Quantitative Ultra-Performance Liquid Chromatography Tandem Mass Spectrometry Method for Comparison of Prochloraz Residue on Garlic Sprouts after Soaking and Spraying Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Postharvest Treatment and Storage
2.3. Sample Preparation
2.3.1. Sample Extraction
2.3.2. Purification
2.3.3. Method Validation
2.4. UPLC-MS/MS Conditions
3. Results and Discussion
3.1. Limit of Detection and LOQ
3.2. Linearity
3.3. Recovery Study
3.4. Actual Sample
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Wei, Q.; Zhang, N.; Zhang, P.; Li, M.G.; Yan, R.X. Timeliness of four bacillus strains against botrytis cinerea of garlic sprouts. Biotechnol. Bull. 2017, 33, 112–120. [Google Scholar]
- Gdula Argasińska, J.; Paśko, P.; Sułkowska Ziaja, K.; Kała, K.; Muszyńska, B. Anti-inflammatory activities of garlic sprouts, a source of α-linolenic acid and 5-hydroxy-l-tryptophan, in raw 264.7 cells. Acta Biochim. Pol. 2017, 64, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Marchese, A.; Barbieri, R.; Sanches-Silva, A.; Daglia, M.; Nabavi, S.F.; Jafari, N.J.; Izadi, M.; Ajami, M.; Nabavi, S.M. Antifungal and antibacterial activities of allicin: A review. Trends Food Sci. Technol. 2016, 52, 49–56. [Google Scholar] [CrossRef]
- Fujisawa, H.; Suma, K.; Origuchi, K.; Kumagai, H.; Seki, T.; Ariga, T. Biological and chemical stability of garlic-derived allicin. J. Agri. Food Chem. 2008, 56, 4229–4235. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, M.; Ide, N.; Ono, K. Changes in organosulfur compounds in garlic cloves during storage. J. Agric. Food Chem. 2006, 54, 4849–4854. [Google Scholar] [CrossRef] [PubMed]
- Bayat, F.; Rezvani, S. Effect of harvesting time and moisture on mechanical properties of garlic (Allium sativum L.) skin. Agric. Eng. Int. 2012, 14, 161–167. [Google Scholar]
- Iliev, A.I.; Ludneva, D.P.; Kalchevakaradzhova, K.D.; Kozarekovayovkova, D.T. Antioxidant activity and polyphenol content in garlic after drying and unsealed storage. In Proceedings of the International Scientific-Practical Conference, Food, Technologies and Health, Plovdiv, Bulgaria, 7–8 November 2013. [Google Scholar]
- Qin, L. Study of no on the preservation of garlic sprouts. Tianjin Agric. Sci. 2010, 16, 57–60. [Google Scholar]
- Gebreyohannes, G.; Gebreyohannes, M. Medicinal values of garlic: A review. Int. J. Med. Med. Sci. 2013, 9, 401–408. [Google Scholar]
- Zhou, X.; Mu, W.; Wang, Q. Effects of three plant extracts on quality of garlic stem during shelf life. J. Agric. 2012, 1, 48–52. [Google Scholar]
- Rejano, L.; Sanchez, A.H.; Ade, C.; Montano, A. Chemical characteristics and storage stability of pickled garlic prepared using different processes. J. Food Sci. 2012, 62, 1120–1123. [Google Scholar] [CrossRef]
- Masood, S.B.; Muhammad, T.S.; Mehmood, S.B.; Javaid, I. Garlic: Nature’s protection against physiological threats. Crit. Rev. Food Sci. 2009, 49, 538–551. [Google Scholar]
- Yu, M.; Wang, J.; Duan, W.Z.; Ai, L.F.; Ma, Y.S.; Li, W. Determination of 86 pesticide residues in sulfur-containing vegetables by GC-NCI/MS. Food Ind. 2016, 37, 246–252. [Google Scholar]
- Besil, N.; Pérez−Parada, A.; Cesio, V.; Varela, P.; Rivas, F.; Heinzen, H. Degradation of imazalil, orthophenylphenol and pyrimethanil in clementine mandarins under conventional postharvest industrial conditions at 4 °C. Food Chem. 2016, 194, 1132–1137. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Luo, T.; Xu, R.W.; Cheng, Y. Dynamic analyses of prochloraz and imazalil residues in citrus during fruit storage. J. Huazhong Agric. Univ. 2016, 35, 17–23. [Google Scholar]
- Yuan, X.Y.; Peng, Z.; Zhou, H.P.; Liu, C. Degradation dynamics and safety evaluation of prochloraz in banana. J. Food Saf. Qual. 2016, 7, 209–214. [Google Scholar]
- Nielsen, F.K.; Hansen, C.H.; Fey, J.A.; Hansen, M.; Halling-Sørensen, B.; Bjorklund, E.; Styrishave, B. Mixture effects of 3 mechanistically different steroidogenic disruptors (prochloraz, genistein, and ketoconazole) in the H295R cell assay. Int. J. Toxicol. 2015, 279, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, E.D.S.; Filho, A.T. Chemical waste risk reduction and environmental impact generated by laboratory activities in research and teaching institutions. Br. J. Pharm. Sci. 2010, 46, 187–198. [Google Scholar] [CrossRef] [Green Version]
- Fang, Q.; Yao, G.; Shi, Y.; Ding, C.; Wang, Y.; Wu, X.; Hua, R.; Cao, H. Residue dynamics and risk assessment of prochloraz and its metabolite 2, 4, 6-trichlorophenol in apple. Molecules 2017, 22, 1780. [Google Scholar] [CrossRef] [PubMed]
- Cengiz, M.F.; Catal, M.; Erler, F.; Bilgin, A.K. Rapid and sensitive determination of the prochloraz residues in the cultivated mushroom, Agaricus bisporus (Lange) Imbach. Anal. Methods 2014, 6, 1970–1976. [Google Scholar] [CrossRef]
- Besil, N.; Cesio, V.; Heinzen, H.; Fernándezalba, A.R. Matrix effects and interferences of different citrus fruits co-extractives in pesticide residue analysis using ultra high-performance liquid chromatography-high resolution mass spectrometry. J. Agric. Food Chem. 2017, 65, 4819–4829. [Google Scholar] [CrossRef] [PubMed]
- Lara-Ortega, F.J.; Robles-Molina, J.; Brandt, S.; Schütz, A.; Gilbert-López, B.; Molina-Día, A.; García-Reyes, J.F.; Franzke, J. Use of dielectric barrier discharge ionization to minimize matrix effects and expand coverage in pesticide residue analysis by liquid chromatography-mass spectrometry. Anal. Chim. Acta 2018, 1020, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Tong, Z.; Wu, Y.C.; Liu, Q.Q.; Shi, Y.H.; Zhou, L.J.; Liu, Z.Y.; Yu, L.S.; Cao, H.Q. Multi-Residue analysis of pesticide residues in crude pollens by UPLC-MS/MS. Molecules 2016, 21, 1652. [Google Scholar] [CrossRef] [PubMed]
- Winther, C.S.; Nielsen, F.K.; Hansen, M.; Styrishave, B. Corticosteroid production in h295r cells during exposure to 3 endocrine disrupters analyzed with lc-ms/ms. Int. J. Toxicol. 2013, 32, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Erney, D.R.; Gillespie, A.M.; Gilvydis, D.M.; Poole, C.F. Explanation of the matrix-induced chromatographic response enhancement of organophosphorus pesticides during open tubular column gas chromatography with splitless or hot on-column injection and flame photometric detection. J. Chromatogr. A 1993, 638, 57–63. [Google Scholar] [CrossRef]
- Erney, D.R.; Pawlowski, T.M.; Poole, C.F. Matrix-Induced peak enhancement of pesticides in gas chromatogrtaphy: Is there a solution? J. Sep. Sci. 2015, 20, 375–378. [Google Scholar] [CrossRef]
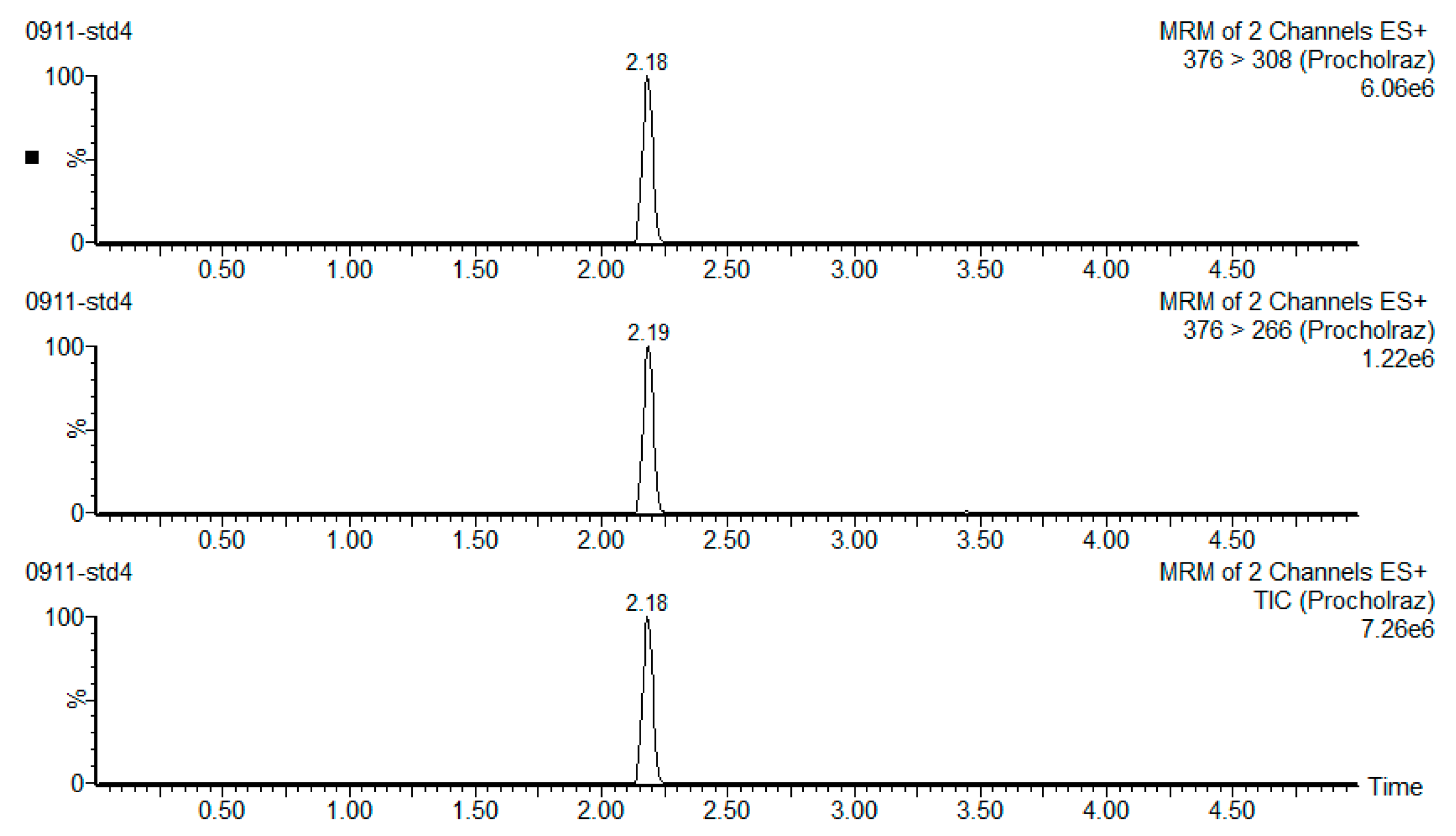
| Compound | Transitions | Dwell Time (s) | Cone Voltage (V) | Collision Energy (eV) |
|---|---|---|---|---|
| Prochloraz | Quantification ion: 376 > 308 | 0.008 | 20 | 15 |
| Confirmation ion: 376 > 266 | 20 | 15 |
| Pesticide | ME (%) | tR (min) | Linear Range (μg/kg) | Linear Regression Equation | Linearity |
|---|---|---|---|---|---|
| Prochloraz | 5.8 | 2.20 | 5–500 | Y = 0.9898X − 1.2624 | 0.9983 |
| Pesticide | LOD (μg/kg) | LOQ (μg/kg) | Concentration (μg/kg) | Measured ± SD (μg/kg) | Recovery (%) | RSD (%) |
|---|---|---|---|---|---|---|
| Prochloraz | 0.0166 | 0.0499 | 5 | 4.7 ± 0.46 | 94.8 | 9.7 |
| 50 | 44.2 ± 1.26 | 88.4 | 2.8 | |||
| 500 | 447.1 ± 11.69 | 89.4 | 2.6 |
| Sampling City | Sampling Time | Soaking (mg/kg) | Spraying (mg/kg) | ||
|---|---|---|---|---|---|
| Whole Plant (Measured ± SD) | Stems (Measured ± SD) | Whole Plant (Measured ± SD) | Stems (Measured ± SD) | ||
| Pingdu | 1 June | 25.14 ± 1.20 | 1.12 ± 0.07 | 5.93 ± 0.36 | 1.11 ± 0.06 |
| 29 June | 22.72 ± 0.94 | 0.80 ± 0.03 | 2.23 ± 0.18 | 0.12 ± 0.01 | |
| 30 July | 21.86 ± 1.13 | 0.58 ± 0.04 | 1.85 ± 0.12 | 0.01 ± 0.001 | |
| Laixi | 1 June | 20.12 ± 0.86 | 1.62 ± 0.05 | 7.89 ± 0.54 | 1.29 ± 0.09 |
| 29 June | 18.70 ± 0.42 | 1.13 ± 0.09 | 5.04 ± 0.30 | 0.15 ± 0.01 | |
| 30 July | 15.76 ± 1.06 | 1.04 ± 0.06 | 4.08 ± 0.20 | 0.03 ± 0.003 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, Q.; Ding, C.; Dong, Z.; Guan, S.; Wu, R.; Wu, X.; Hua, R.; Cao, H. Quantitative Ultra-Performance Liquid Chromatography Tandem Mass Spectrometry Method for Comparison of Prochloraz Residue on Garlic Sprouts after Soaking and Spraying Treatment. Int. J. Environ. Res. Public Health 2018, 15, 1552. https://doi.org/10.3390/ijerph15071552
Fang Q, Ding C, Dong Z, Guan S, Wu R, Wu X, Hua R, Cao H. Quantitative Ultra-Performance Liquid Chromatography Tandem Mass Spectrometry Method for Comparison of Prochloraz Residue on Garlic Sprouts after Soaking and Spraying Treatment. International Journal of Environmental Research and Public Health. 2018; 15(7):1552. https://doi.org/10.3390/ijerph15071552
Chicago/Turabian StyleFang, Qingkui, Chenchun Ding, Zhan Dong, Shuai Guan, Ruifeng Wu, Xiangwei Wu, Rimao Hua, and Haiqun Cao. 2018. "Quantitative Ultra-Performance Liquid Chromatography Tandem Mass Spectrometry Method for Comparison of Prochloraz Residue on Garlic Sprouts after Soaking and Spraying Treatment" International Journal of Environmental Research and Public Health 15, no. 7: 1552. https://doi.org/10.3390/ijerph15071552





