Is a Water Content of 60% Maximum Water Holding Capacity Suitable for Folsomia candida Reproduction Tests? A Study with Silver Nanoparticles and AgNO3
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Toxicity Test
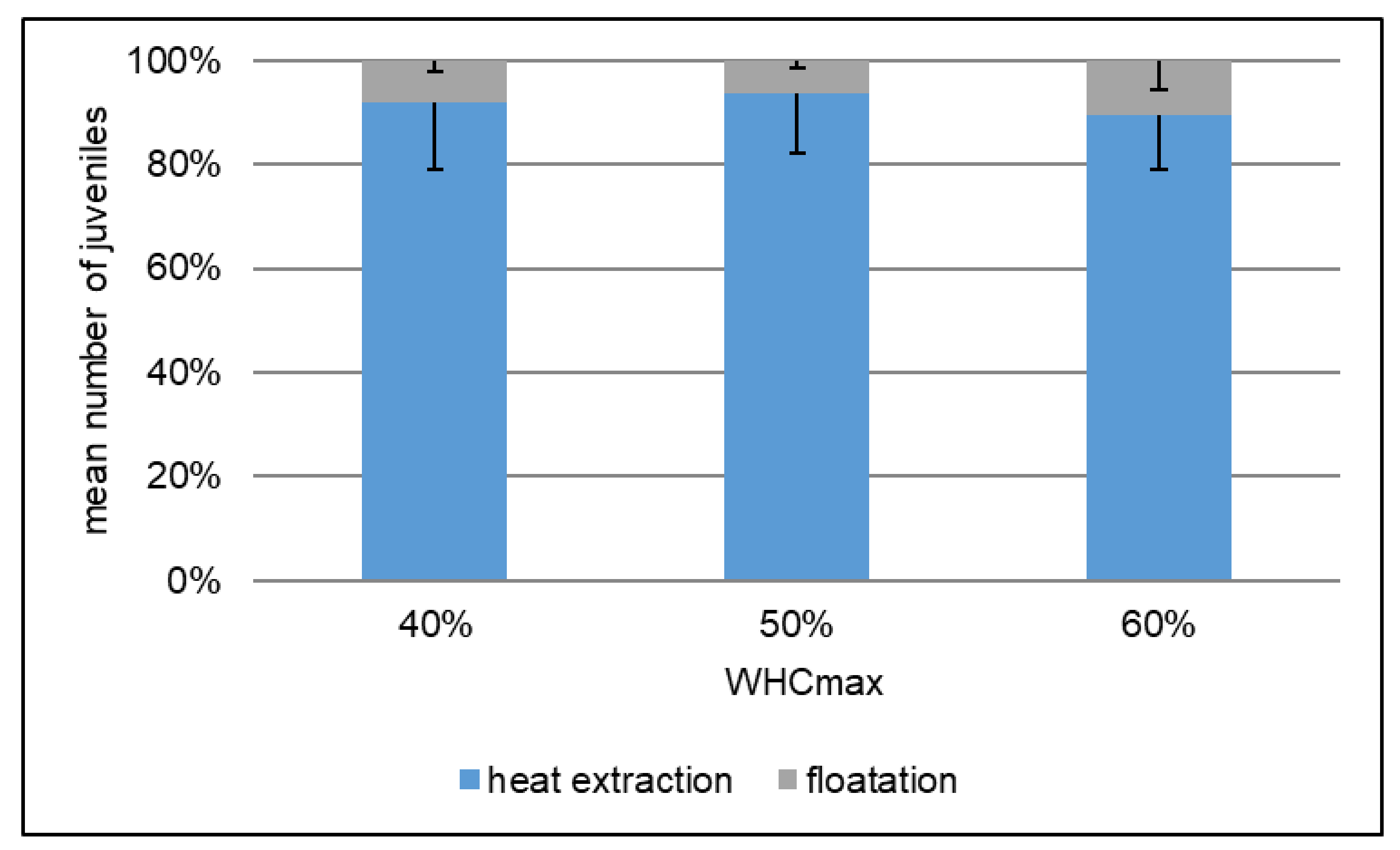
2.3. Pre-Test of Extraction Method
2.4. Data Analysis
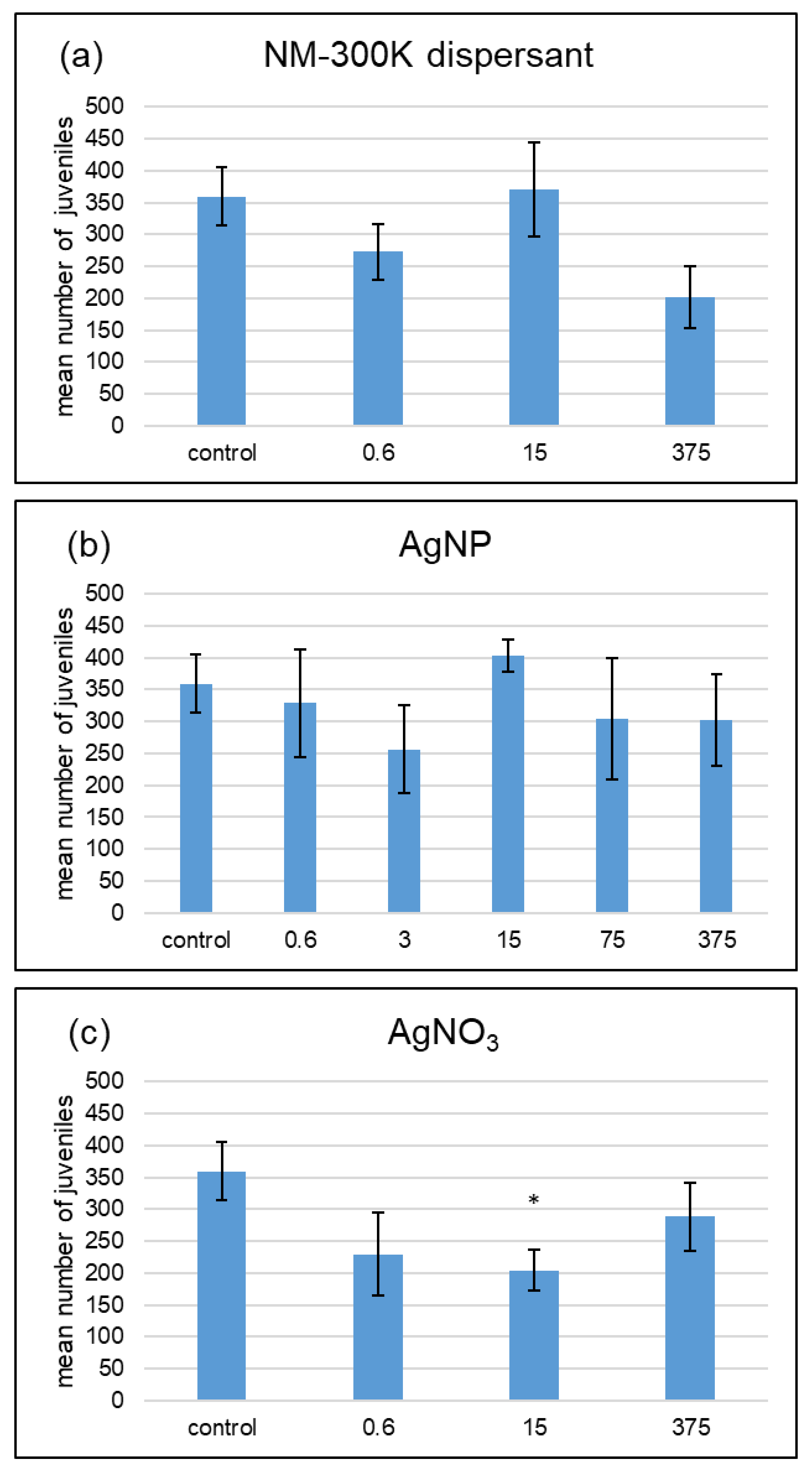
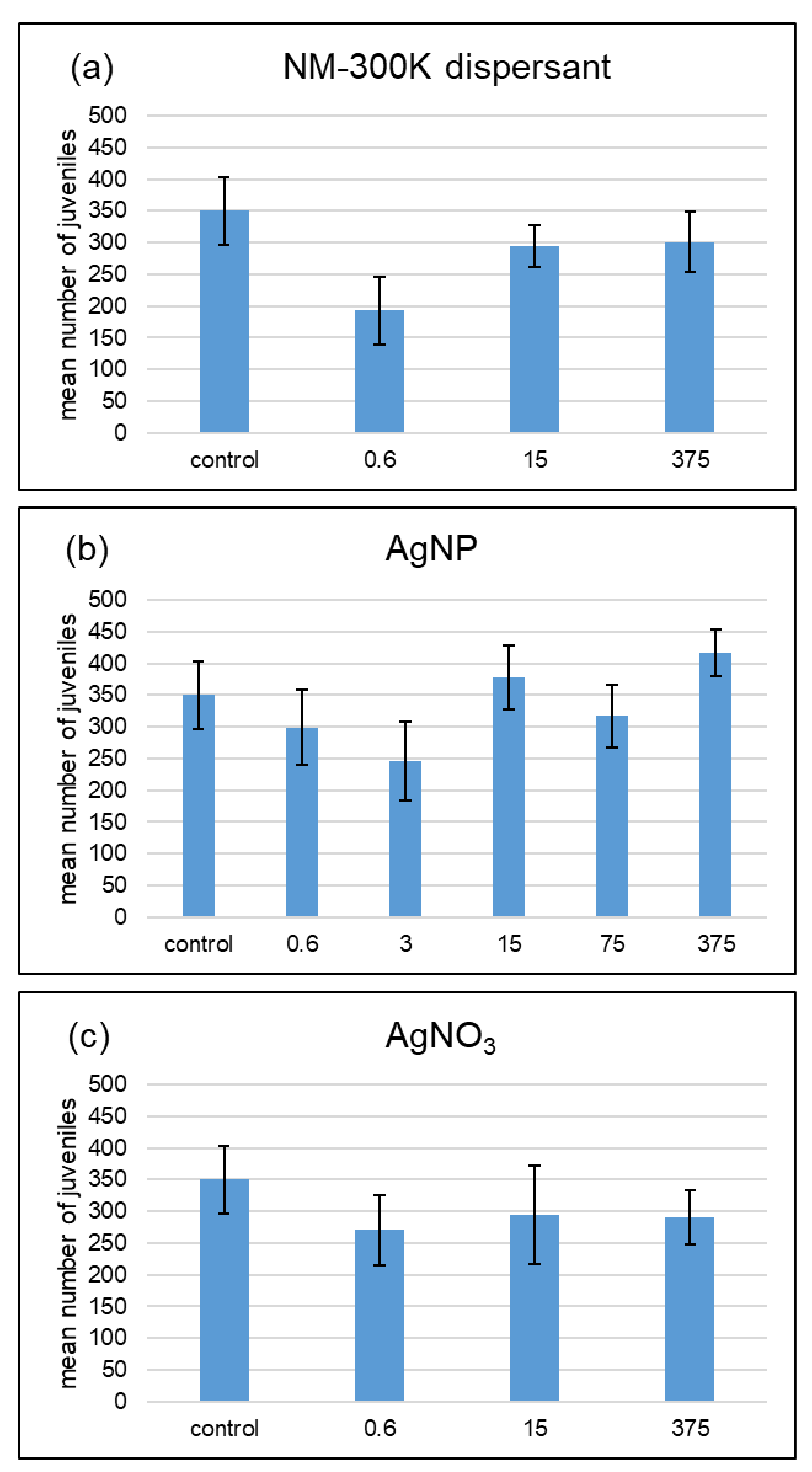
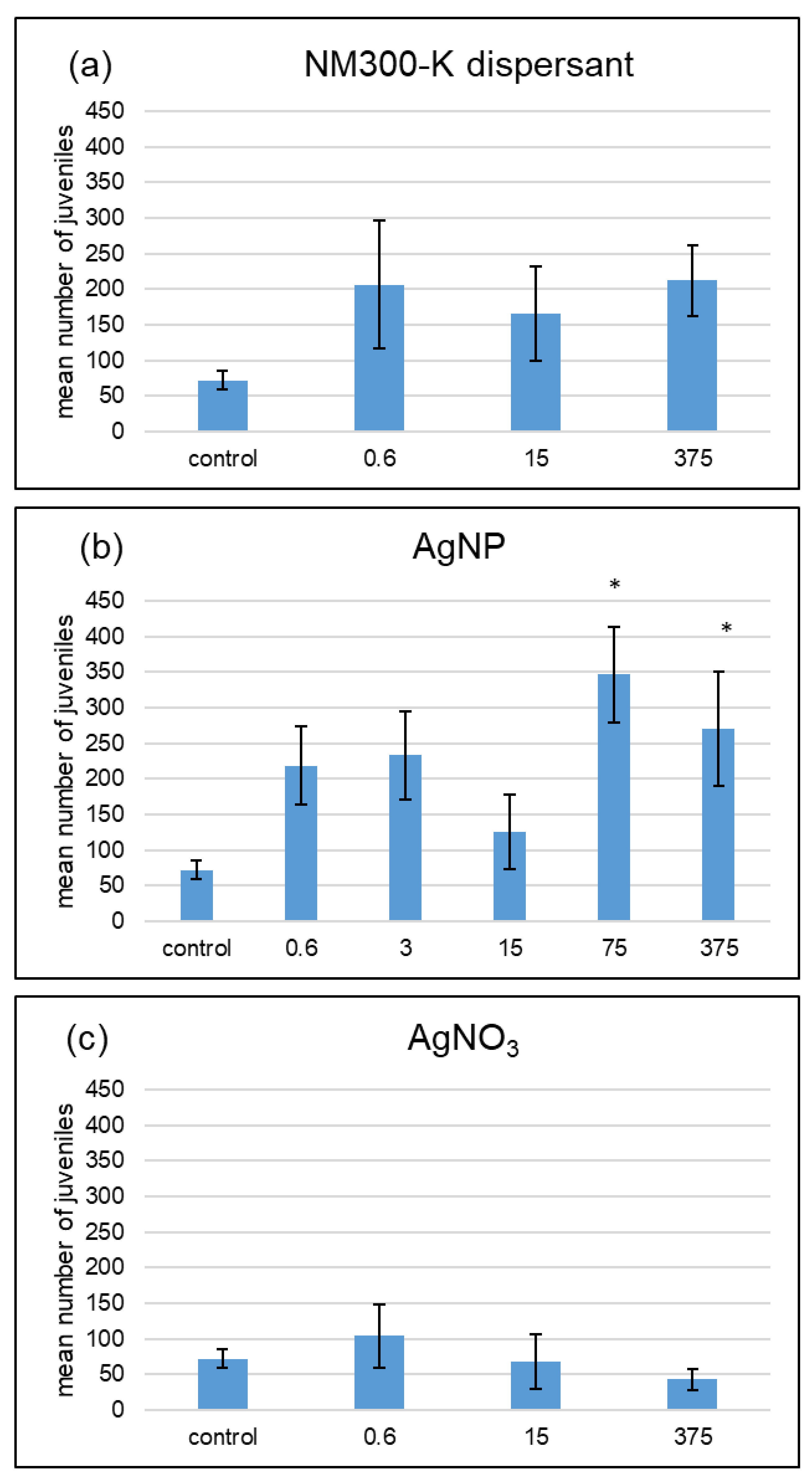
3. Results
4. Discussion
4.1. No Dose-Response Curves for Silver Treatments
4.2. Different Effects of AgNP and AgNO3 with Different Soil Water Contents
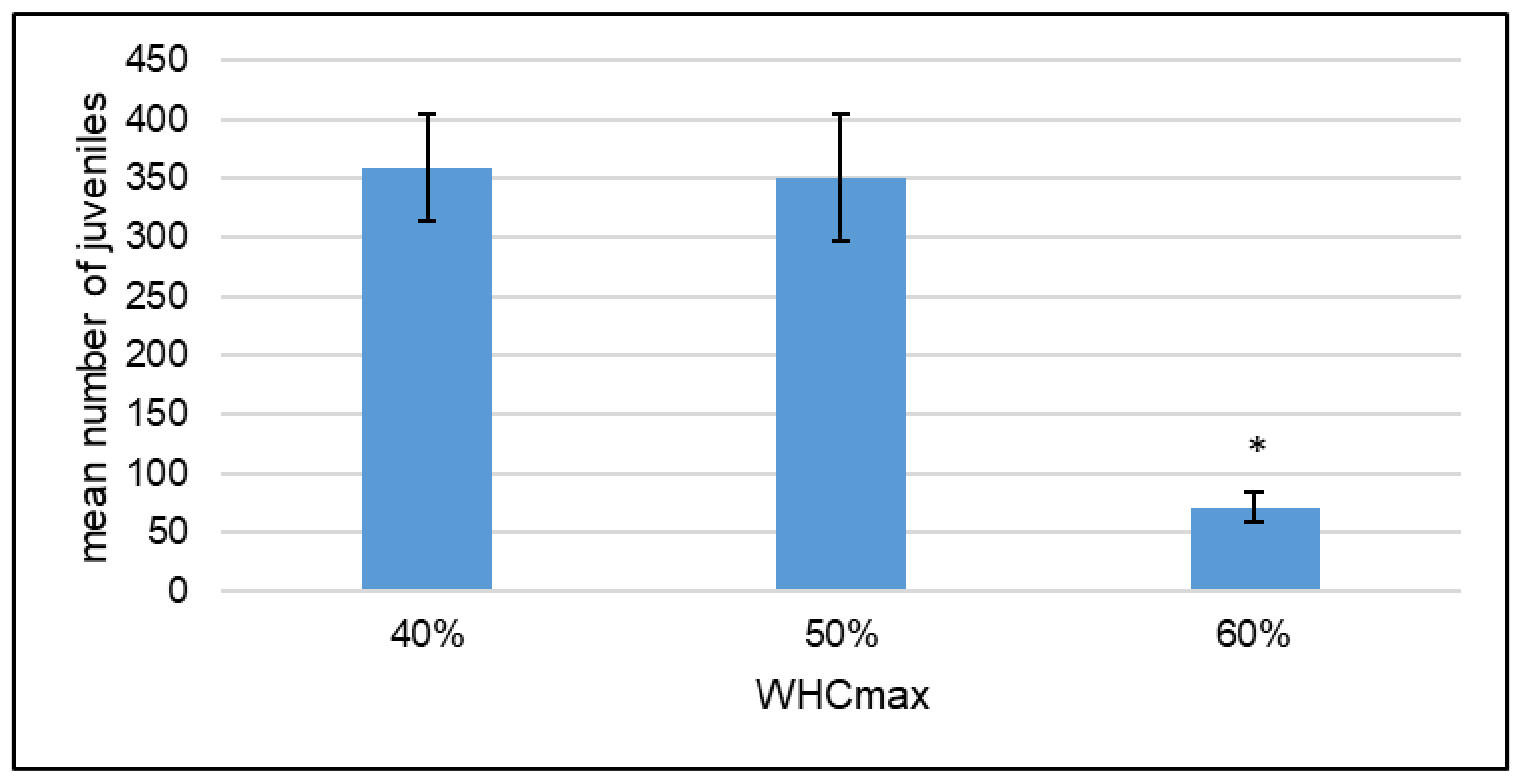
4.3. Less Suitable Conditions for F. candida with 60% WHC
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Voelker, D.; Schlich, K.; Hohndorf, L.; Koch, W.; Kuehnen, U.; Polleichtner, C.; Kussatz, C.; Hund-Rinke, K. Approach on environmental risk assessment of nanosilver released from textiles. Environ. Res. 2015, 140, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Piccinno, F.; Gottschalk, F.; Seeger, S.; Nowack, B. Industrial production quantities and uses of ten engineered nanomaterials in Europe and the world. J. Nanopart. Res. 2012, 14, 1109. [Google Scholar] [CrossRef]
- Gottschalk, F.; Lassen, C.; Kjoelholt, J.; Christensen, F.; Nowack, B. Modeling Flows and Concentrations of Nine Engineered Nanomaterials in the Danish Environment. Int. J. Environ. Res. Public Health 2015, 12, 5581–5602. [Google Scholar] [CrossRef] [PubMed]
- Tourinho, P.S.; van Gestel, C.A.M.; Morgan, A.J.; Kille, P.; Svendsen, C.; Jurkschat, K.; Mosselmans, J.F.W.; Soares, A.M.V.M.; Loureiro, S. Toxicokinetics of Ag in the terrestrial isopod Porcellionides pruinosus exposed to Ag NPs and AgNO3 via soil and food. Ecotoxicology 2016, 25, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Schlich, K.; Klawonn, T.; Terytze, K.; Hund-Rinke, K. Effects of silver nanoparticles and silver nitrate in the earthworm reproduction test. Environ. Toxicol. Chem. 2013, 32, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Van der Ploeg, M.J.C.; Handy, R.D.; Waalewijn-Kool, P.L.; van den Berg, J.H.J.; Herrera Rivera, Z.E.; Bovenschen, J.; Molleman, B.; Baveco, J.M.; Tromp, P.; Peters, R.J.B.; et al. Effects of silver nanoparticles (NM-300K) on Lumbricus rubellus earthworms and particle characterization in relevant test matrices including soil. Environ. Toxicol. Chem. 2014, 33, 743–752. [Google Scholar] [CrossRef] [PubMed]
- McKee, M.S.; Engelke, M.; Zhang, X.; Lesnikov, E.; Köser, J.; Eickhorst, T.; Filser, J. Collembola Reproduction Decreases with Aging of Silver Nanoparticles in a Sewage Sludge-Treated Soil. Front. Environ. Sci. 2017, 5, 19. [Google Scholar] [CrossRef]
- Novo, M.; Lahive, E.; Díez-Ortiz, M.; Matzke, M.; Morgan, A.J.; Spurgeon, D.J.; Svendsen, C.; Kille, P. Different routes, same pathways: Molecular mechanisms under silver ion and nanoparticle exposures in the soil sentinel Eisenia fetida. Environ. Pollut. 2015, 205, 385–393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Velicogna, J.R.; Ritchie, E.E.; Scroggins, R.P.; Princz, J.I. A comparison of the effects of silver nanoparticles and silver nitrate on a suite of soil dwelling organisms in two field soils. Nanotoxicology 2016, 10, 1144–1151. [Google Scholar] [CrossRef] [PubMed]
- Cornelis, G.; Hund-Rinke, K.; Kuhlbusch, T.; Van den Brink, N.; Nickel, C. Fate and bioavailability of engineered nanoparticles in soils: A review. Crit. Rev. Environ. Sci. Technol. 2014, 44, 2720–2764. [Google Scholar] [CrossRef]
- McKee, M.S.; Filser, J. Impacts of metal-based engineered nanomaterials on soil communities. Environ. Sci. Nano 2016, 3, 506–533. [Google Scholar] [CrossRef]
- Shoults-Wilson, W.A.; Zhurbich, O.I.; McNear, D.H.; Tsyusko, O.V.; Bertsch, P.M.; Unrine, J.M. Evidence for avoidance of Ag nanoparticles by earthworms (Eisenia fetida). Ecotoxicology 2011, 20, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Topuz, E.; van Gestel, C.A.M. The effect of soil properties on the toxicity and bioaccumulation of Ag nanoparticles and Ag ions in Enchytraeus crypticus. Ecotoxicol. Environ. Saf. 2017, 144, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Tourinho, P.S.; van Gestel, C.A.M.; Lofts, S.; Svendsen, C.; Soares, A.M.V.M.; Loureiro, S. Metal-based nanoparticles in soil: Fate, behavior, and effects on soil invertebrates. Environ. Toxicol. Chem. 2012, 31, 1679–1692. [Google Scholar] [CrossRef] [PubMed]
- Kühnel, D.; Nickel, C. The OECD expert meeting on ecotoxicology and environmental fate—Towards the development of improved OECD guidelines for the testing of nanomaterials. Sci. Total Environ. 2014, 472, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Hund-Rinke, K.; Baun, A.; Cupi, D.; Fernandes, T.F.; Handy, R.; Kinross, J.H.; Navas, J.M.; Peijnenburg, W.; Schlich, K.; Shaw, B.J.; et al. Regulatory ecotoxicity testing of nanomaterials-proposed modifications of OECD test guidelines based on laboratory experience with silver and titanium dioxide nanoparticles. Nanotoxicology 2016, 10, 1442–1447. [Google Scholar] [CrossRef] [PubMed]
- Organisation for Economic Co-operation and Development. Guideline 232: Collembolan Reproduction Test in Soil. In OECD Guidelines for the Testing of Chemicals; OECD: Paris, France, 2016. [Google Scholar]
- Klein, C.; Comero, S.; Stahlmecke, B.; Romazanov, J.; Kuhlbusch, T.; Van Doren, E.; De Temmerman, P.-J.; Mast, J.; Wick, P.; Krug, H.; et al. NM-Series of Representative Manufactured Nanomaterials: NM-300 Silver Characterisation, Stability, Homogeneity; Publications Office of the European Union: Luxembourg, 2011. [Google Scholar]
- Hoppe, M.; Mikutta, R.; Utermann, J.; Duijnisveld, W.; Guggenberger, G. Remobilization of sterically stabilized silver nanoparticles from farmland soils determined by column leaching. Eur. J. Soil Sci. 2015, 66, 898–909. [Google Scholar] [CrossRef]
- Köser, J.; Engelke, M.; Hoppe, M.; Nogowski, A.; Filser, J.; Thöming, J. Predictability of silver nanoparticle speciation and toxicity in ecotoxicological media. Environ. Sci. Nano 2017, 4, 1470–1483. [Google Scholar] [CrossRef]
- Filser, J.; Wiegmann, S.; Schröder, B. Collembola in ecotoxicology-Any news or just boring routine? Appl. Soil Ecol. 2014, 83, 193–199. [Google Scholar] [CrossRef]
- Waalewijn-Kool, P.L.; Klein, K.; Forniés, R.M.; van Gestel, C.A.M. Bioaccumulation and toxicity of silver nanoparticles and silver nitrate to the soil arthropod Folsomia candida. Ecotoxicology 2014, 23, 1629–1637. [Google Scholar] [CrossRef] [PubMed]
- Mendes, L.A.; Maria, V.L.; Scott-Fordsmand, J.J.; Amorim, M.J.B. Ag nanoparticles (Ag NM300K) in the terrestrial environment: Effects at population and cellular level in Folsomia candida (Collembola). Int. J. Environ. Res. Public Health 2015, 12, 12530–12542. [Google Scholar] [CrossRef] [PubMed]
- Simonin, M.; Martins, J.M.F.; Le Roux, X.; Uzu, G.; Calas, A.; Richaume, A. Toxicity of TiO2 nanoparticles on soil nitrification at environmentally relevant concentrations: Lack of classical dose–response relationships. Nanotoxicology 2017, 11, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Iavicoli, I.; Leso, V.; Fontana, L.; Calabrese, E. Nanoparticle exposure and hormetic dose–responses: An update. Int. J. Mol. Sci. 2018, 19, 805. [Google Scholar] [CrossRef] [PubMed]
- Dromph, K.M.; Vestergaard, S. Pathogenicity and attractiveness of entomopathogenic hyphomycete fungi to collembolans. Appl. Soil Ecol. 2002, 21, 197–210. [Google Scholar] [CrossRef]
- Zhang, X. Ecotoxical Effects of Silver Nanoparticles: The Relevance of Test Species and Test Conditions. Ph.D. Thesis, Universitat Bremen, Bremen, Germany, 2017. [Google Scholar]
- Fountain, M.T.; Hopkin, S.P. Folsomia candida (Collembola): A “Standard” Soil Arthropod. Annu. Rev. Entomol. 2005, 50, 201–222. [Google Scholar] [CrossRef] [PubMed]
- Carbone, S.; Vittori Antisari, L.; Gaggia, F.; Baffoni, L.; Di Gioia, D.; Vianello, G.; Nannipieri, P. Bioavailability and biological effect of engineered silver nanoparticles in a forest soil. J. Hazard. Mater. 2014, 280, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Sweet, M.J.; Singleton, I. Silver Nanoparticles: A Microbial Perspective. Adv. Appl. Microbiol. 2011, 77, 115–133. [Google Scholar] [CrossRef] [PubMed]
- Drenovsky, R.E.; Vo, D.; Graham, K.J.; Scow, K.M. Soil water content and organic carbon availability are major determinants of soil microbial community composition. Microb. Ecol. 2004, 48, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Van Gestel, C.A.M.; van Diepen, A.M.F. The Influence of Soil Moisture Content on the Bioavailability and Toxicity of Cadmium forFolsomia candidaWillem (Collembola: Isotomidae). Ecotoxicol. Environ. Saf. 1997, 36, 123–132. [Google Scholar] [CrossRef] [PubMed]
| Treatment | Concentration (µg·kg−1) | 40%–50% | 40%–60% | 50%–60% |
|---|---|---|---|---|
| AgNP | 375 | n.s. | n.s. | n.s. |
| AgNP | 75 | n.s. | n.s. | n.s. |
| AgNP | 15 | n.s. | 0.003 | 0.007 |
| AgNP | 3 | n.s. | n.s. | n.s. |
| AgNP | 0.6 | n.s. | n.s. | n.s. |
| dispersant | 375 | n.s. | n.s. | n.s. |
| dispersant | 15 | n.s. | n.s. | n.s. |
| dispersant | 0.6 | n.s. | n.s. | n.s. |
| AgNO3 | 375 | n.s. | 0.004 | 0.004 |
| AgNO3 | 15 | n.s. | n.s. | 0.036 |
| AgNO3 | 0.6 | n.s. | n.s. | n.s. |
| Treatment | 40% WHC | 50% WHC | 60% WHC |
|---|---|---|---|
| AgNP | 0 | 0 | ++ |
| AgNO3 | − | 0 | 0 |
| dispersant | 0 | 0 | 0 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
McKee, M.S.; Megía Guerrero, A.; Filser, J. Is a Water Content of 60% Maximum Water Holding Capacity Suitable for Folsomia candida Reproduction Tests? A Study with Silver Nanoparticles and AgNO3. Int. J. Environ. Res. Public Health 2018, 15, 652. https://doi.org/10.3390/ijerph15040652
McKee MS, Megía Guerrero A, Filser J. Is a Water Content of 60% Maximum Water Holding Capacity Suitable for Folsomia candida Reproduction Tests? A Study with Silver Nanoparticles and AgNO3. International Journal of Environmental Research and Public Health. 2018; 15(4):652. https://doi.org/10.3390/ijerph15040652
Chicago/Turabian StyleMcKee, Moira S., Amelia Megía Guerrero, and Juliane Filser. 2018. "Is a Water Content of 60% Maximum Water Holding Capacity Suitable for Folsomia candida Reproduction Tests? A Study with Silver Nanoparticles and AgNO3" International Journal of Environmental Research and Public Health 15, no. 4: 652. https://doi.org/10.3390/ijerph15040652





