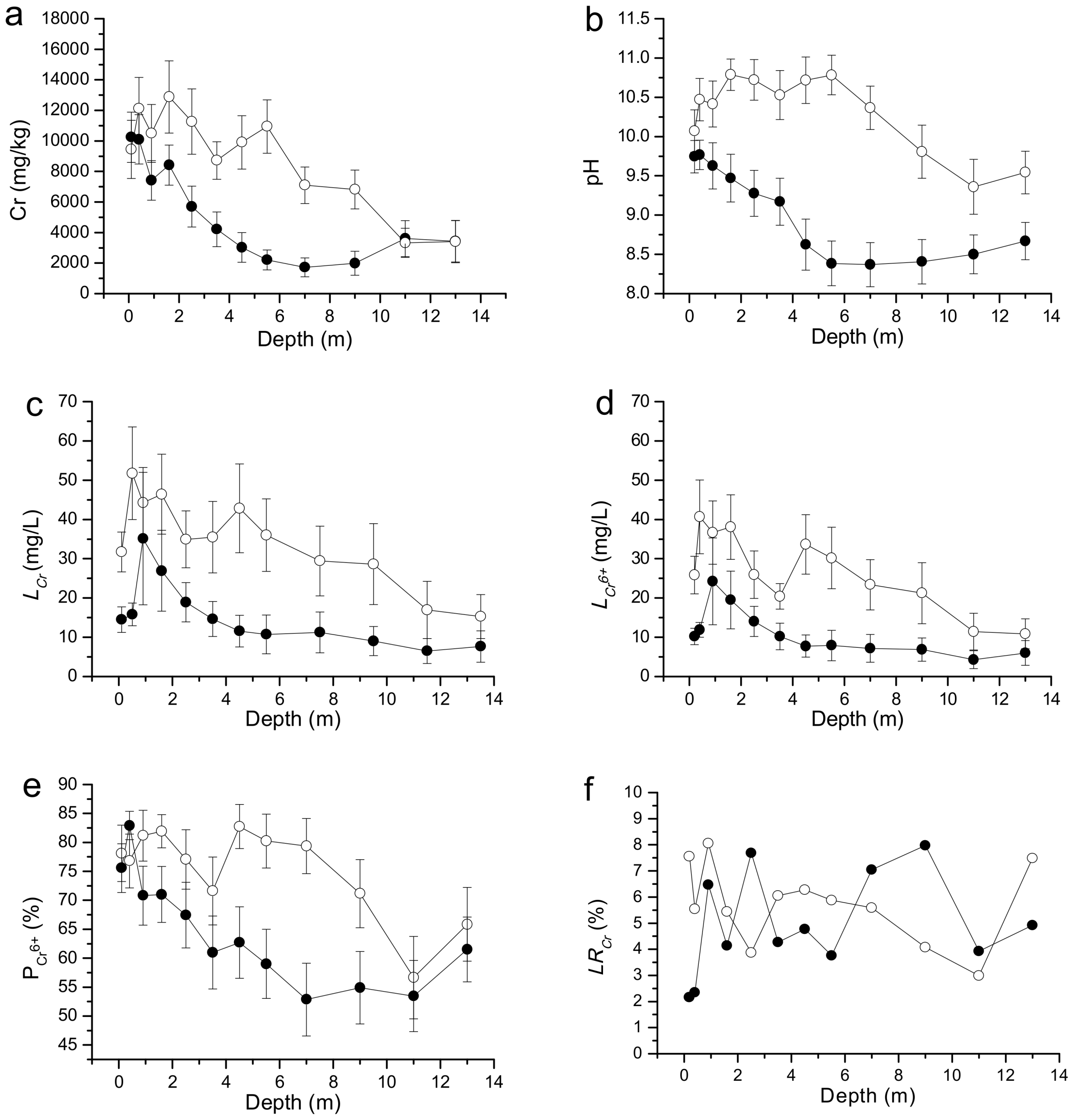
3.3. Distribution of the Cr Concentration in Soil Profiles
3.4. General Characteristics of Soil Profiles Obeying a Binomial Distribution
3.5. General Characteristics of Different Soil Layers
4. Discussion
4.1. Horizontal Distribution of Cr in Soil
4.2. Vertical Distribution of Cr in Soil
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
| Depth (m) | pH | Cr (mg/kg) | LCr6+ (mg/L) | LCr (mg/L) | LRCr (%) | PCr6+ (%) |
|---|---|---|---|---|---|---|
| 0.3–0.5 | 5.15 ± 0.7 | 68.7 ± 11.6 | 0.008 ± 0.006 | 0.102 ± 0.008 | 1.52 ± 0.29 | 8.4 ± 6.6 |
| 3–3.2 | 5.35 ± 0.8 | 64.0 ± 11.3 | 0.009 ± 0.009 | 0.094 ± 0.011 | 1.35 ± 0.04 | 9.1 ± 9.7 |
| 6–6.2 | 5.46 ± 1.1 | 71.6 ± 5.2 | 0.006 ± 0.003 | 0.088 ± 0.010 | 1.17 ± 0.10 | 6.6 ± 3.1 |
| 12–12.2 | 5.32 ± 0.4 | 66.8 ± 22.6 | 0.010 ± 0.004 | 0.085 ± 0.005 | 1.38 ± 0.45 | 10.3 ± 5.5 |
| Average values | 5.30 ± 0.75 | 67.8 ± 14.4 | 0.008 ± 0.006 | 0.092 ± 0.010 | 1.36 ± 0.22 | 8.6 ± 6.24 |
References
- Du, J.; Lu, J.; Wu, Q.; Jing, C. Reduction and immobilization of chromate in chromite ore processing residue with nanoscale zero-valent iron. J. Hazard. Mater. 2012, 215, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Bewley, R.J.F.; Jeffries, R.; Watson, S.; Granger, D. An overview of chromium contamination issues in the south-east of Glasgow and the potential for remediation. Environ. Geochem. Health 2001, 23, 267–271. [Google Scholar] [CrossRef]
- Zhang, H.J.; Wang, X.R.; Chen, C.Y.; Liu, X.; Wang, Q. The Health Risk Assessmentand Remediation Guide Limitvalue of Typical Chromium Slag Contaminated Sites. Acta Sci. Circumst. 2010, 30, 1445–1450. [Google Scholar]
- Narin, I.; Surme, Y.; Soylak, M.; Dogan, M. Speciation of Cr(III) and Cr(VI) in environmental samples by solid phase extraction on Ambersorb 563 resin. J. Hazard. Mater. 2006, 136, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Dhal, B.; Thatoi, H.N.; Das, N.N.; Pandey, B.D. Chemical and microbial remediation of hexavalent chromium from contaminated soil and mining/metallurgical solid waste: A review. J. Hazard. Mater. 2013, 250, 272–291. [Google Scholar] [CrossRef] [PubMed]
- Zayed, A.M.; Terry, N. Chromium in the environment: Factors affecting biological remediation. Plant Soil 2003, 249, 139–156. [Google Scholar] [CrossRef]
- Chattopadhyay, B.; Datta, S.; Chatterjee, A.; Mukhopadhyay, S.K. The environmental impact of waste chromium of tannery agglomerates in the east Calcutta wetland ecosystem. J. Soc. Leather Technol. Chem. 2000, 84, 94–100. [Google Scholar]
- Eary, L.E.; Davis, A. Geochemistry of an acidic chromium sulfate plume. Applied geochemistry. Appl. Geochem. 2007, 22, 357–369. [Google Scholar] [CrossRef]
- Jean-Soro, L.; Bordas, F.; Bollinger, J.C. Column leaching of chromium and nickel from a contaminated soil using EDTA and citric acid. Environ. Pollut. 2012, 164, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Banks, M.K.; Schwab, A.P.; Henderson, C. Leaching and reduction of chromium in soil as affected by soil organic content and plants. Chemosphere 2006, 62, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.F.; Huang, C.Y.; Tu, Y.T.; Chen, J.R. Cr-removal efficiency as affected by the Cr-bonding fractionation in soil treated with trivalent and hexavalent chromium. Int. J. Appl. Sci. Eng. 2012, 10, 319–331. [Google Scholar]
- Pei, T.Q.; Wang, L.; Zhong, S.; Gong, X. Pollution characteristics and treatment analysis of chromium residue and soil chromium in typical chromium residue simple stock. Chin. J. Environ. Eng. 2008, 2, 994–999. (In Chinese) [Google Scholar]
- Luo, J.F.; Qu, D. Study on the soil Cr pollution in residue piling yard in Qinghai Province. Acta Agric. Boreali-Occident. Sin. 2006, 15, 244–247. (In Chinese) [Google Scholar]
- Deng, H.Y. Chromium Pollution and Research on Bioremediation of Chromium Contaminated Soil in a Plant. Ph.D. Thesis, Chongqing University, Chongqing, China, 2016. [Google Scholar]
- Cheng, J.L.; Zhou, S.H.I.; Zhu, Y.W. Assessment and mapping of environmental quality in agricultural soils of Zhejiang Province, China. J. Environ. Sci. 2007, 19, 50–54. [Google Scholar] [CrossRef]
- Liu, F.; Wang, Q.; Huang, Q.F.; Li, L.; Zhou, D.J.; Duan, H.B.; Dai, T.Y. Study on the Standard Methods of Leaching Toxicity of Solid Waste. Res. Environ. Sci. 2008, 21, 9–15. (In Chinese) [Google Scholar]
- Freese, K.; Miller, R.; Cutright, T.; Senko, J. Review of chromite ore processing residue (COPR): Past practices, environmental impact and potential remediation methods. Curr. Environ. Eng. 2014, 1, 82–90. [Google Scholar] [CrossRef]
- Hillier, S.; Roe, M.J.; Geelhoed, J.S.; Fraser, A.R.; Farmer, J.G.; Paterson, E. Role of quantitative mineralogical analysis in the investigation of sites contaminated by chromite ore processing residue. Sci. Total Environ. 2003, 308, 195–210. [Google Scholar] [CrossRef]
- Chrysochoou, M.; Dermatas, D.; Grubb, D.G.; Moon, D.H.; Christodoulatos, C. Importance of mineralogy in the geoenvironmental characterization and treatment of chromite ore processing residue. J. Geotech. Geoenviron. Eng. 2009, 136, 510–521. [Google Scholar] [CrossRef]
- Chrysochoou, M.; Dermatas, D.; Moon, D.H.; Christodoulatos, C.; Wazne, M.; French, C.; Kaouris, M. Investigation of barium treatment of chromite ore processing residue (COPR). ASTM Int. 2006, 3. [Google Scholar] [CrossRef]
- Zeng, F.; Ali, S.; Zhang, H.; Ouyang, Y.; Qiu, B.; Wu, F.; Zhang, G. The influence of pH and organic matter content in paddy soil on heavy metal availability and their uptake by rice plants. Environ. Pollut. 2011, 159, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Bradl, H.B. Adsorption of heavy metal ions on soils and soils constituents. J. Colloid Interface Sci. 2004, 277, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, L.; Ruiz, E.; Alonso-Azcárate, J.; Rincón, J. Heavy metal distribution and chemical speciation in tailings and soils around a Pb–Zn mine in Spain. J. Environ. Manag. 2009, 90, 1106–1116. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Wang, C.; Yang, L. Characterizing spatial distribution and sources of heavy metals in the soils from mining-smelting activities in Shuikoushan, Hunan Province, China. J. Environ. Sci. 2009, 21, 1230–1236. [Google Scholar]
- Ljung, K.; Otabbong, E.; Selinus, O. Natural and anthropogenic metal inputs to soils in urban Uppsala, Sweden. Environ. Geochem. Health 2006, 28, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Rock, M.L.; James, B.R.; Helz, G.R. Hydrogen peroxide effects on chromium oxidation state and solubility in four diverse, chromium-enriched soils. Environ. Sci. Technol. 2001, 35, 4054–4059. [Google Scholar] [CrossRef] [PubMed]
- Tzou, Y.M.; Loeppert, R.H.; Wang, M.K. Light-catalyzed chromium(VI) reduction by organic compounds and soil minerals. J. Environ. Qual. 2003, 32, 2076–2084. [Google Scholar] [CrossRef] [PubMed]
- Khérici-Bousnoubra, H.; Khérici, N.; Derradji, E.F. Behaviour of chromium VI in a multilayer aquifer in the industrial zone of Annaba, Algeria. Environ. Geol. 2009, 57, 1619–1624. [Google Scholar] [CrossRef]
- Chrysochoou, M.; Ferreira, D.R.; Johnston, C.P. Calcium polysulfide treatment of Cr(VI)-contaminated soil. J. Hazard. Mater. 2010, 179, 650–657. [Google Scholar] [CrossRef] [PubMed]
- Maskall, J.; Whitehead, K.; Thornton, I. Heavy metal migration in soils and rocks at historical smelting sites. Environ. Geochem. Health 1995, 17, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Meegoda, J.N.; Kamolpornwijit, W.; Vaccari, D.A.; Ezeldin, A.S.; Noval, B.A.; Mueller, R.T.; Santora, S. Remediation of chromium-contaminated soils: Bench-scale investigation. Pract. Period. Hazard. Toxic Radioact. Waste Manag. 1999, 3, 124–131. [Google Scholar] [CrossRef]

| Equation | R | R2adj | p |
|---|---|---|---|
| lgCr = 3.934 − 0.008 × distance | 0.616 | 0.374 | <0.001 |
| lgCr = 3.927 × exp(−0.003 × distance) | 0.598 | 0.353 | <0.001 |
| lgCr = 9.403 − 0.155 × elevation | 0.567 | 0.315 | <0.001 |
| lgCr = 27.482 × exp(−0.055 × elevation) | 0.570 | 0.319 | <0.001 |
| lgCr = 0.311 + 0.298 × pH | 0.675 | 0.451 | <0.001 |
| lgCr = 1.102 × exp(0.106 × pH) | 0.681 | 0.460 | <0.001 |
| lgCr = 4.436 + 0.217 × pH − 0.003 × distance − 0.07 × elevation | 0.800 | 0.632 | <0.001 |
| Functions | Equation | R2 | R2adj | RMSE |
|---|---|---|---|---|
| Linear Function | Predicted = 1.197 + 0.649 lgCr | 0.649 | 0.648 | 0.320 |
| Exponential Function | Predicted = 1.162 + 0.659 lgCr | 0.656 | 0.655 | 0.320 |
| Binomial Distribution Function | Predicted = 0.878 + 0.743 lgCr | 0.743 | 0.742 | 0.293 |
| Binomial Type | Positive Binomial Distribution (n = 29) | Negative Binomial Distribution (n = 22) |
|---|---|---|
| a | 0.016 ± 0.012 | −0.007 ± 0.006 |
| b | −0.268 ± 0.133 | 0.032 ± 0.085 |
| c | 3.973 ± 0.597 | 3.862 ± 0.467 |
| (, ) | (8.4 ± 2.2, 2.7 ± 0.4) | (−2.0 ± 4.4, 4.1 ± 0.4) |
| equations | lgCr = 0.013 × d2 − 0.026 × d + 3.805 | lgCr = −0.006 × d2 + 0.027 × d + 3.829 |
| R2 | 0.218 | 0.208 |
| p | <0.001 | <0.001 |
| Elevation | Distance | pH | Cr | LCr6+ | LCr | PCr6+ | |
|---|---|---|---|---|---|---|---|
| Distance | 0.607 ** | ||||||
| pH | −0.303 ** | −0.394 ** | |||||
| Cr | −0.402 ** | −0.523 ** | 0.526 ** | ||||
| LCr6+ | −0.322 ** | −0.454 ** | 0.485 ** | 0.642 ** | |||
| LCr | −0.351 ** | −0.489 ** | 0.504 ** | 0.654 ** | 0.965 ** | ||
| PCr6+ | −0.515 ** | −0.499 ** | 0.520 ** | 0.501 ** | 0.525 ** | 0.466 ** | |
| LRCr | −0.257 ** | −0.160 | 0.100 | −0.090 | 0.282 ** | 0.273 ** | 0.356 ** |
| Parameter | Depth (m) | P-BD (n = 29) | N-BD (n = 22) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pH | LCr6+ | LCr | PCr6+ | LRCr | lgCr | pH | LCr6+ | LCr | PCr6+ | LRCr | lgCr | ||
| b | 0.0–0.2 | 0.07 | 0.15 | 0.16 | −0.12 | 0.32 | −0.35 | −0.16 | −0.45 * | −0.48 * | 0.01 | 0.28 | −0.38 |
| 0.2–0.6 | −0.14 | −0.15 | −0.07 | −0.07 | 0.22 | −0.43 * | −0.04 | −0.23 | −0.21 | 0.07 | 0.58 ** | −0.60 ** | |
| 0.6–1.2 | −0.18 | 0.30 | 0.29 | −0.06 | 0.34 | −0.3 | 0.02 | −0.45 * | −0.41 * | −0.26 | −0.16 | −0.53 * | |
| 1.2–2.0 | −0.07 | 0.25 | 0.26 | −0.10 | 0.31 | −0.44 * | −0.20 | −0.18 | −0.09 | −0.52 * | 0.65 ** | −0.63 ** | |
| 2.0–3.0 | −0.13 | 0.22 | 0.24 | 0.02 | 0.27 | −0.14 | −0.37 | 0.12 | 0.07 | −0.01 | 0.49 * | −0.34 | |
| 3.0–4.0 | −0.13 | 0.37 * | 0.34 | 0.26 | 0.47 * | 0.04 | −0.3 | 0.18 | 0.39 | −0.09 | 0.54 * | −0.13 | |
| 4.0–5.0 | −0.09 | 0.40 * | 0.40 * | 0.23 | 0.48 * | 0.06 | −0.26 | 0.58 ** | 0.57 ** | 0.10 | 0.56 ** | −0.14 | |
| 5.0–6.0 | −0.01 | 0.39 * | 0.38 * | 0.59 ** | 0.48 * | 0.28 | −0.27 | 0.58 ** | 0.62 ** | −0.38 | 0.37 | −0.03 | |
| 6.0–8.0 | −0.02 | 0.41 * | 0.40 * | 0.38 * | 0.33 | 0.21 | 0.16 | 0.47 * | 0.48 * | −0.42 | 0.26 | 0.10 | |
| 8.0–10.0 | −0.33 | 0.42 * | 0.43 * | 0.11 | 0.33 | −0.10 | 0.21 | 0.56 ** | 0.53 * | 0.06 | 0.62 ** | −0.13 | |
| 10.0–12.0 | −0.24 | 0.35 * | 0.38 * | −0.02 | 0.35 * | −0.02 | 0.26 | 0.47 * | 0.47 * | 0.41 | 0.30 | 0.09 | |
| 12.0–14.0 | −0.28 | 0.38 * | 0.38 * | 0.26 | 0.39 * | −0.02 | −0.17 | 0.58 ** | 0.57 ** | 0.40 | 0.56 ** | 0.06 | |
| c | 0.0–0.2 | 0.35 * | 0.41 * | 0.28 | 0.56 ** | −0.08 | 0.69 ** | 0.47 * | 0.57 ** | 0.63 ** | −0.11 | −0.36 | 0.54 * |
| 0.2–0.6 | 0.59 ** | 0.47 * | 0.42 * | 0.07 | −0.03 | 0.88 ** | 0.46 * | 0.44 * | 0.45 * | −0.27 | −0.70 ** | 0.92 ** | |
| 0.6–1.2 | 0.57 ** | 0.13 | 0.12 | 0.56 ** | −0.05 | 0.91 ** | 0.44 * | 0.62 ** | 0.62 ** | 0.02 | −0.07 | 0.80 ** | |
| 1.2–2.0 | 0.50 ** | 0.08 | 0.06 | 0.30 | −0.03 | 0.85 ** | 0.67 ** | 0.40 | 0.37 | 0.34 | −0.64 ** | 0.84** | |
| 2.0–3.0 | 0.71 ** | 0.29 | 0.28 | 0.34 | 0.09 | 0.73 ** | 0.80 ** | 0.25 | 0.35 | −0.14 | −0.60 ** | 0.82 ** | |
| 3.0–4.0 | 0.73 ** | 0.19 | 0.19 | −0.06 | −0.23 | 0.60 ** | 0.71 ** | −0.07 | −0.03 | −0.13 | −0.74 ** | 0.71 ** | |
| 4.0–5.0 | 0.55 ** | 0.06 | 0.04 | −0.03 | −0.13 | 0.56 ** | 0.63 ** | −0.30 | −0.23 | −0.13 | −0.75 ** | 0.60 ** | |
| 5.0–6.0 | 0.56 ** | 0.01 | 0.01 | −0.01 | −0.13 | 0.43 * | 0.64 ** | −0.52 * | −0.58 ** | 0.26 | −0.74 ** | 0.57 ** | |
| 6.0–8.0 | 0.53 ** | 0.01 | 0.01 | −0.13 | −0.04 | 0.19 | 0.31 | −0.15 | −0.15 | 0.20 | −0.28 | 0.27 | |
| 8.0–10.0 | 0.59 ** | −0.05 | −0.04 | 0.02 | −0.09 | 0.30 | 0.01 | −0.30 | −0.27 | 0.17 | −0.60 ** | 0.45 * | |
| 10.0–12.0 | 0.38 * | −0.06 | −0.03 | −0.12 | −0.27 | 0.41 * | −0.07 | −0.17 | −0.15 | −0.25 | −0.10 | 0.15 | |
| 12.0–14.0 | 0.32 | −0.07 | −0.08 | −0.25 | −0.15 | 0.03 | 0.31 | −0.38 | −0.29 | −0.41 | −0.51 * | 0.25 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, B.; Huang, D.; Wu, J.; Zhu, Q.; Zhu, H. Horizontal and Vertical Distributions of Chromium in a Chromate Production District of South Central China. Int. J. Environ. Res. Public Health 2018, 15, 571. https://doi.org/10.3390/ijerph15040571
Zhou B, Huang D, Wu J, Zhu Q, Zhu H. Horizontal and Vertical Distributions of Chromium in a Chromate Production District of South Central China. International Journal of Environmental Research and Public Health. 2018; 15(4):571. https://doi.org/10.3390/ijerph15040571
Chicago/Turabian StyleZhou, Bin, Daoyou Huang, Jinshui Wu, Qihong Zhu, and Hanhua Zhu. 2018. "Horizontal and Vertical Distributions of Chromium in a Chromate Production District of South Central China" International Journal of Environmental Research and Public Health 15, no. 4: 571. https://doi.org/10.3390/ijerph15040571