Association between Cervical Spondylosis and Migraine: A Nationwide Retrospective Cohort Study
Abstract
:1. Introduction
2. Methods
2.1. Data Source
2.2. Sampled Participants
2.3. Outcome and Comorbidities
2.4. Statistical Analysis
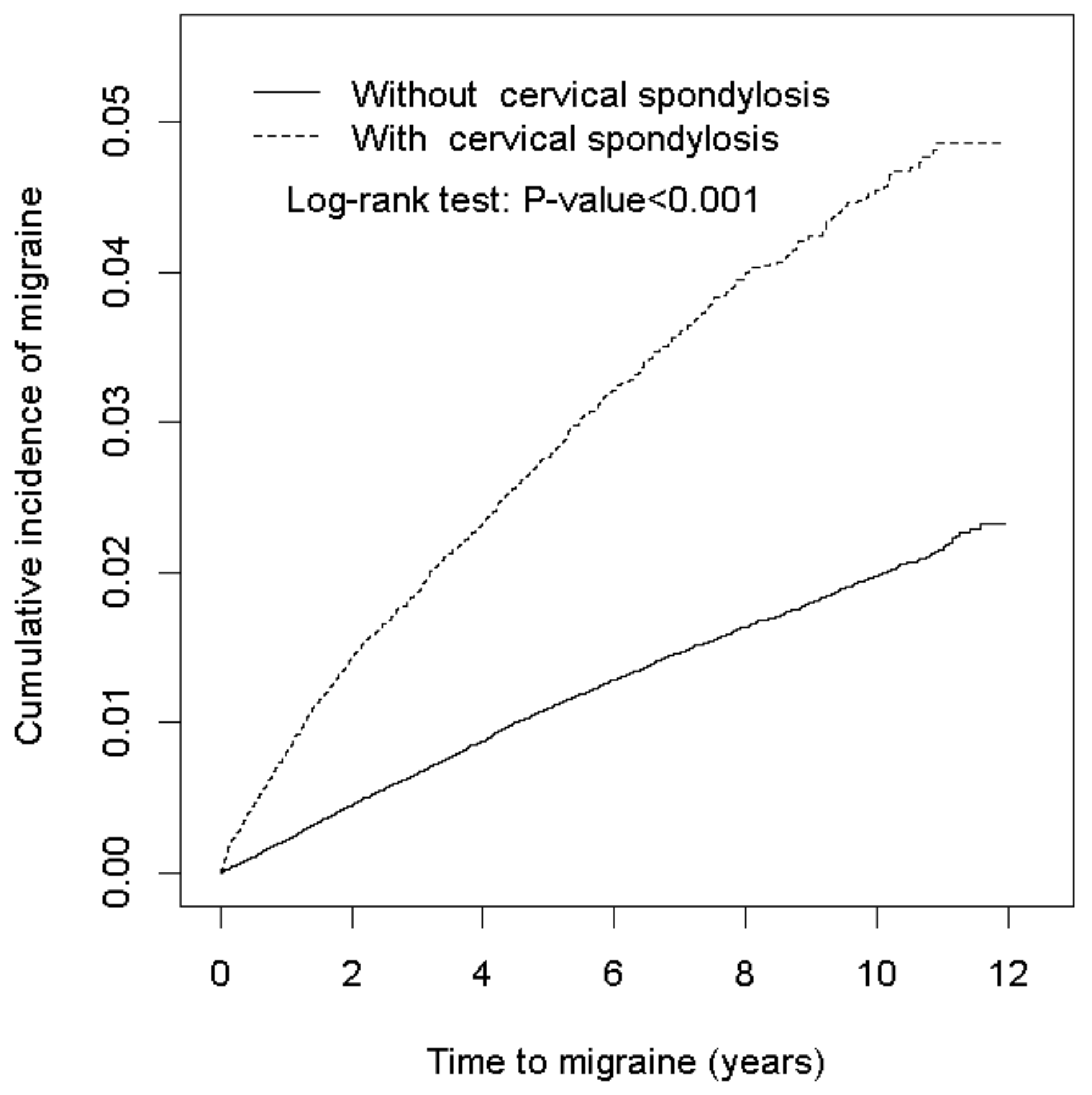
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| CS | cervical spondylosis |
| HR | hazard ratio |
| CI | confidence interval |
| NHIRD | National Health Insurance Research Database |
| LHID2000 | Longitudinal Health Insurance Database 2000 |
| ICD-9-CM | International Classification of Diseases, Ninth Revision, Clinical Modification |
References
- Vos, T.; Abajobir, A.A.; Abbafati, C.; Abate, K.H.; Abbas, K.M.; Abd-Allah, F.; Abdulkader, R.S.; Abdulle, A.M.; Abebo, T.A.; Abera, S.F.; et al. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1211–1259. [Google Scholar] [CrossRef]
- Steiner, T.J.; Stovner, L.J.; Vos, T.; Jensen, R.; Katsarava, Z. Migraine is first cause of disability in under 50s: Will health politicians now take notice? J. Headache Pain 2018, 19, 17. [Google Scholar] [CrossRef] [PubMed]
- Bloudek, L.M.; Stokes, M.; Buse, D.C.; Wilcox, T.K.; Lipton, R.B.; Goadsby, P.J.; Varon, S.F.; Blumenfeld, A.M.; Katsarava, Z.; Pascual, J.; et al. Cost of healthcare for patients with migraine in five European countries: Results from the International Burden of Migraine Study (IBMS). J. Headache Pain 2012, 13, 361–378. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.; Tozzi, A.; Rainero, I.; Cupini, L.M.; Calabresi, P.; Ayata, C.; Sarchielli, P. Cortical spreading depression as a target for anti-migraine agents. J. Headache Pain 2013, 14, 62. [Google Scholar] [CrossRef] [PubMed]
- Akerman, S.; Holland, P.R.; Goadsby, P.J. Diencephalic and brainstem mechanisms in migraine. Nat. Rev. Neurosci. 2011, 12, 570–584. [Google Scholar] [CrossRef] [PubMed]
- Olesen, J.; Burstein, R.; Ashina, M.; Tfelt-Hansen, P. Origin of pain in migraine: Evidence for peripheral sensitisation. Lancet Neurol. 2009, 8, 679–690. [Google Scholar] [CrossRef]
- Gasparini, C.F.; Smith, R.A.; Griffiths, L.R. Genetic and biochemical changes of the serotonergic system in migraine pathobiology. J. Headache Pain 2017, 18, 20. [Google Scholar] [CrossRef] [PubMed]
- Tajti, J.; Vecsei, L. The mechanism of peripheral and central sensitization in migraine. A literature review. Neuropsychopharmacol. Hung. 2009, 11, 15–21. [Google Scholar] [PubMed]
- Martin, P.R. Behavioral Management of Migraine Headache Triggers: Learning to Cope with Triggers. Curr. Pain Headache Rep. 2010, 14, 221–227. [Google Scholar] [PubMed]
- Levy, D.; Strassman, A.M.; Burstein, R. A Critical View on the Role of Migraine Triggers in the Genesis of Migraine Pain. Headache 2009, 49, 953–957. [Google Scholar] [CrossRef] [PubMed]
- Biondi, D.M. Cervicogenic headache: A review of diagnostic and treatment strategies. J. Am. Osteopath. Assoc. 2005, 105, 16S–22S. [Google Scholar] [PubMed]
- Kaniecki, R.G. Migraine and tension-type headache—An assessment of challenges in diagnosis. Neurology 2002, 58, S15–S20. [Google Scholar] [PubMed]
- Escher, C.M.; Paracka, L.; Dressler, D.; Kollewe, K. Botulinum toxin in the management of chronic migraine: Clinical evidence and experience. Ther. Adv. Neurol. Disord. 2017, 10, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Szok, D.; Csati, A.; Vecsei, L.; Tajti, J. Treatment of Chronic Migraine with OnabotulinumtoxinA: Mode of Action, Efficacy and Safety. Toxins 2015, 7, 2659–2673. [Google Scholar] [CrossRef] [PubMed]
- Robbins, M.S.; Kuruvilla, D.; Blumenfeld, A.; Charleston, L.; Sorrell, M.; Robertson, C.E.; Grosberg, B.M.; Bender, S.D.; Napchan, U.; Ashkenazi, A. Trigger point injections for headache disorders: Expert consensus methodology and narrative review. Headache 2014, 54, 1441–1459. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-de-las-Penas, C.; Cuadrado, M.L. Therapeutic options for cervicogenic headache. Expert Rev. Neurother. 2014, 14, 39–49. [Google Scholar] [PubMed]
- Martinovic, Z.; Buder, N.; Velickovic, R.; Milovanovic, M. Comorbidity of migraine and somatic diseases. Med. Pregl. 2005, 58, 342–346. [Google Scholar] [PubMed]
- Database NHIR. Taiwan. 2016. Available online: http://nhird.nhri.org.tw/en/index.html (accessed on 28 June 2016).
- Hu, W.S.; Lin, C.L. CHA2DS2-VASc score for ischaemic stroke risk stratification in patients with chronic obstructive pulmonary disease with and without atrial fibrillation: A nationwide cohort study. EP Eur. 2017. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.W.; Lin, C.L.; Lin, P.Y.; Thielke, S.; Su, K.P.; Kao, C.H. Antidepressants and risk of dementia in migraine patients: A population-based case-control study. Prog. Neuropsychopharmacol. Biol. Psychiatry 2017, 77, 83–89. [Google Scholar] [PubMed]
- Wang, S.J.; Fuh, J.L.; Huang, S.Y.; Yang, S.S.; Wu, Z.A.; Hsu, C.H.; Wang, C.H.; Yu, H.Y.; Wang, P.J.; Taiwan MAP Study Group. Diagnosis and development of screening items for migraine in neurological practice in Taiwan. J. Formos. Med. Assoc. 2008, 107, 485–494. [Google Scholar] [PubMed]
- Blau, J.N.; MacGregor, E.A. Migraine and the neck. Headache 1994, 34, 88–90. [Google Scholar] [CrossRef] [PubMed]
- Haldeman, S.; Dagenais, S. Cervicogenic headaches: A critical review. Spine J. 2001, 1, 31–46. [Google Scholar] [CrossRef]
- Schrot, R.J.; Mathew, J.S.; Li, Y.; Beckett, L.; Bae, H.W.; Kim, K.D. Headache relief after anterior cervical discectomy: Post hoc analysis of a randomized investigational device exemption trial. J. Neurosurg. Spine 2014, 21, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Riina, J.; Anderson, P.A.; Holly, L.T.; Flint, K.; Davis, K.E.; Riew, K.D. The Effect of an Anterior Cervical Operation for Cervical Radiculopathy or Myelopathy on Associated Headaches. J. Bone Joint Surg. Am. 2009, 91A, 1919–1923. [Google Scholar] [CrossRef] [PubMed]
- Marcus, D.A.; Scharff, L.; Mercer, S.; Turk, D.C. Musculoskeletal abnormalities in chronic headache: A controlled comparison of headache diagnostic groups. Headache 1999, 39, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Vetvik, K.G.; MacGregor, E.A. Sex differences in the epidemiology, clinical features, and CrossMark pathophysiology of migraine. Lancet Neurol. 2017, 16, 76–87. [Google Scholar] [CrossRef]
- Wang, C.; Tian, F.; Zhou, Y.; He, W.; Cai, Z. The incidence of cervical spondylosis decreases with aging in the elderly, and increases with aging in the young and adult population: A hospital-based clinical analysis. Clin. Interv. Aging 2016, 11, 47–53. [Google Scholar] [PubMed]
- Woolf, C.J. Central sensitization: Implications for the diagnosis and treatment of pain. Pain 2011, 152, S2–S15. [Google Scholar] [CrossRef] [PubMed]
- Takano, M.; Kawabata, S.; Komaki, Y.; Shibata, S.; Hikishima, K.; Toyama, Y.; Okano, H.; Nakamura, M. Inflammatory cascades mediate synapse elimination in spinal cord compression. J. Neuroinflamm. 2014, 11, 40. [Google Scholar] [CrossRef] [PubMed]
- Beattie, M.S.; Manley, G.T. Tight squeeze, slow burn: Inflammation and the aetiology of cervical myelopathy. Brain 2011, 134, 1259–1261. [Google Scholar] [CrossRef] [PubMed]
- Murphy, R.K.J.; Sun, P.; Xu, J.Q.; Wang, Y.; Sullivan, S.; Gamble, P.; Wagner, J.; Wright, N.N.; Dorward, I.G.; Riew, D.; et al. Magnetic Resonance Imaging Biomarker of Axon Loss Reflects Cervical Spondylotic Myelopathy Severity. Spine 2016, 41, 751–756. [Google Scholar] [CrossRef] [PubMed]
- Allison, D.J.; Ditor, D.S. Immune dysfunction and chronic inflammation following spinal cord injury. Spinal Cord 2015, 53, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Steilen, D.; Hauser, R.; Woldin, B.; Sawyer, S. Chronic neck pain: Making the connection between capsular ligament laxity and cervical instability. Open Orthop. J. 2014, 8, 326–345. [Google Scholar] [PubMed]
- Menezes, A.H. Craniovertebral junction database analysis: Incidence, classification, presentation, and treatment algorithms. Childs Nerv. Syst. 2008, 24, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J. Vertebral Artery Blood flow Velocity Changes Associated with Cervical Spine rotation: A Meta-Analysis of the Evidence with implications for Professional Practice. J. Man. Manip. Ther. 2009, 17, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Watson, D.H.; Drummond, P.D. Head pain referral during examination of the neck in migraine and tension-type headache. Headache 2012, 52, 1226–1235. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-de-Las-Penas, C.; Cuadrado, M.L.; Pareja, J.A. Myofascial trigger points, neck mobility and forward head posture in unilateral migraine. Cephalalgia 2006, 26, 1061–1070. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-de-Las-Penas, C. Myofascial Head Pain. Curr. Pain Headache Rep. 2015, 19, 28. [Google Scholar] [CrossRef] [PubMed]
- Noseda, R.; Constandil, L.; Bourgeais, L.; Chalus, M.; Villanueva, L. Changes of meningeal excitability mediated by corticotrigeminal networks: A link for the endogenous modulation of migraine pain. J. Neurosci. 2010, 30, 14420–14429. [Google Scholar] [CrossRef] [PubMed]
- Sauro, K.M.; Becker, W.J. The stress and migraine interaction. Headache 2009, 49, 1378–1386. [Google Scholar] [CrossRef] [PubMed]
- Revanappa, K.K.; Moorthy, R.K.; Alexander, M.; Rajshekhar, V. Recovery of sympathetic skin response after central corpectomy in patients with moderate and severe cervical spondylotic myelopathy. Br. J. Neurosurg. 2017, 31, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Gu, T.; Yang, H.; Liang, L.; Jiang, D.J.; Wang, Z.C.; Yuan, W.; Wang, X.W. Sympathetic nerve innervation in cervical posterior longitudinal ligament as a potential causative factor in cervical spondylosis with sympathetic symptoms and preliminary evidence. Med. Hypotheses 2014, 82, 631–635. [Google Scholar] [CrossRef] [PubMed]
- Gu, Q.G.; Jiang, D.J.; Wang, X.W.; Chen, D.Y.; Yuan, W. Chronic compression of the posterior longitudinal ligament of the cervical spine is associated with abnormal discharge of middle cervical ganglion. Int. J. Clin. Exp. Med. 2014, 7, 4316–4321. [Google Scholar] [PubMed]
- Peng, K.P.; Wang, S.J. Migraine diagnosis: Screening items, instruments, and scales. Acta Anaesthesiol. Taiwan 2012, 50, 69–73. [Google Scholar] [CrossRef] [PubMed]
| Variable | Cervical Spondylosis | ||
|---|---|---|---|
| No | Yes | Standard Mean Difference | |
| (N =111,720) | (N =27,930) | ||
| Sex | 0.99 | ||
| Women | 63,592(56.9) | 15,898(56.9) | |
| Men | 48,128(43.1) | 12,032(43.1) | |
| Age stratified | 0.99 | ||
| ≤49 | 40,452(36.2) | 10,113(36.2) | |
| 50–64 | 41,880(37.5) | 10,470(37.5) | |
| 65+ | 29,388(26.3) | 7347(26.3) | |
| Age, mean ± SD a | 55.1(14.0) | 55.6(13.6) | <0.001 |
| Comorbidity | |||
| Hypertension | 36,886(33.0) | 11,658(41.7) | <0.001 |
| Hyperlipidemia | 22,730(20.4) | 8887(31.8) | <0.001 |
| Depression | 4498(4.03) | 2252(8.06) | <0.001 |
| Coronary artery disease | 17,160(15.4) | 6941(24.9) | <0.001 |
| Anxiety | 10,300(9.22) | 5607(20.1) | <0.001 |
| Sleep disorder | 20,851(18.7) | 9450(33.8) | <0.001 |
| Irritable bowel syndrome | 4969(4.45) | 2487(8.90) | <0.001 |
| Diabetes | 10,876(9.74) | 3182(11.4) | <0.001 |
| Fibromyalgia | 4599(4.12) | 2984(10.7) | <0.001 |
| Variable | Event | PY | Rate # | Crude HR (95% CI) | Adjusted HR & (95% CI) |
|---|---|---|---|---|---|
| Cervical spondylosis | |||||
| No | 1414 | 677,913 | 2.09 | 1.00 | 1.00 |
| Yes | 883 | 171,179 | 5.16 | 2.48(2.28, 2.69) *** | 2.03(1.86, 2.22) *** |
| Age group, year | |||||
| 20−49 | 997 | 330,050 | 3.02 | 1.46(1.31, 1.64) *** | 1.81(1.58, 2.06) *** |
| 50−64 | 874 | 317,220 | 2.76 | 1.32(1.18, 1.48) *** | 1.40(1.24, 1.58) *** |
| ≥65 | 426 | 201,822 | 2.11 | 1.00 | 1.00 |
| Sex | |||||
| Female | 1719 | 494,959 | 3.47 | 2.14(1.95, 2.35) *** | 1.92(1.74, 2.11) *** |
| Male | 578 | 354,133 | 1.63 | 1.00 | 1.00 |
| Comorbidity | |||||
| Hypertension | |||||
| No | 1465 | 570,275 | 2.57 | 1.00 | 1.00 |
| Yes | 832 | 278,816 | 2.98 | 1.15(1.06, 1.25) ** | 1.06(0.95, 1.18) |
| Hyperlipidemia | |||||
| No | 1698 | 668,229 | 2.54 | 1.00 | 1.00 |
| Yes | 599 | 180,862 | 3.31 | 1.29(1.17, 1.41) *** | 1.01(0.91, 1.12) |
| Depression | |||||
| No | 2090 | 813,829 | 2.57 | 1.00 | 1.00 |
| Yes | 207 | 35,263 | 5.87 | 2.24(1.94, 2.58) *** | 1.13(0.97, 1.32) |
| Coronary artery disease | |||||
| No | 1800 | 711,569 | 2.53 | 1.00 | 1.00 |
| Yes | 497 | 137,523 | 3.61 | 1.42(1.28, 1.56) *** | 1.20(1.07, 1.35) ** |
| Anxiety | |||||
| No | 1785 | 764,303 | 2.34 | 1.00 | 1.00 |
| Yes | 512 | 84,789 | 6.04 | 2.53(2.30, 2.80) *** | 1.48(1.32, 1.66) *** |
| Sleep disorder | |||||
| No | 1444 | 690,750 | 2.09 | 1.00 | 1.00 |
| Yes | 853 | 158,342 | 5.39 | 2.52(2.31, 2.74) *** | 1.81(1.64, 1.99) *** |
| Irritable bowel syndrome | |||||
| No | 2105 | 809,522 | 2.60 | 1.00 | 1.00 |
| Yes | 192 | 39,569 | 4.85 | 1.83(1.57, 2.12) *** | 1.24(1.06,1.44) ** |
| Diabetes | |||||
| No | 2112 | 775,714 | 2.72 | 1.00 | 1.00 |
| Yes | 185 | 73,378 | 2.52 | 0.91(0.78, 1.05) | - |
| Fibromyalgia | |||||
| No | 2127 | 808,699 | 2.63 | 1.00 | 1.00 |
| Yes | 170 | 4093 | 4.21 | 1.57(1.34, 1.83) *** | 1.08(0.92, 1.27) |
| Variable | Cervical spondylosis | |||||||
|---|---|---|---|---|---|---|---|---|
| No | Yes | Crude HR (95% CI) | Adjusted HR & (95% CI) | |||||
| Event | PY | Rate # | Event | PY | Rate # | |||
| Sex | ||||||||
| Women | 1082 | 395,676 | 2.73 | 637 | 99,282 | 6.42 | 2.35(2.13, 2.59) *** | 1.93(1.74, 2.14) *** |
| Men | 332 | 282,237 | 1.18 | 246 | 71,896 | 3.42 | 2.91(2.47, 3.44) *** | 2.32(1.95, 2.76) *** |
| Stratify age | ||||||||
| ≤49 | 620 | 264,087 | 2.35 | 377 | 65,963 | 5.72 | 2.44(2.14, 2.77) *** | 1.94(1.70, 2.23) *** |
| 50–64 | 532 | 253,619 | 2.10 | 342 | 63,600 | 5.38 | 2.56(2.24, 2.94) *** | 2.05(1.78, 2.36) *** |
| 65+ | 377 | 160,207 | 1.64 | 164 | 41,615 | 3.94 | 2.42(1.99, 2.94) *** | 2.08(1.70, 2.55) *** |
| Comorbidity ‡ | ||||||||
| No | 520 | 342,246 | 1.52 | 180 | 49,454 | 3.64 | 2.40(2.02, 2.84) *** | 2.36(1.99, 2.80) *** |
| Yes | 894 | 335,667 | 2.66 | 703 | 121,725 | 5.78 | 2.19(1.98, 2.41) *** | 2.10(1.91, 2.32) *** |
| Variable | N | Events | PYs | Rate # | Crude HR (95% CI) | Adjusted HR & 95% CI) |
|---|---|---|---|---|---|---|
| Without cervical spondylosis | 111,720 | 1414 | 677,913 | 2.09 | 1.00 | 1.00 |
| Type of Cervical spondylosis | ||||||
| Cervical spondylosis without myelopathy | 24,287 | 771 | 150,298 | 5.13 | 2.47(2.26, 2.69 ) *** | 2.01(1.83, 2.20) *** |
| Cervical spondylosis with myelopathy | 3643 | 112 | 20,880 | 5.36 | 2.55(2.10, 3.09) *** | 2.19(1.80, 2.66) *** |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, W.-S.; Huang, T.-F.; Chuang, T.-Y.; Lin, C.-L.; Kao, C.-H. Association between Cervical Spondylosis and Migraine: A Nationwide Retrospective Cohort Study. Int. J. Environ. Res. Public Health 2018, 15, 587. https://doi.org/10.3390/ijerph15040587
Lin W-S, Huang T-F, Chuang T-Y, Lin C-L, Kao C-H. Association between Cervical Spondylosis and Migraine: A Nationwide Retrospective Cohort Study. International Journal of Environmental Research and Public Health. 2018; 15(4):587. https://doi.org/10.3390/ijerph15040587
Chicago/Turabian StyleLin, Wang-Sheng, Tung-Fu Huang, Tien-Yow Chuang, Cheng-Li Lin, and Chia-Hung Kao. 2018. "Association between Cervical Spondylosis and Migraine: A Nationwide Retrospective Cohort Study" International Journal of Environmental Research and Public Health 15, no. 4: 587. https://doi.org/10.3390/ijerph15040587





