Toxic Effects of Bisphenol A, Propyl Paraben, and Triclosan on Caenorhabditis elegans
Abstract
:1. Introduction
2. Materials and Methods
2.1. Nematodes and Bacteria
2.2. Solutions and Exposure
2.3. Lethality Assay
2.4. Growth Assay
2.5. Reproduction Assay
2.6. Gene Expression through Fluorescence Measuring
2.7. q-ORO Assay
2.8. Statistical Analysis
3. Results
3.1. Lethality
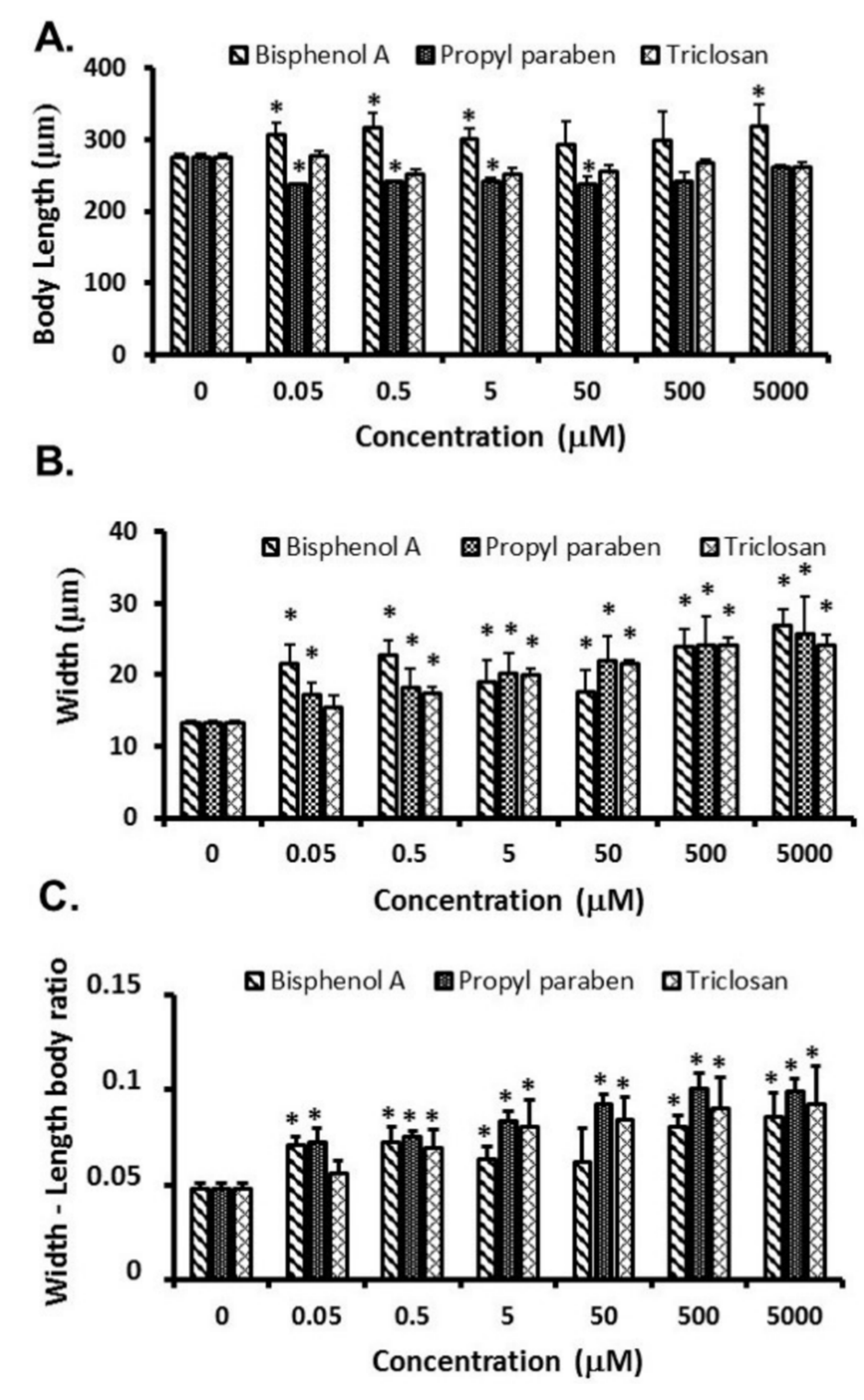
3.2. Growth
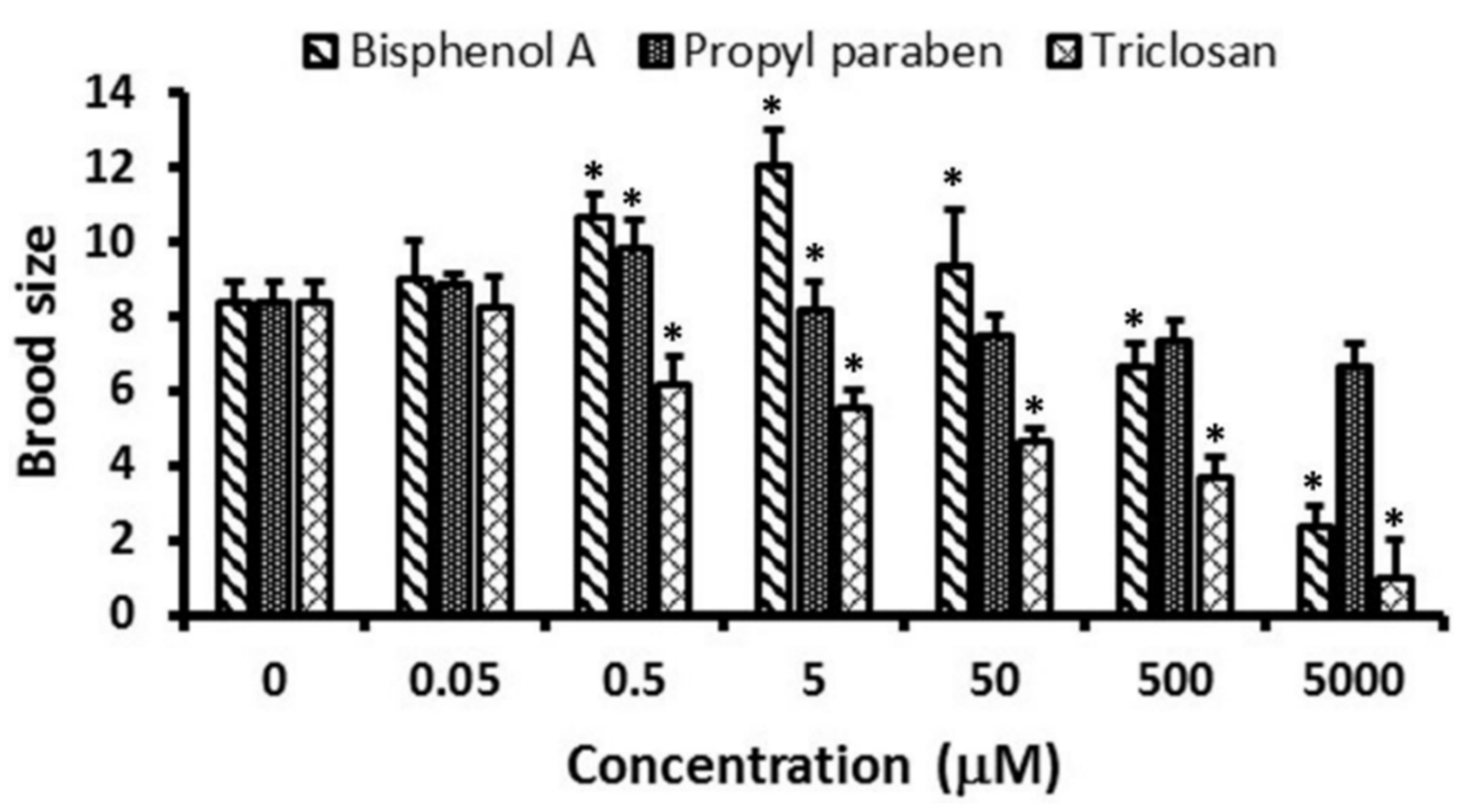
3.3. Reproduction
3.4. Changes in Gene Expression
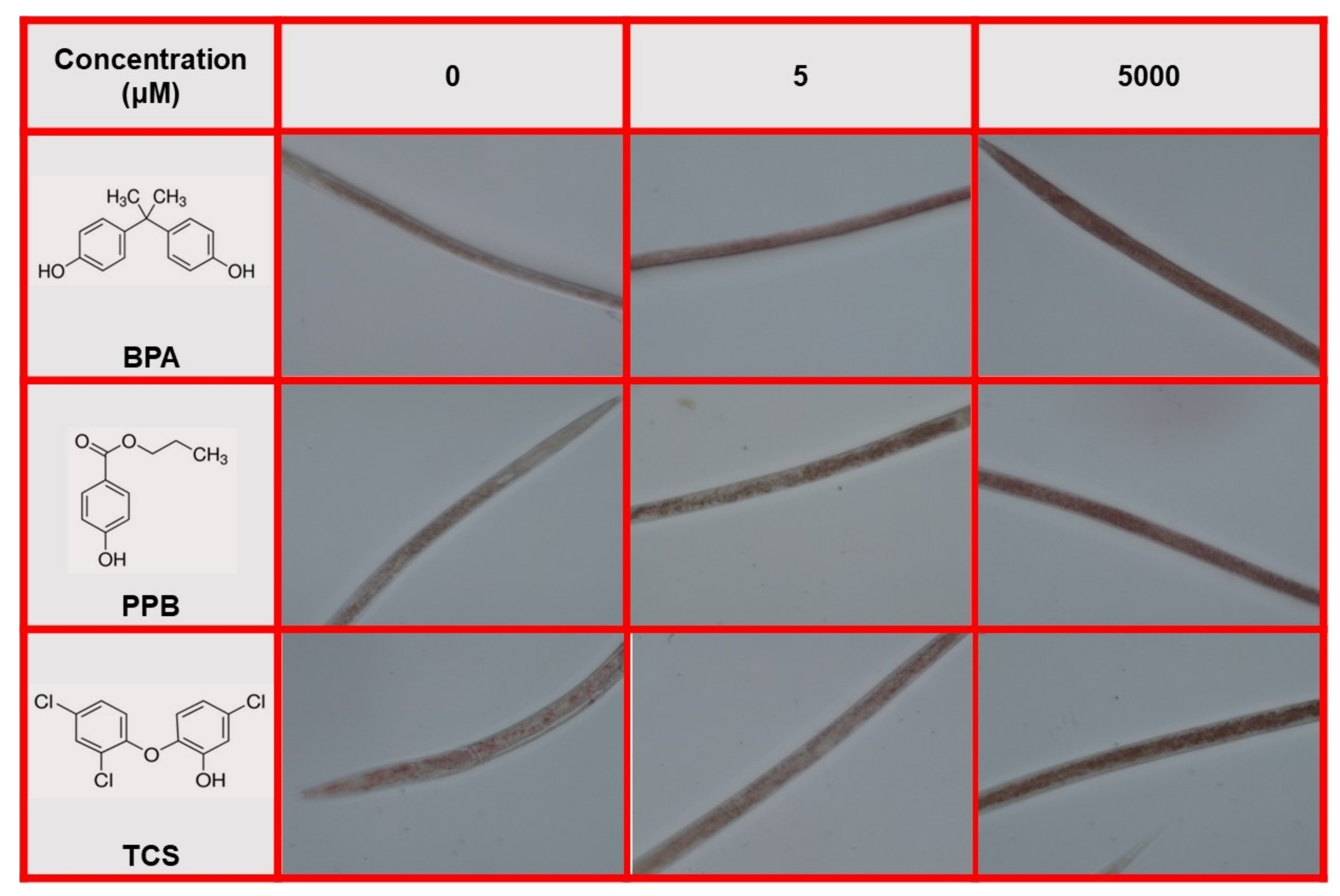
3.5. q-ORO Stain
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Michałowicz, J. Bisphenol A-sources, toxicity and biotransformation. Environ. Toxicol. Pharmacol. 2014, 37, 738–758. [Google Scholar] [CrossRef] [PubMed]
- Schug, T.T.; Janesick, A.; Blumberg, B.; Heindel, J.J. Endocrine disrupting chemicals and disease susceptibility. J. Steroid Biochem. Mol. Biol. 2011, 127, 204–215. [Google Scholar] [CrossRef] [PubMed]
- Careghini, A.; Mastorgio, A.F.; Saponaro, S.; Sezenna, E. Bisphenol A, nonylphenols, benzophenones, and benzotriazoles in soils, groundwater, surface water, sediments, and food: A review. Environ. Sci. Pollut. Res. 2015, 22, 5711–5741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sauvé, S.; Desrosiers, M. A review of what is an emerging contaminant. Chem. Cent. J. 2014, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Björnsdotter, M.K.; de Boer, J.; Ballesteros-Gómez, A. Bisphenol A and replacements in thermal paper: Aa review. Chemosphere 2017, 182, 691–706. [Google Scholar] [CrossRef] [PubMed]
- Flint, S.; Markle, T.; Thompson, S.; Wallace, E. Bisphenol A exposure, effects, and policy: A wildlife perspective. J. Environ. Manag. 2012, 104, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Hoekstra, E.J.; Simoneau, C. Release of Bbisphenol A from polycarbonate—A review. Crit. Rev. Food Sci. Nutr. 2013, 53, 386–402. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.Q.; Wong, C.K.C.; Zheng, J.S.; Bouwman, H.; Barra, R.; Wahlström, B.; Neretin, L.; Wong, M.H. Bisphenol A (BPA) in China: A review of sources, environmental levels, and potential human health impacts. Environ. Int. 2012, 42, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Spagnuolo, M.L.; Marini, F.; Sarabia, L.A.; Ortiz, M.C. Migration test of bisphenol A from polycarbonate cups using excitation-emission fluorescence data with parallel factor analysis. Talanta 2017, 167, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Beltifa, A.; Feriani, A.; Machreki, M.; Ghorbel, A.; Ghazouani, L.; Di Bella, G.; Van Loco, J.; Reyns, T.; Mansour, H. Ben Plasticizers and bisphenol A, in packaged foods sold in the Tunisian markets: Study of their acute in vivo toxicity and their environmental fate. Environ. Sci. Pollut. Res. 2017, 24, 22382–22392. [Google Scholar] [CrossRef] [PubMed]
- Ndaw, S.; Remy, A.; Jargot, D.; Robert, A. Occupational exposure of cashiers to bisphenol A via thermal paper: Urinary biomonitoring study. Int. Arch. Occup. Environ. Health 2016, 89, 935–946. [Google Scholar] [CrossRef] [PubMed]
- Hines, C.J.; Jackson, M.V.; Christianson, A.L.; Clark, J.C.; Arnold, J.E.; Pretty, J.R.; Deddens, J.A. Air, hand wipe, and surface wipe sampling for bisphenol A (BPA) among workers in industries that manufacture and use BPA in the United States. J. Occup. Environ. Hyg. 2017, 14, 882–897. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Sun, M.-Z.; Li, X.; Zhang, S.-H.; Dai, L.-T.; Liu, X.-Y.; Zhao, X.; Chen, D.-Y.; Feng, X.-Z. Impact of low-dose chronic exposure to bisphenol A (BPA) on adult male zebrafish adaption to the environmental complexity: Disturbing the color preference patterns and reliving the anxiety behavior. Chemosphere 2017, 186, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Martin, J.W. Prolonged Exposure to bisphenol A from single dermal contact events. Environ. Sci. Technol. 2017, 51, 9940–9949. [Google Scholar] [CrossRef] [PubMed]
- Luigi, V.; Giuseppe, M.; Claudio, R. Emerging and priority contaminants with endocrine active potentials in sediments and fish from the River Po (Italy). Environ. Sci. Pollut. Res. 2015, 22, 14050–14066. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Luo, Y.; Nie, X.-P.; Liao, W.; Yang, Y.-F.; Ying, G.-G. Toxic effects of triclosan on the detoxification system and breeding of Daphnia magna. Ecotoxicology 2013, 22, 1384–1394. [Google Scholar] [CrossRef] [PubMed]
- Vandenberg, L.N.; Colborn, T.; Hayes, T.B.; Heindel, J.J.; Jacobs, D.R.; Lee, D.-H.; Shioda, T.; Soto, A.M.; vom Saal, F.S.; Welshons, W.V.; et al. Hormones and endocrine-disrupting chemicals: Low-dose effects and nonmonotonic dose responses. Endocr. Rev. 2012, 33, 378–455. [Google Scholar] [CrossRef] [PubMed]
- Kouidhi, W.; Thannimalay, L.; Soon, C.S.; Ali Mohd, M. Occupational exposure to bisphenol A (BPA) in a plastic injection molding factory in Malaysia. Int. J. Occup. Med. Environ. Health 2017, 30, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Nehring, I.; Staniszewska, M.; Falkowska, L. Human hair, Baltic Grey Seal (Halichoerus grypus) fur and Herring Gull (Larus argentatus) feathers as accumulators of bisphenol A and alkylphenols. Arch. Environ. Contam. Toxicol. 2017, 72, 552–561. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Liu, M. Adipose tissue in control of metabolism. J. Endocrinol. 2016, 231, R77–R99. [Google Scholar] [CrossRef] [PubMed]
- LaPensee, E.W.; Tuttle, T.R.; Fox, S.R.; Ben-Jonathan, N. Bisphenol A at low nanomolar doses confers chemoresistance in estrogen receptor-α-positive and -negative breast cancer cells. Environ. Health Perspect. 2009, 117, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, D.; Chung, Y.M.; Hu, M.C.T. Effects of low-dose Bisphenol A on DNA damage and proliferation of breast cells: The role of c-Myc. Environ. Health Perspect. 2015, 123, 1271–1279. [Google Scholar] [CrossRef] [PubMed]
- Mansour, S.A.; Mohamed, D.A.; Sutra, J.F. Which exposure stage (gestation or lactation) is more vulnerable to atrazine toxicity? Studies on mouse dams and their pups. Toxicol. Rep. 2014, 1, 53–68. [Google Scholar] [CrossRef] [PubMed]
- Pu, Y.; Gingrich, J.; Steibel, J.P.; Veiga-Lopez, A. Sex-specific modulation of fetal adipogenesis by gestational Bisphenol A and Bisphenol S exposure. Endocrinology 2017, 158, 3844–3858. [Google Scholar] [CrossRef] [PubMed]
- Rubin, B.S.; Paranjpe, M.; DaFonte, T.; Schaeberle, C.; Soto, A.M.; Obin, M.; Greenberg, A.S. Perinatal BPA exposure alters body weight and composition in a dose specific and sex specific manner: The addition of peripubertal exposure exacerbates adverse effects in female mice. Reprod. Toxicol. 2017, 68, 130–144. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hollis-Hansen, K.; Ren, X.; Qiu, Y.; Qu, W. Do environmental pollutants increase obesity risk in humans? Obes. Rev. 2016, 17, 1179–1197. [Google Scholar] [CrossRef] [PubMed]
- Golden, R.; Gandy, J.; Vollmer, G. A Review of the endocrine activity of parabens and implications for potential risks to human health. Crit. Rev. Toxicol. 2005, 35, 435–458. [Google Scholar] [CrossRef] [PubMed]
- Calafat, A.M.; Ye, X.; Wong, L.-Y.; Bishop, A.M.; Needham, L.L. Urinary concentrations of four parabens in the U.S. population: NHANES 2005–2006. Environ. Health Perspect. 2010, 118, 679–685. [Google Scholar] [CrossRef] [PubMed]
- Kodani, S.D.; Overby, H.B.; Morisseau, C.; Chen, J.; Zhao, L.; Hammock, B.D. Parabens inhibit fatty acid amide hydrolase: A potential role in paraben-enhanced 3T3-L1 adipocyte differentiation. Toxicol. Lett. 2016, 262, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Szeląg, S.; Zabłocka, A.; Trzeciak, K.; Drozd, A.; Baranowska-Bosiacka, I.; Kolasa, A.; Goschorska, M.; Chlubek, D.; Gutowska, I. Propylparaben-induced disruption of energy metabolism in human HepG2 cell line leads to increased synthesis of superoxide anions and apoptosis. Toxicol. Vitr. 2016, 31, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Soni, M.G.; Burdock, G.A.; Taylor, S.L.; Greenberg, N.A. Safety assessment of propyl paraben: A review of the published literature. Food Chem. Toxicol. 2001, 39, 513–532. [Google Scholar] [CrossRef]
- Butkovskyi, A.; Rijnaarts, H.H.M.; Zeeman, G.; Hernandez Leal, L. Fate of personal care and household products in source separated sanitation. J. Hazard. Mater. 2016, 320, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Siddique, S.; Kubwabo, C.; Harris, S.A. A review of the role of emerging environmental contaminants in the development of breast cancer in women. Emerg. Contam. 2016, 2, 204–219. [Google Scholar] [CrossRef]
- Routledge, E.J.; Parker, J.; Odum, J.; Ashby, J.; Sumpter, J.P. Some alkyl hydroxy benzoate preservatives (parabens) are estrogenic. Toxicol. Appl. Pharmacol. 1998, 153, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Huo, W.; Li, Y.; Zhang, B.; Wan, Y.; Zheng, T.; Zhou, A.; Chen, Z.; Qian, M.; Zhu, Y.; et al. Maternal urinary paraben levels and offspring size at birth from a Chinese birth cohort. Chemosphere 2017, 172, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Ng, M.; Fleming, T.; Robinson, M.; Thomson, B.; Graetz, N.; Margono, C.; Mullany, E.C.; Biryukov, S.; Abbafati, C.; Abera, S.F.; et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014, 384, 766–781. [Google Scholar] [CrossRef]
- Martín, J.M.P.; Peropadre, A.; Herrero, Ó.; Freire, P.F.; Labrador, V.; Hazen, M.J. Oxidative DNA damage contributes to the toxic activity of propylparaben in mammalian cells. Mutat. Res. Toxicol. Environ. Mutagen. 2010, 702, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Faniband, M.; Lindh, C.H.; Jönsson, B.A.G. Human biological monitoring of suspected endocrine-disrupting compounds. Asian J. Androl. 2014, 16, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.L.; Dusenbery, D.B. Aquatic toxicity testing using the nematode, Caenorhabditis elegans. Environ. Toxicol. Chem. 1990, 9, 1285–1290. [Google Scholar] [CrossRef]
- Hines, E.P.; Mendola, P.; von Ehrenstein, O.S.; Ye, X.; Calafat, A.M.; Fenton, S.E. Concentrations of environmental phenols and parabens in milk, urine and serum of lactating North Carolina women. Reprod. Toxicol. 2015, 54, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Philippat, C.; Wolff, M.S.; Calafat, A.M.; Ye, X.; Bausell, R.; Meadows, M.; Stone, J.; Slama, R.; Engel, S.M. Prenatal exposure to environmental phenols: Concentrations in amniotic fluid and variability in urinary concentrations during pregnancy. Environ. Health Perspect. 2013, 121, 1225–1231. [Google Scholar] [CrossRef] [PubMed]
- Paulose, T.; Speroni, L.; Sonnenschein, C.; Soto, A.M. Estrogens in the wrong place at the wrong time: Fetal BPA exposure and mammary cancer. Reprod. Toxicol. 2015, 54, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Wróbel, A.M.; Gregoraszczuk, E.Ł. Action of methyl-, propyl- and butylparaben on GPR30 gene and protein expression, cAMP levels and activation of ERK1/2 and PI3K/Akt signaling pathways in MCF-7 breast cancer cells and MCF-10A non-transformed breast epithelial cells. Toxicol. Lett. 2015, 238, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Legler, J.; Fletcher, T.; Govarts, E.; Porta, M.; Blumberg, B.; Heindel, J.J.; Trasande, L. Obesity, diabetes, and associated costs of exposure to endocrine-disrupting chemicals in the european union. J. Clin. Endocrinol. Metab. 2015, 100, 1278–1288. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Chen, X.; Whitener, R.J.; Boder, E.T.; Jones, J.O.; Porollo, A.; Chen, J.; Zhao, L. Effects of parabens on adipocyte differentiation. Toxicol. Sci. 2013, 131, 56–70. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Lee, M.; Ahn, C.; Kang, H.Y.; Tran, D.N.; Jeung, E.B. Parabens accelerate ovarian dysfunction in a 4-vinylcyclohexene diepoxide-induced ovarian failure model. Int. J. Environ. Res. Public Health 2017, 14, 161. [Google Scholar] [CrossRef] [PubMed]
- Pycke, B.F.G.; Geer, L.A.; Dalloul, M.; Abulafia, O.; Halden, R.U. Maternal and fetal exposure to parabens in a multiethnic urban U.S. population. Environ. Int. 2015, 84, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Bao-Liang, S.; Hai-Ying, L.; Dun-Ren, P. In vitro spermicidal activity of parabens against human spermatozoa. Contraception 1989, 39, 331–335. [Google Scholar] [CrossRef]
- Shibata, M.-A.; Yamada, M.; Hirose, M.; Asakawa, E.; Tatematsu, M.; Ito, N. Early proliferative responses of forestomach and glandular stomach of rats treated with five different phenolic antioxidants. Carcinogenesis 1990, 11, 425–429. [Google Scholar] [CrossRef] [PubMed]
- Hirose, M.; Inoue, T.; Asamoto, M.; Tagawa, Y.; Ito, N. Comparison of the effects of 13 phenolic compounds in induction of proliferative lesions of the forestomach and increase in the labelling indices of the glandular stomach and urinary bladder epithelium of Syrian golden hamsters. Carcinogenesis 1986, 7, 1285–1289. [Google Scholar] [CrossRef] [PubMed]
- Oishi, S. Effects of propyl paraben on the male reproductive system. Food Chem. Toxicol. 2002, 40, 1807–1813. [Google Scholar] [CrossRef]
- Pedersen, K.L.; Pedersen, S.N.; Christiansen, L.B.; Korsgaard, B.; Bjerregaard, P. The preservatives ethyl-, propyl- and butylparaben are oestrogenic in an in vivo fish assay. Pharmacol. Toxicol. 2008, 86, 110–113. [Google Scholar] [CrossRef]
- Inui, M.; Adachi, T.; Takenaka, S.; Inui, H.; Nakazawa, M.; Ueda, M.; Watanabe, H.; Mori, C.; Iguchi, T.; Miyatake, K. Effect of UV screens and preservatives on vitellogenin and choriogenin production in male medaka (Oryzias latipes). Toxicology 2003, 194, 43–50. [Google Scholar] [CrossRef]
- Dambal, V.Y.; Selvan, K.P.; Lite, C.; Barathi, S.; Santosh, W. Developmental toxicity and induction of vitellogenin in embryo-larval stages of zebrafish (Danio rerio) exposed to methyl paraben. Ecotoxicol. Environ. Saf. 2017, 141, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Barra Caracciolo, A.; Topp, E.; Grenni, P. Pharmaceuticals in the environment: Biodegradation and effects on natural microbial communities. A review. J. Pharm. Biomed. Anal. 2015, 106, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Lishman, L.; Smyth, S.A.; Sarafin, K.; Kleywegt, S.; Toito, J.; Peart, T.; Lee, B.; Servos, M.; Beland, M.; Seto, P. Occurrence and reductions of pharmaceuticals and personal care products and estrogens by municipal wastewater treatment plants in Ontario, Canada. Sci. Total Environ. 2006, 367, 544–558. [Google Scholar] [CrossRef] [PubMed]
- Montes-Grajales, D.; Fennix-Agudelo, M.; Miranda-Castro, W. Occurrence of personal care products as emerging chemicals of concern in water resources: A review. Sci. Total Environ. 2017, 595, 601–614. [Google Scholar] [CrossRef] [PubMed]
- Rüdel, H.; Böhmer, W.; Müller, M.; Fliedner, A.; Ricking, M.; Teubner, D.; Schröter-Kermani, C. Retrospective study of triclosan and methyl-triclosan residues in fish and suspended particulate matter: Results from the German Environmental Specimen Bank. Chemosphere 2013, 91, 1517–1524. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, J.P.R.; Lapworth, D.J.; Nkhuwa, D.C.W.; Stuart, M.E.; Gooddy, D.C.; Bell, R.A.; Chirwa, M.; Kabika, J.; Liemisa, M.; Chibesa, M.; et al. Emerging contaminants in urban groundwater sources in Africa. Water Res. 2015, 72, 51–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perez, A.L.; De Sylor, M.A.; Slocombe, A.J.; Lew, M.G.; Unice, K.M.; Donovan, E.P. Triclosan occurrence in freshwater systems in the United States (1999–2012): A meta-analysis. Environ. Toxicol. Chem. 2013, 32, 1479–1487. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.M.; Murugesan, K.; Schmidt, S.; Bokare, V.; Jeon, J.R.; Kim, E.J.; Chang, Y.S. Triclosan susceptibility and co-metabolism-a comparison for three aerobic pollutant-degrading bacteria. Bioresour. Technol. 2011, 102, 2206–2212. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.R.R.; Cardoso, D.N.; Cruz, A.; Lourenço, J.; Mendo, S.; Soares, A.M.V.M.; Loureiro, S. Ecotoxicity and genotoxicity of a binary combination of triclosan and carbendazim to Daphnia magna. Ecotoxicol. Environ. Saf. 2015, 115, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Kovacevic, V.; Simpson, A.J.; Simpson, M.J. 1H NMR-based metabolomics of Daphnia magna responses after sub-lethal exposure to triclosan, carbamazepine and ibuprofen. Comp. Biochem. Physiol. Part D Genom. Proteom. 2016, 19, 199–210. [Google Scholar] [CrossRef]
- Orvos, D.R.; Versteeg, D.J.; Inauen, J.; Capdevielle, M.; Rothenstein, A.; Cunningham, V. Aquatic toxicity of triclosan. Environ. Toxicol. Chem. 2002, 21, 1338–1349. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-W.; Ishibashi, H.; Yamauchi, R.; Ichikawa, N.; Takao, Y.; Hirano, M.; Koga, M.; Arizono, K. Acute toxicity of pharmaceutical and personal care products on freshwater crustacean (Thamnocephalus platyurus) and fish (Oryzias latipes). J. Toxicol. Sci. 2009, 34, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Perron, M.M.; Ho, K.T.; Cantwell, M.G.; Burgess, R.M.; Pelletier, M.C. Effects of triclosan on marine benthic and epibenthic organisms. Environ. Toxicol. Chem. 2012, 31, 1861–1866. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Lu, Y.; Zhang, D.; Wang, Y.; Zhou, X.; Xu, H.; Mei, Y. Toxic Assessment of triclosan and triclocarban on Artemia salina. Bull. Environ. Contam. Toxicol. 2015, 95, 728–733. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Li, Y.; Zhou, Q.; Xu, Y.; Wang, D. Effect of triclosan on reproduction, DNA damage and heat shock protein gene expression of the earthworm Eisenia fetida. Ecotoxicology 2014, 23, 1826–1832. [Google Scholar] [CrossRef] [PubMed]
- Gillis, J.D.; Price, G.W.; Prasher, S. Lethal and sub-lethal effects of triclosan toxicity to the earthworm Eisenia fetida assessed through GC–MS metabolomics. J. Hazard. Mater. 2017, 323, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, Z.; Wang, W.; Yan, Z.; Zhang, C.; Wang, W.; Chen, L. Assessment of toxic effects of triclosan on the terrestrial snail (Achatina fulica). Chemosphere 2014, 108, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Binelli, A.; Cogni, D.; Parolini, M.; Riva, C.; Provini, A. In vivo experiments for the evaluation of genotoxic and cytotoxic effects of triclosan in Zebra mussel hemocytes. Aquat. Toxicol. 2009, 91, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Paz, P.; Morales, M.; Martín, R.; Martínez-Guitarte, J.L.; Morcillo, G. Characterization of the small heat shock protein Hsp27 gene in Chironomus riparius (Diptera) and its expression profile in response to temperature changes and xenobiotic exposures. Cell Stress Chaperones 2014, 19, 529–540. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Paz, P.; Morales, M.; Martínez-Guitarte, J.L.; Morcillo, G. Genotoxic effects of environmental endocrine disruptors on the aquatic insect Chironomus riparius evaluated using the comet assay. Mutat. Res. Toxicol. Environ. Mutagen. 2013, 758, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Paz, P.; Morales, M.; Urien, J.; Morcillo, G.; Martínez-Guitarte, J.L. Endocrine-related genes are altered by antibacterial agent triclosan in Chironomus riparius aquatic larvae. Ecotoxicol. Environ. Saf. 2017, 140, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Xin, X.; Huang, G.; Liu, X.; An, C.; Yao, Y.; Weger, H.; Zhang, P.; Chen, X. Molecular toxicity of triclosan and carbamazepine to green algae Chlorococcum sp.: A single cell view using synchrotron-based Fourier transform infrared spectromicroscopy. Environ. Pollut. 2017, 226, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Rowett, C.J.; Hutchinson, T.H.; Comber, S.D.W. The impact of natural and anthropogenic Dissolved Organic Carbon (DOC), and pH on the toxicity of triclosan to the crustacean Gammarus pulex (L.). Sci. Total Environ. 2016, 565, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Park, J.C.; Han, J.; Lee, M.-C.; Seo, J.S.; Lee, J.-S. Effects of triclosan (TCS) on fecundity, the antioxidant system, and oxidative stress-mediated gene expression in the copepod Tigriopus japonicus. Aquat. Toxicol. 2017, 189, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Chai, L.; Chen, A.; Luo, P.; Zhao, H.; Wang, H. Histopathological changes and lipid metabolism in the liver of Bufo gargarizans tadpoles exposed to triclosan. Chemosphere 2017, 182, 255–266. [Google Scholar] [CrossRef] [PubMed]
- González-Pleiter, M.; Rioboo, C.; Reguera, M.; Abreu, I.; Leganés, F.; Cid, Á.; Fernández-Piñas, F. Calcium mediates the cellular response of Chlamydomonas reinhardtii to the emerging aquatic pollutant triclosan. Aquat. Toxicol. 2017, 186, 50–66. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-F.; Tian, Y. Reproductive endocrine-disrupting effects of triclosan: Population exposure, present evidence and potential mechanisms. Environ. Pollut. 2015, 206, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Lenz, K.A.; Pattison, C.; Ma, H. Triclosan (TCS) and triclocarban (TCC) induce systemic toxic effects in a model organism the nematode Caenorhabditis elegans. Environ. Pollut. 2017, 231, 462–470. [Google Scholar] [CrossRef] [PubMed]
- Wyatt, L.H.; Luz, A.L.; Cao, X.; Maurer, L.L.; Blawas, A.M.; Aballay, A.; Pan, W.K.Y.; Meyer, J.N. Effects of methyl and inorganic mercury exposure on genome homeostasis and mitochondrial function in Caenorhabditis elegans. DNA Repair 2017, 52, 31–48. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Zhang, M.; Hu, J.; Li, Z.; Wu, T.; Bao, J.; Wu, S.; Lei, L.; He, D. Behavioral deficits and neural damage of Caenorhabditis elegans induced by three rare earth elements. Chemosphere 2017, 181, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Bailey, D.C.; Todt, C.E.; Orfield, S.E.; Denney, R.D.; Snapp, I.B.; Negga, R.; Montgomery, K.M.; Bailey, A.C.; Pressley, A.S.; Traynor, W.L.; et al. Caenorhabditis elegans chronically exposed to a Mn/Zn ethylene-bis-dithiocarbamate fungicide show mitochondrial Complex I inhibition and increased reactive oxygen species. Neurotoxicology 2016, 56, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Negi, H.; Saikia, S.K.; Kanaujia, R.; Jaiswal, S.; Pandey, R. 3β-Hydroxy-urs-12-en-28-oic acid confers protection against ZnONPs induced adversity in Caenorhabditis elegans. Environ. Toxicol. Pharmacol. 2017, 53, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Jacques, M.T.; Oliveira, J.L.; Campos, E.V.R.; Fraceto, L.F.; Ávila, D.S. Safety assessment of nanopesticides using the roundworm Caenorhabditis elegans. Ecotoxicol. Environ. Saf. 2017, 139, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Li, Q.; Shi, J.; Shi, L.; Li, B.; Xu, A.; Zhao, G.; Wu, L. Perfluorooctane sulfonate exposure causes gonadal developmental toxicity in Caenorhabditis elegans through ROS-induced DNA damage. Chemosphere 2016, 155, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Meyer, D.; Williams, P.L. Toxicity Testing of Neurotoxic Pesticides in Caenorhabditis elegans. J. Toxicol. Environ. Health Part B 2014, 17, 284–306. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.; Pears, C.; Woollard, A. An enhanced C. elegans based platform for toxicity assessment. Sci. Rep. 2017, 7, 9839. [Google Scholar] [CrossRef] [PubMed]
- Tejeda-Benitez, L.; Flegal, R.; Odigie, K.; Olivero-Verbel, J. Pollution by metals and toxicity assessment using Caenorhabditis elegans in sediments from the Magdalena River, Colombia. Environ. Pollut. 2016, 212, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Höss, S.; Jänsch, S.; Moser, T.; Junker, T.; Römbke, J. Assessing the toxicity of contaminated soils using the nematode Caenorhabditis elegans as test organism. Ecotoxicol. Environ. Saf. 2009, 72, 1811–1818. [Google Scholar] [CrossRef] [PubMed]
- Roh, J.-Y.; Park, Y.-K.; Park, K.; Choi, J. Ecotoxicological investigation of CeO2 and TiO2 nanoparticles on the soil nematode Caenorhabditis elegans using gene expression, growth, fertility, and survival as endpoints. Environ. Toxicol. Pharmacol. 2010, 29, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Xiao, J.; Ye, H.; Wang, D. Toxicity evaluation in nematode Caenorhabditis elegans after chronic metal exposure. Environ. Toxicol. Pharmacol. 2009, 28, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Tejeda-Benitez, L.; Olivero-Verbel, J. Caenorhabditis elegans, a biological model for research in toxicology. Rev. Environ. Contam. Toxicol. 2016, 237, 1–35. [Google Scholar] [PubMed]
- Höss, S.; Menzel, R.; Gessler, F.; Nguyen, H.T.; Jehle, J.A.; Traunspurger, W. Effects of insecticidal crystal proteins (Cry proteins) produced by genetically modified maize (Bt maize) on the nematode Caenorhabditis elegans. Environ. Pollut. 2013, 178, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Leelaja, B.C.; Rajini, P.S. Biochemical and physiological responses in Caenorhabditis elegans exposed to sublethal concentrations of the organophosphorus insecticide, monocrotophos. Ecotoxicol. Environ. Saf. 2013, 94, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Rui, Q.; Zhao, Y.; Wu, Q.; Tang, M.; Wang, D. Biosafety assessment of titanium dioxide nanoparticles in acutely exposed nematode Caenorhabditis elegans with mutations of genes required for oxidative stress or stress response. Chemosphere 2013, 93, 2289–2296. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.W.; Souter, I.; Dimitriadis, I.; Ehrlich, S.; Williams, P.L.; Calafat, A.M.; Hauser, R. Urinary paraben concentrations and ovarian aging among women from a fertility center. Environ. Health Perspect. 2013, 121, 1299–1305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tejeda-Benítez, L.; Noguera-Oviedo, K.; Aga, D.S.; Olivero-Verbel, J. Toxicity profile of organic extracts from Magdalena River sediments. Environ. Sci. Pollut. Res. 2018, 25, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Altincicek, B.; Fischer, M.; Fischer, M.; Lüersen, K.; Boll, M.; Wenzel, U.; Vilcinskas, A. Role of matrix metalloproteinase ZMP-2 in pathogen resistance and development in Caenorhabditis elegans. Dev. Comp. Immunol. 2010, 34, 1160–1169. [Google Scholar] [CrossRef] [PubMed]
- Anbalagan, C.; Lafayette, I.; Antoniou-Kourounioti, M.; Gutierrez, C.; Martin, J.R.; Chowdhuri, D.K.; De Pomerai, D.I. Use of transgenic GFP reporter strains of the nematode Caenorhabditis elegans to investigate the patterns of stress responses induced by pesticides and by organic extracts from agricultural soils. Ecotoxicology 2013, 22, 72–85. [Google Scholar] [CrossRef] [PubMed]
- Power, R.S.; De Pomerai, D.I. Effect of single and paired metal inputs in soil on a stress-inducible transgenic nematode. Arch. Environ. Contam. Toxicol. 1999, 37, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Turbyville, T.J.; Wijeratne, E.M.K.; Whitesell, L.; Gunatilaka, A.A.L. The anticancer activity of the fungal metabolite terrecyclic acid A is associated with modulation of multiple cellular stress response pathways. Mol. Cancer Ther. 2005, 4, 1569–1576. [Google Scholar] [CrossRef] [PubMed]
- Wählby, C.; Lee Conery, A.; Bray, M.A.; Kamentsky, L.; Larkins-Ford, J.; Sokolnicki, K.L.; Veneskey, M.; Michaels, K.; Carpenter, A.E.; O’Rourke, E.J. High- and low-throughput scoring of fat mass and body fat distribution in C. elegans. Methods 2014, 68, 492–499. [Google Scholar] [CrossRef] [PubMed]
- De Andrade, A.L.C.; Soares, P.R.L.; da Silva, S.C.B.L.; da Silva, M.C.G.; Santos, T.P.; Cadena, M.R.S.; Soares, P.C.; Cadena, P.G. Evaluation of the toxic effect of endocrine disruptor bisphenol A (BPA) in the acute and chronic toxicity tests with Pomacea lineata gastropod. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2017, 197, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Matsushima, A.; Ryan, K.; Shimohigashi, Y.; Meinertzhagen, I.A. An endocrine disruptor, bisphenol A, affects development in the protochordate Ciona intestinalis: Hatching rates and swimming behavior alter in a dose-dependent manner. Environ. Pollut. 2013, 173, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Ura, K.; Kai, T.; Sakata, S.; Iguchi, T.; Arizono, K. Aquatic acute toxicity testing using the nematode Caenorhabditis elegans. J. Health Sci. 2002, 48, 583–586. [Google Scholar] [CrossRef]
- Dobbins, L.L.; Usenko, S.; Brain, R.A.; Brooks, B.W. Probabilistic ecological hazard assessment of parabens using Daphnia magna and Pimephales promelas. Environ. Toxicol. Chem. 2009, 28, 2744–2753. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Wang, S.; Wang, Y.; He, M.; Liu, D. Bisphenol A exposure accelerated the aging process in the nematode Caenorhabditis elegans. Toxicol. Lett. 2015, 235, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Yang, J.; Li, H.; Cui, C.; Yu, Y.; Liu, Y.; Lin, K. The chronic toxicity of bisphenol A to Caenorhabditis elegans after long-term exposure at environmentally relevant concentrations. Chemosphere 2016, 154, 546–551. [Google Scholar] [CrossRef] [PubMed]
- Solecki, R.; Kortenkamp, A.; Bergman, Å.; Chahoud, I.; Degen, G.H.; Dietrich, D.; Greim, H.; Håkansson, H.; Hass, U.; Husoy, T.; et al. Scientific principles for the identification of endocrine-disrupting chemicals: A consensus statement. Arch. Toxicol. 2017, 91, 1001–1006. [Google Scholar] [CrossRef] [PubMed]
- Pjanic, M. The role of polycarbonate monomer bisphenol-A in insulin resistance. PeerJ 2017, 5, 3809. [Google Scholar] [CrossRef] [PubMed]
- Killeen, A.; Marin de Evsikova, C. Effects of sub-lethal teratogen exposure during larval development on egg laying and egg quality in adult Caenorhabditis elegans. F1000Research 2016, 5, 2925. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Shu, L.; Qiu, Z.; Lee, D.Y.; Settle, S.J.; Que Hee, S.; Telesca, D.; Yang, X.; Allard, P. Exposure to the BPA-substitute bisphenol S causes unique alterations of germline function. PLoS Genet. 2016, 12, e1006223. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Song, S.; Wu, H.; Zhang, J.; Ma, E. Antioxidant enzymes and their role in phoxim and carbaryl stress in Caenorhabditis elegans. Pestic. Biochem. Physiol. 2017, 138, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Braeckman, B.P.; Smolders, A.; Back, P.; De Henau, S. In Vivo detection of reactive oxygen species and redox status in Caenorhabditis elegans. Antioxid. Redox Signal. 2016, 25, 577–592. [Google Scholar] [CrossRef] [PubMed]
- Avila, D.S.; Benedetto, A.; Au, C.; Bornhorst, J.; Aschner, M. Involvement of heat shock proteins on Mn-induced toxicity in Caenorhabditis elegans. BMC Pharmacol. Toxicol. 2016, 17, 54. [Google Scholar] [CrossRef] [PubMed]
- Thondamal, M.; Witting, M.; Schmitt-Kopplin, P.; Aguilaniu, H. Steroid hormone signalling links reproduction to lifespan in dietary-restricted Caenorhabditis elegans. Nat. Commun. 2014, 5, 4879. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.-H.; Xia, J.; Sun, X.-C.; Li, X.-N.; Zhang, C.; Zhao, H.-S.; Zhu, S.-Y.; Li, J.-L. A novel nuclear xenobiotic receptors (AhR/PXR/CAR)-mediated mechanism of DEHP-induced cerebellar toxicity in quails (Coturnix japonica) via disrupting CYP enzyme system homeostasis. Environ. Pollut. 2017, 226, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Du, Z.-H.; Zhu, S.-Y.; Li, X.-N.; Li, N.; Guo, J.-A.; Li, J.-L.; Zhang, Y. Atrazine triggers developmental abnormality of ovary and oviduct in quails (Coturnix coturnix) via disruption of hypothalamo-pituitary-ovarian axis. Environ. Pollut. 2015, 207, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Mimoto, A.; Fujii, M.; Usami, M.; Shimamura, M.; Hirabayashi, N.; Kaneko, T.; Sasagawa, N.; Ishiura, S. Identification of an estrogenic hormone receptor in Caenorhabditis elegans. Biochem. Biophys. Res. Commun. 2007, 364, 883–888. [Google Scholar] [CrossRef] [PubMed]
- Montes-Grajales, D.; Olivero-Verbel, J. Computer-aided identification of novel protein targets of bisphenol A. Toxicol. Lett. 2013, 222, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.Y.; Wu, L.; Shi, Z.; Zhang, X.J.; Englert, N.A.; Zhang, S.Y. Upregulation of human CYP2C9 expression by Bisphenol A via estrogen receptor alpha (ERα) and Med25. Environ. Toxicol. 2017, 32, 970–978. [Google Scholar] [CrossRef] [PubMed]
- An, J.H.; Blackwell, T.K. SKN-1 links C. elegans mesendodermal specification to a conserved oxidative stress response. Genes Dev. 2003, 17, 1882–1893. [Google Scholar] [CrossRef] [PubMed]
- Yoon, D.S.; Choi, Y.; Cha, D.S.; Zhang, P.; Choi, S.M.; Alfhili, M.A.; Polli, J.R.; Pendergrass, D.; Taki, F.A.; Kapalavavi, B.; et al. Triclosan disrupts SKN-1/Nrf2-mediated oxidative stress response in C. elegans and human mesenchymal stem cells. Sci. Rep. 2017, 7, 12592. [Google Scholar] [CrossRef] [PubMed]
- Legeay, S.; Faure, S. Is bisphenol A an environmental obesogen? Fundam. Clin. Pharmacol. 2017, 31, 594–609. [Google Scholar] [CrossRef] [PubMed]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Espiñeira, M.C.; Tejeda-Benítez, L.P.; Olivero-Verbel, J. Toxic Effects of Bisphenol A, Propyl Paraben, and Triclosan on Caenorhabditis elegans. Int. J. Environ. Res. Public Health 2018, 15, 684. https://doi.org/10.3390/ijerph15040684
García-Espiñeira MC, Tejeda-Benítez LP, Olivero-Verbel J. Toxic Effects of Bisphenol A, Propyl Paraben, and Triclosan on Caenorhabditis elegans. International Journal of Environmental Research and Public Health. 2018; 15(4):684. https://doi.org/10.3390/ijerph15040684
Chicago/Turabian StyleGarcía-Espiñeira, María Cecilia, Lesly Patricia Tejeda-Benítez, and Jesus Olivero-Verbel. 2018. "Toxic Effects of Bisphenol A, Propyl Paraben, and Triclosan on Caenorhabditis elegans" International Journal of Environmental Research and Public Health 15, no. 4: 684. https://doi.org/10.3390/ijerph15040684




