Fine Particulate Matter Pollution and Risk of Community-Acquired Sepsis
Abstract
:1. Background
2. Methods
2.1. Study Design
2.2. Data Source
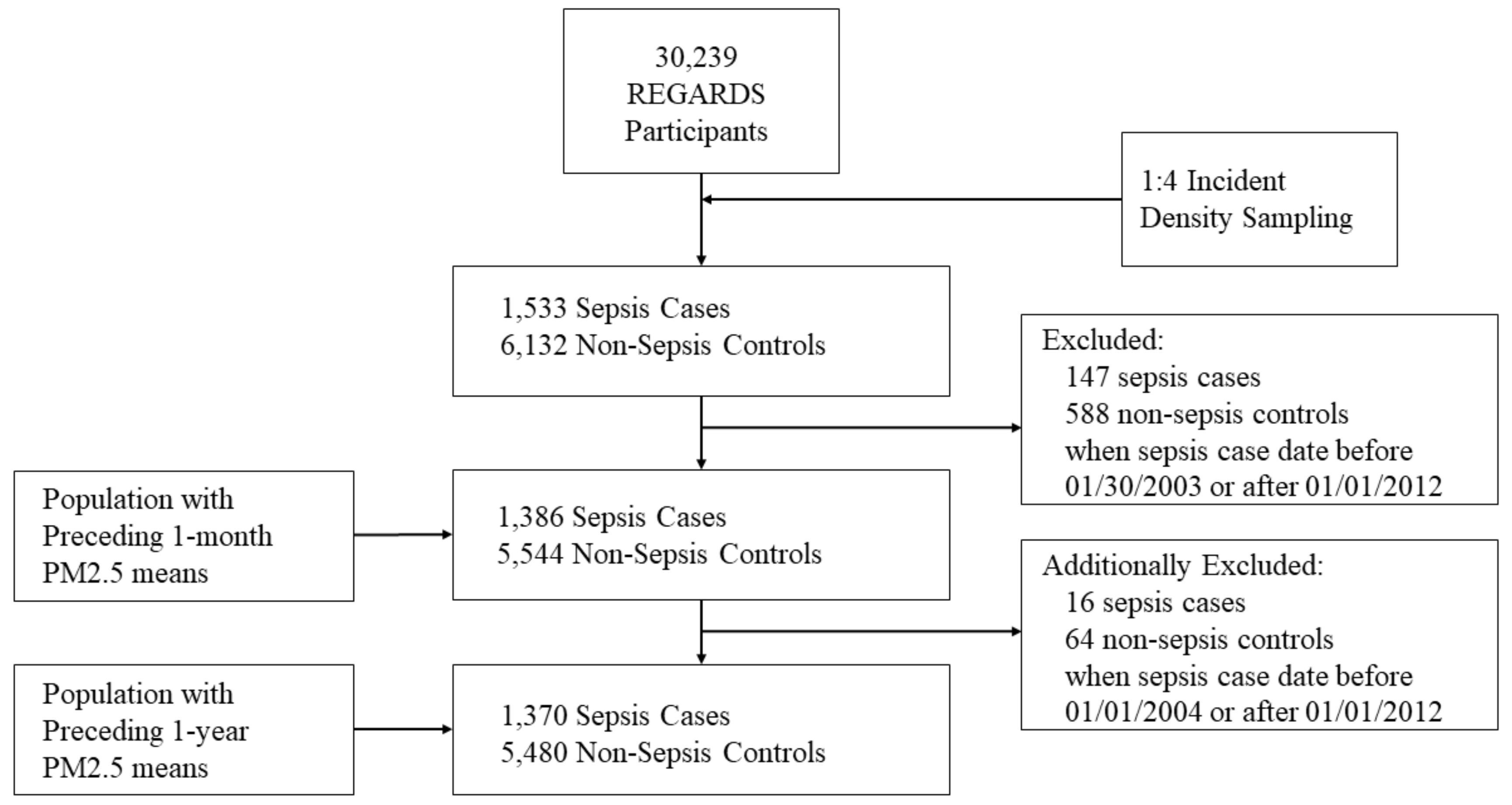
2.3. Selection of Sepsis Cases
2.4. Selection of Matched Controls
2.5. Measurement of Exposure (PM2.5)
2.6. Participant Characteristics
2.7. Community Characteristics
2.8. Statistical Analysis
3. Results
4. Discussion
Limitations
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A.
| Characteristics | Definition and/or Technical Information |
|---|---|
| Sociodemographics | |
| Age | Age in years |
| Gender | Male; female |
| Race | African-American; white |
| Education | Participant reported:
|
| Income | Participant reported:
|
| Geographic Region | Participant residence:
|
| Health Behaviors | |
| Smoking Status | Participant reported:
|
| Alcohol use | Participant reported:
|
| Chronic Medical Conditions | |
| Atrial Fibrillation | Participant reported history of atrial fibrillation. |
| Chronic Lung Disease | Participant use of pulmonary medications (beta agonists, leukotriene inhibitors, inhaled corticosteroids, combination inhalers, ipratropium, cromolyn, aminophylline and theophylline) as a surrogate for chronic lung disease. |
| Coronary Artery Disease | Participant reported history of myocardial infarction, coronary artery bypass grafting, or cardiac angioplasty or stenting, or baseline electrocardiographic evidence of myocardial infarction. |
| Diabetes | Fasting glucose ≥126 mg/L (or a glucose ≥200 mg/L for those not fasting) or participant reported use of insulin or oral hypoglycemic agents. |
| Deep Vein Thrombosis | Participant reported history of deep vein thrombosis. |
| Dyslipidemia | Low-density lipoprotein cholesterol >130 mg/dL or participant reported use of lipid lowering medications. |
| Hypertension | Systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg, or participant reported antihypertensive agent use. |
| Myocardial Infarction | Participant reported history of myocardial infarction or baseline electrocardiographic evidence of myocardial infarction. |
| Obesity | {Waist circumference [>102 cm for males or >88 cm for females]} or {body mass index ≥30 kg/m2}. |
| Peripheral Artery Disease | Participant reported history of lower extremity arterial bypass or leg amputation. |
| Stroke | Participant reported history of stroke or transient ischemic attack. |
| Unadjusted | Model 1 a | Model 2 b | Model 3 c | |
|---|---|---|---|---|
| Preceding 30-Day Mean OR (95% CI) | ||||
| Particulate Matter 2.5 | ||||
| Per SD µg/m3 increase (SD** = 3.04) | 1.01 (0.91, 1.12) | 0.99 (0.89, 1.11) | 1.00 (0.90, 1.12) | 0.99 (0.87, 1.12) |
| p-value | 0.87 | 0.90 | 0.98 | 0.84 |
| Particulate Matter 2.5 | ||||
| 1st Quartile (3.27–10.53 µg/m3) | Referent | Referent | Referent | Referent |
| 2nd Quartile (10.53–11.97 µg/m3) | 1.18 (0.93, 1.49) | 1.12 (0.88, 1.43) | 1.16 (0.90, 1.49) | 1.16 (0.87, 1.54) |
| 3rd Quartile (11.97–13.78 µg/m3) | 0.91 (0.71, 1.17) | 0.85 (0.65, 1.10) | 0.87 (0.66, 1.14) | 0.85 (0.63, 1.14) |
| 4th Quartile (13.78–28.11 µg/m3) | 1.06 (0.81, 1.40) | 1.04 (0.78, 1.38) | 1.09 (0.81, 1.47) | 1.04 (0.75, 1.42) |
| p-valuetrend | 0.17 | 0.16 | 0.13 | 0.18 |
| Preceding One-Year Mean OR (95% CI) | ||||
| Particulate Matter 2.5 | ||||
| Per SD µg/m3 increase (SD** = 1.73) | 1.02 (0.94, 1.12) | 0.99 (0.90, 1.09) | 0.99 (0.90, 1.09) | 0.98 (0.88, 1.09) |
| p-value | 0.62 | 0.80 | 0.78 | 0.67 |
| Particulate Matter 2.5 | ||||
| 1st Quartile (5.88–11.48 µg/m3) | Referent | Referent | Referent | Referent |
| 2nd Quartile (11.48–12.58 µg/m3) | 1.00 (0.79, 1.26) | 0.91 (0.71, 1.17) | 0.87 (0.67, 1.13) | 0.83 (0.62, 1.11) |
| 3rd Quartile (12.58–13.53 µg/m3) | 1.02 (0.80, 1.29) | 0.92 (0.72, 1.19) | 0.95 (0.73, 1.23) | 0.92 (0.68, 1.23) |
| 4th Quartile (13.53–20.18 µg/m3) | 1.03 (0.80, 1.32) | 0.93 (0.72, 1.22) | 0.91 (0.69, 1.20) | 0.85 (0.63, 1.16) |
| p-valuetrend | 1.00 | 0.89 | 0.77 | 0.60 |
References
- Dellinger, R.P.; Levy, M.M.; Rhodes, A.; Annane, D.; Gerlach, H.; Opal, S.M.; Sevransky, J.E.; Sprung, C.L.; Douglas, I.S.; Jaeschke, R.; et al. Surviving Sepsis Campaign: International guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013, 39, 165–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angus, D.C.; Linde-Zwirble, W.T.; Lidicker, J.; Clermont, G.; Carcillo, J.; Pinsky, M.R. Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and associated costs of care. Crit. Care Med. 2001, 29, 1303–1310. [Google Scholar] [CrossRef] [PubMed]
- Brook, R.D.; Rajagopalan, S.; Pope, C.A., 3rd; Brook, J.R.; Bhatnagar, A.; Diez-Roux, A.V.; Holguin, F.; Hong, Y.; Luepker, R.V.; Mittleman, M.A.; et al. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation 2010, 121, 2331–2378. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.L., Jr. Relative effects of air pollution on lungs and heart. Circulation 2004, 109, 5–7. [Google Scholar] [CrossRef] [PubMed]
- Kent, S.T.; Kabagambe, E.K.; Wadley, V.G.; Howard, V.J.; Crosson, W.L.; Al-Hamdan, M.Z.; Judd, S.E.; Peace, F.; McClure, L.A. The relationship between long-term sunlight radiation and cognitive decline in the REGARDS cohort study. Int. J. Biometeorol. 2014, 58, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Mittleman, M.A. Air pollution, exercise, and cardiovascular risk. N. Engl. J. Med. 2007, 357, 1147–1149. [Google Scholar] [CrossRef] [PubMed]
- Neupane, B.; Jerrett, M.; Burnett, R.T.; Marrie, T.; Arain, A.; Loeb, M. Long-term exposure to ambient air pollution and risk of hospitalization with community-acquired pneumonia in older adults. Am. J. Respir. Crit. Care Med. 2010, 181, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Peng, R.D.; Chang, H.H.; Bell, M.L.; McDermott, A.; Zeger, S.L.; Samet, J.M.; Dominici, F. Coarse particulate matter air pollution and hospital admissions for cardiovascular and respiratory diseases among Medicare patients. JAMA 2008, 299, 2172–2179. [Google Scholar] [CrossRef] [PubMed]
- Pope, C.A., 3rd; Burnett, R.T.; Thurston, G.D.; Thun, M.J.; Calle, E.E.; Krewski, D.; Godleski, J.J. Cardiovascular mortality and long-term exposure to particulate air pollution: Epidemiological evidence of general pathophysiological pathways of disease. Circulation 2004, 109, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, N.S.; Donnelly, J.P.; Moore, J.X.; Baddley, J.W.; Safford, M.M.; Wang, H.E. Association of baseline steroid use with long-term rates of infection and sepsis in the REGARDS cohort. Crit. Care 2017, 21, 185. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.E.; Devereaux, R.S.; Yealy, D.M.; Safford, M.M.; Howard, G. National variation in United States sepsis mortality: A descriptive study. Int. J. Health Geogr. 2010, 9, 9. [Google Scholar] [CrossRef] [PubMed]
- Howard, V.J.; Cushman, M.; Pulley, L.; Gomez, C.R.; Go, R.C.; Prineas, R.J.; Graham, A.; Moy, C.S.; Howard, G. The Reasons for Geographic and Racial Differences in Stroke Study: Objectives and Design. Neuroepidemiology 2005, 25, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Bone, R.C. The sepsis syndrome. Definition and general approach to management. Clin. Chest Med. 1996, 17, 175–181. [Google Scholar] [CrossRef]
- Moore, J.X.; Donnelly, J.P.; Griffin, R.; Safford, M.M.; Howard, G.; Baddley, J.; Wang, H.E. Black-white racial disparities in sepsis: A prospective analysis of the REasons for Geographic And Racial Differences in Stroke (REGARDS) cohort. Crit. Care 2015, 19, 279. [Google Scholar] [CrossRef] [PubMed]
- Mayr, F.B.; Yende, S.; Angus, D.C. Epidemiology of severe sepsis. Virulence 2014, 5, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Breslow, N.E.; Day, N.E. Statistical Methods in Cancer Research. Volume I—The Analysis of Case-Control Studies; International Agency for Research on Cancer: Lyon, France, 1980. [Google Scholar]
- Al-Hamdan, M.Z.; Crosson, W.L.; Economou, S.A.; Estes, M.G., Jr.; Estes, S.M.; Hemmings, S.N.; Kent, S.T.; Puckett, M.; Quattrochi, D.A.; Rickman, D.L.; et al. Environmental Public Health Applications Using Remotely Sensed Data. Geocarto Int. 2014, 29, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Al-Hamdan, M.Z.; Crosson, W.L.; Limaye, A.S.; Rickman, D.L.; Quattrochi, D.A.; Estes, M.G., Jr.; Qualters, J.R.; Sinclair, A.H.; Tolsma, D.D.; Adeniyi, K.A.; et al. Methods for characterizing fine particulate matter using ground observations and remotely sensed data: Potential use for environmental public health surveillance. J. Air Waste Manag. Assoc. 2009, 59, 865–881. [Google Scholar] [CrossRef] [PubMed]
- Meteorological Versus Astronomical Seasons. Available online: https://www.ncei.noaa.gov/news/meteorological-versus-astronomical-seasons (accessed on 22 September 2016).
- American Community Survey. Available online: https://www.census.gov/programs-surveys/acs/news/data-releases/2010/release.html (accessed on 1 January 2016).
- Gruca, T.S.; Pyo, T.H.; Nelson, G.C. Improving Rural Access to Orthopaedic Care through Visiting Consultant Clinics. J. Bone Jt. Surg. Am. 2016, 98, 768–774. [Google Scholar] [CrossRef] [PubMed]
- Kirchhoff, A.C.; Hart, G.; Campbell, E.G. Rural and urban primary care physician professional beliefs and quality improvement behaviors. J. Rural Health 2014, 30, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Shimizu, Y.; Wu, X.; Kelly, G.T.; Xu, X.; Wang, L.; Qian, Z.; Chen, Y.; Garcia, J.G.N. Particulate matter disrupts human lung endothelial cell barrier integrity via Rho-dependent pathways. Pulm. Circ. 2017, 7, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.E.; Shapiro, N.I.; Griffin, R.; Safford, M.M.; Judd, S.; Howard, G. Chronic medical conditions and risk of sepsis. PLoS ONE 2012, 7, e48307. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.E.; Shapiro, N.I.; Griffin, R.; Safford, M.M.; Judd, S.; Howard, G. Inflammatory and endothelial activation biomarkers and risk of sepsis: A nested case-control study. J. Crit. Care 2013, 28, 549–555. [Google Scholar] [CrossRef] [PubMed]
- National Institute on Alcohol Abuse and Alcoholism. Helping Patients Who Drink Too Much, a Clinician’s Guide. Available online: http://pubs.niaaa.nih.gov/publications/Practitioner/CliniciansGuide2005/guide.pdf (accessed on 13 February 2018).

| Sepsis Cases (n =1386) | Non-Sepsis Controls * (n = 5544) | p value ** | |
|---|---|---|---|
| Age † | 68.2 (9.3) | 67.9 (9.1) | - |
| Sex (%) | |||
| Male | 714 (51.5) | 2856 (51.5) | - |
| Female | 672 (48.5) | 2688 (48.5) | |
| Race (%) | |||
| Black | 463 (33.4) | 1852 (33.4) | - |
| White | 923 (66.6) | 3692 (66.6) | |
| < High School Education (%) | 231 (16.7) | 580 (10.5) | <0.01 |
| Income ≤ $20 000 (%) | 332 (24.0) | 872 (15.7) | <0.01 |
| Geographic Region (%) | |||
| Stroke Belt a | 527 (38.0) | 1833 (33.1) | <0.01 |
| Buckle b | 294 (21.2) | 136 (20.5) | |
| Non-Stroke Belt c | 565 (40.8) | 2575 (46.5) | |
| Temperature 30-Day Mean (°F) € | 59.5 (48.6–65.5) | 59.1 (47.4–72.8) | <0.01 |
| Temperature 365-Day Mean (°F) € | 62.4 (56.7–65.7) | 61.9 (54.9–65.6) | 0.03 |
| Season | 1.0 | ||
| Fall | 301 (21.7) | 1204 (21.7) | |
| Spring | 371 (26.8) | 1484 (26.8) | |
| Summer | 309 (22.3) | 1236 (22.3) | |
| Winter | 405 (29.2) | 1620 (29.2) | |
| Urbanicity (%) | |||
| Mixed | 455 (32.9) | 1582 (28.6) | <0.01 |
| Rural | 84 (6.1) | 278 (5.0) | |
| Urban | 844 (61.0) | 3670 (61.0) | |
| Tobacco Use (%) | |||
| Never | 485 (35.2) | 2539 (46.0) | <0.01 |
| Past | 648 (47.0) | 2364 (42.8) | |
| Current | 245 (17.8) | 619 (11.2) | |
| Alcohol Use (%) | |||
| Never | 916 (67.5) | 3341(61.4)) | <0.01 |
| Past | 390 (28.7) | 1846 (34.0) | |
| Current | 52 (3.8) | 251 (4.6) | |
| Baseline Medical Condition (%) | |||
| Atrial fibrillation | 185 (13.6) | 499 (9.2) | <0.01 |
| Chronic lung disease | 263 (19.0) | 453 (8.2) | <0.01 |
| Coronary artery disease | 390 (28.9) | 1089 (20.0) | <0.01 |
| Deep vein thrombosis | 114 (8.3) | 295 (5.3) | <0.01 |
| Diabetes | 447 (32.3) | 1163 (21.0) | <0.01 |
| Dyslipidemia | 865 (65.1) | 3242 (60.4)) | <0.01 |
| Hypertension | 945 (68.5) | 3261 (59.0) | <0.01 |
| Myocardial infarction | 279 (20.6) | 760 (14.0) | <0.01 |
| Obesity | 846 (61.2) | 2718 (49.1) | <0.01 |
| Peripheral artery disease | 64 (4.6) | 94 (1.7) | <0.01 |
| Stroke | 150 (10.9) | 347 (6.3) | <0.01 |
| Unadjusted | Model 1 a | Model 2 b | Model 3 c | |
|---|---|---|---|---|
| Preceding 30-Day Mean OR (95% CI) | ||||
| Particulate Matter 2.5 | ||||
| Per SD µg/m3 increase (SD** = 3.04) | 1.06 (0.98, 1.13) | 1.04 (0.96, 1.12) | 1.03 (0.96, 1.11) | 1.04 (0.96, 1.13) |
| p-Value | 0.13 | 0.32 | 0.40 | 0.36 |
| Particulate Matter 2.5 | ||||
| 1st Quartile (3.27–10.53 µg/m3) | Referent | Referent | Referent | Referent |
| 2nd Quartile (10.53–11.97 µg/m3) | 1.05 (0.88, 1.25) | 0.98 (0.82, 1.17) | 0.98 (0.82, 1.18) | 0.93 (0.76, 1.14) |
| 3rd Quartile (11.97–13.78 µg/m3) | 0.97 (0.82, 1.16) | 0.91 (0.76, 1.09) | 0.90 (0.75, 1.09) | 0.86 (0.70, 1.05) |
| 4th Quartile (13.78–28.11 µg/m3) | 1.14 (0.94, 1.38) | 1.09 (0.89, 1.32) | 1.08 (0.88, 1.32) | 1.06 (0.85, 1.32) |
| p-valuetrend | 0.36 | 0.31 | 0.31 | 0.22 |
| Preceding One-Year Mean OR (95% CI) | ||||
| Particulate Matter 2.5 | ||||
| Per SD µg/m3 increase (SD** = 1.73) | 1.03 (0.96, 1.09) | 0.99 (0.92, 1.06) | 0.98 (0.92, 1.05) | 0.98 (0.91, 1.05) |
| p-value | 0.45 | 0.71 | 0.59 | 0.56 |
| Particulate Matter 2.5 | ||||
| 1st Quartile (5.88–11.48 µg/m3) | Referent | Referent | Referent | Referent |
| 2nd Quartile (11.48–12.58 µg/m3) | 1.10 (0.93, 1.30) | 1.00 (0.84, 1.19) | 0.99 (0.82, 1.18) | 0.99 (0.82, 1.21) |
| 3rd Quartile (12.58–13.53 µg/m3) | 1.15 (0.97, 1.36) | 1.05 (0.88, 1.26) | 1.05 (0.87, 1.26) | 1.04 (0.85, 1.27) |
| 4th Quartile (13.53–20.18 µg/m3) | 1.10 (0.92, 1.32) | 1.00 (0.83, 1.21) | 0.98 (0.81, 1.26) | 0.96 (0.78, 1.18) |
| p-valuetrend | 0.44 | 0.92 | 0.88 | 0.87 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarmiento, E.J.; Moore, J.X.; McClure, L.A.; Griffin, R.; Al-Hamdan, M.Z.; Wang, H.E. Fine Particulate Matter Pollution and Risk of Community-Acquired Sepsis. Int. J. Environ. Res. Public Health 2018, 15, 818. https://doi.org/10.3390/ijerph15040818
Sarmiento EJ, Moore JX, McClure LA, Griffin R, Al-Hamdan MZ, Wang HE. Fine Particulate Matter Pollution and Risk of Community-Acquired Sepsis. International Journal of Environmental Research and Public Health. 2018; 15(4):818. https://doi.org/10.3390/ijerph15040818
Chicago/Turabian StyleSarmiento, Elisa J., Justin Xavier Moore, Leslie A. McClure, Russell Griffin, Mohammad Z. Al-Hamdan, and Henry E. Wang. 2018. "Fine Particulate Matter Pollution and Risk of Community-Acquired Sepsis" International Journal of Environmental Research and Public Health 15, no. 4: 818. https://doi.org/10.3390/ijerph15040818






