Bioavailability and Uptake of Lead by Coffeeweed (Sesbania exaltata Raf.)
Abstract
:Introduction
Materials and Methods
Soil Preparation
Plant Establishment and Maintenance
Metal Extraction from Plant Tissues
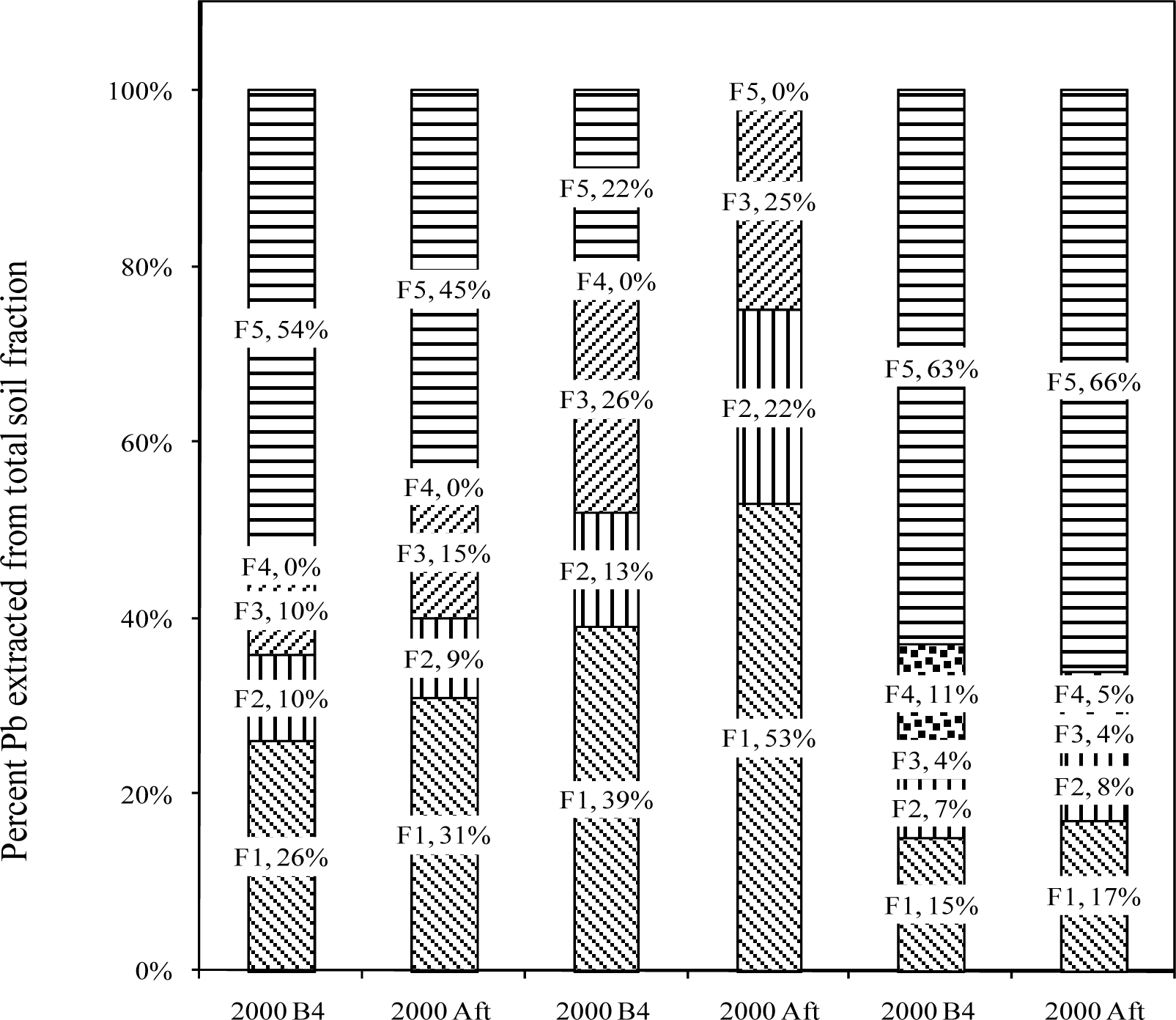
Sequential Extraction of Pb from Soil Samples
Fraction 1 – Exchangeable
Fraction 2 – Carbonate
Fraction 3 – Fe-Mn Oxides
Fraction 4 – Bound to Organic Matter
Fraction 5 – Residual
Statistical Analysis
Results and Discussion
Conclusions


References
- Davis, A; Drexler, JW; Ruby, MV; Nicholson, A. Micromineralogy of mine wastes in relation to lead bioavailability. Environ. Sci. Technol. 1993, 27, 1415–1425. [Google Scholar]
- Zhang, M; Alva, AK; Li, YC; Calvert, DV. Chemical association of Cu, Zn, Mn, and Pb in selected sandy citrus soils. Soil Sci 1997, 162, 181–188. [Google Scholar]
- Gray, CW; McLaren, RG; Roberts, AHC; Condron, LM. Solubility, sorption and desorption of native added cadmium in relation to properties of soils in New Zealand. Eur. J. Soil Sci. 1999, 50, 127–137. [Google Scholar]
- McBride, MB. Environmental chemistry of soils; Oxford University Press, 1994. [Google Scholar]
- Blaylock, MJ; Salt, DE; Dushenkov, S; Zakharova, O; Gussman, C; Kapulnik, Y; Ensley, BD; Raskin, I. Enhanced accumulation of Pb in Indian mustard by soil-applied chelating agents. Environ. Sci. Technol. 1997, 31(3), 860–865. [Google Scholar]
- Huang, JW; Chen, J; Berti, WR; Cunningham, SD. Phytoremediation of lead- contaminated soils: Role of synthetic chelates in lead phytoextraction. Environ. Sci. Technol. 1997, 31(3), 800–805. [Google Scholar]
- Huang, JW; Cunningham, SD. Lead phytoextraction: Species variation in lead uptake and translocation. New Phytologist 1996, 134, 75–84. [Google Scholar]
- Ebbs, SD; Kochian, LV. Phytoextraction of zinc by oat (Avena sativa), barley (Hordeum vulgare) and Indian mustard (Brassica juncea). Environ. Sci. Technol. 1998, 32, 802–806. [Google Scholar]
- Kayser, A; Wenger, K; Keller, A; Attinger, W; Felix, HR; Gupta, SK; Schulin, R. Enhancement of phytoextraction of Zn, Cd, and Cu from calcareous soil: The use of NTA and sulfur amendments. Environ. Sci. Technol. 2000, 34, 1778–1783. [Google Scholar]
- Miller, G; Begonia, G; Begonia, M; Ntoni, J; Hundley, O. Assessment of the efficacy of chelate-assisted phytoextraction of lead by coffeeweed (Sesbania exaltata Raf). International Journal Environ. Res. Public Health 2008, 428–435. [Google Scholar]
- Tessier, A; Campbell, PGC; Bisson, M. Sequential extraction procedure for the speciation of particulate traces metals. Analytical Chemistry 1979, 51(7), 844–851. [Google Scholar]
- Salomons, W; Förstner, U. Trace metal analysis on polluted sediments. Part II. Evaluation of environmental impact. Environ Technol Lett 1980, 1, 506–517. [Google Scholar]
- Pueyo, M; Sastre, J; Hernández, E; Vidal, M; López-Sánchez, JF; Rauret, G. Heavy metals in the environment. J Environ Qual 2003, 32, 2054–2066. [Google Scholar]
- Chaudhuri, D; Tripathy, S; Powell, HVA; Hart, BR. Relationship of chemical fractions of heavy metals with microial and enzyme activities in sludge and ash-amended acid lateritic soil from India. Environ. Geol. 2003, 44, 419–432. [Google Scholar]
- Kaasalainen, M; Yli-Halla, M. Use of sequential extraction to assess metal partitioning in soils. Environ. Pollut. 2003, 126, 225–233. [Google Scholar]
- McLaren, RG; Clucas, LM. Fractionation of copper, nickel and zinc in metal-spiked sewage sludge. J Environ Qual 2001, 30, 1968–1975. [Google Scholar]
- Ma, LQ; Rao, GN. Chemical fractionation of cadmium, copper, nickel and zinc in contaminated soils. J Environ Qual 1997, 26, 259–264. [Google Scholar]
- Tlustoš, P; Szárková, J; Stárková, A; Pavlǐková, D. A comparison of sequential extraction procedures for fractionation of arsenic, cadmium, lead, and zinc in soil. Central European Journal of Chemistry 2005, 3(4), 830–851. [Google Scholar]
- Lim, TT; Tay, JH; Wang, JY. Chelating-agent-enhanced heavy metal extraction from a contaminated acidic soil. J. Environ. Eng. 2004, 130, 59–66. [Google Scholar]
- Filgueiras, AV; Lavilla, I; Vendicho, C. Chemical sequential extraction for metal partitioning in environmental solid samples. J. Environ. Monit. 2002, 4, 823–857. [Google Scholar]
- Keltjens, WG; van Beusichem, ML. Phytochelatins as biomarkers for heavy metal toxicity in maize: Single metal effects of copper and cadmium. J. Plant Nutr. 1998, 21(4), 635–648. [Google Scholar]
- Dushenkov, V; Kumar, NPBA; Motto, H; Raskin, I. Rhizofiltration - the use of plants to remove heavy metals from aqueous streams. Environ. Sci. Technol. 1995, 29, 1239–1245. [Google Scholar]
© 2008 MDPI All rights reserved.
Share and Cite
Miller, G.; Begonia, G.; Begonia, M.; Ntoni, J. Bioavailability and Uptake of Lead by Coffeeweed (Sesbania exaltata Raf.). Int. J. Environ. Res. Public Health 2008, 5, 436-440. https://doi.org/10.3390/ijerph5050436
Miller G, Begonia G, Begonia M, Ntoni J. Bioavailability and Uptake of Lead by Coffeeweed (Sesbania exaltata Raf.). International Journal of Environmental Research and Public Health. 2008; 5(5):436-440. https://doi.org/10.3390/ijerph5050436
Chicago/Turabian StyleMiller, Gloria, Gregorio Begonia, Maria Begonia, and Jennifer Ntoni. 2008. "Bioavailability and Uptake of Lead by Coffeeweed (Sesbania exaltata Raf.)" International Journal of Environmental Research and Public Health 5, no. 5: 436-440. https://doi.org/10.3390/ijerph5050436



