Gamma Hydroxybutyric Acid (GHB) for the Treatment of Alcohol Dependence: A Review
Abstract
:1. Introduction
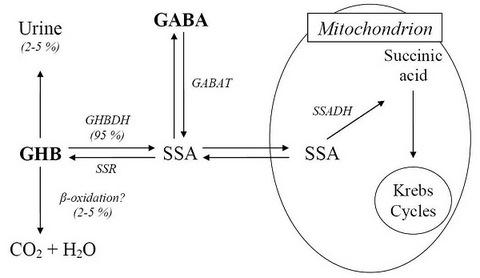
2. Metabolism
3. Neuro-Modulatory Properties
4. GHB for the Treatment of Alcohol Withdrawal Syndrome
5. GHB as an Anti-Craving Drug in the Maintenance of Alcohol Abstinence
5.1. Studies with GHB as Mono-Therapy
5.2. Comparative Studies
5.3. Combined Studies
6. Craving for and Abuse of GHB in Clinical Studies
7. Conclusions
References
- Carter, LP; Koek, W; France, CP. Behavioral analyses of GHB: receptor mechanisms. Pharmacol. Ther 2009, 121, 100–114. [Google Scholar]
- Gianoulakis, C. Implication of endogenous opioids and dopamine in alcoholism: human and basic science studies. Alcohol Alcoholism 1996, 31, 33–42. [Google Scholar]
- Bessmann, SP; Fishbein, WM. Gamma-hydroxybutyric, a normal brain metabolite. Nature 1963, 200, 1207–1208. [Google Scholar]
- Laborit, H; Jouany, JM; Gerard, J; Fabiani, F. Summary of an experimental and clinical study on a metabolic substrate with inhibitory central action: sodium 4-hydroxybutyrate. Presse Medicale 1960, 68, 1867–1869. [Google Scholar]
- Benavides, J; Rumigny, JF; Bourguignon, JJ; Cash, C; Wermuth, CG; Mandel, P; Vincendon, G; Maitre, M. High affinity binding sites for gamma-hydroxybutyric acid in rat brain. Life Sci 1982, 30, 953–961. [Google Scholar]
- Snead, OC; Liu, CC. Gamma-hydroxybutyric acid binding sites in rat and human brain synaptosomal membranes. Biochem. Pharmacol 1984, 33, 2587–2590. [Google Scholar]
- Andriamampandry, C; Taleb, O; Viry, S; Muller, C; Humbert, JP; Gobaille, S; Aunis, D; Maitre, M. Cloning and characterization of a rat brain receptor that binds the endogenous neuromodulator gamma-hydroxybutyrate (GHB). FASEB J 2003, 17, 1691–1693. [Google Scholar]
- Andriamampandry, C; Taleb, O; Kemmel, V; Humbert, JP; Aunis, D; Maitre, M. Cloning and functional characterization of a gamma-hydroxybutyrate receptor identified in the human brain. FASEB. J 2007, 21, 885–895. [Google Scholar]
- Aldrete, JA; Barnes, DP. 4-Hydroxybutyrate anaesthesia for cardiovascular surgery. A comparison with halothane. Anaesthesia 1968, 23, 558–565. [Google Scholar]
- Kleinschmidt, S; Grundmann, U; Janneck, U; Kreienmeyer, J; Kulosa, R; Larsen, R. Total intravenous anaesthesia using propofol, gamma-hydroxybutyrate or midazolam in combination with sufentanil for patients undergoing coronary artery bypass surgery. Eur. J. Anaesthesiol 1997, 14, 590–599. [Google Scholar]
- Kleinschmidt, S; Grundmann, U; Knocke, T; Silomon, M; Bach, F; Larsen, R. Total intravenous anaesthesia with gamma-hydroxybutyrate (GHB) and sufentanil in patients undergoing coronary artery bypass graft surgery: a comparison in patients with unimpaired and impaired left ventricular function. Eur. J. Anaesthesiol 1998, 15, 559–564. [Google Scholar]
- Kemmel, V; Miehe, M; Roussel, G; Taleb, O; Nail-Boucherie, K; Marchand, C; Stutz, C; Andriamampandry, C; Aunis, D; Maitre, M. Immunohistochemical localization of a GHB receptor-like protein isolated from rat brain. J. Comp. Neurol 2006, 498, 508–524. [Google Scholar]
- Carai, MA; Colombo, G; Brunetti, G; Melis, S; Serra, S; Vacca, G; Mastinu, S; Pistuddi, AM; Solinas, C; Cignarella, G; Minardi, G; Gessa, GL. Role of GABAB receptors in the sedative/hypnotic effect of γ-hydroxybutyric acid. Eur. J. Pharmacol 2001, 428, 315–321. [Google Scholar]
- Mamelak, M; Escrui, JM; Stokan, O. The effects of γ-hydroxybutyrate on sleep. Biol. Psychiatry 1977, 12, 273–278. [Google Scholar]
- Mamelak, M; Scharf, MB; Woods, M. Treatment of narcolepsy with gamma-hydroxybutyrate. A review of clinical and sleep laboratory findings. Sleep 1986, 9, 285–289. [Google Scholar]
- Carai, MA; Colombo, G; Reali, R; Serra, S; Mocci, I; Castelli, MP; Cignarella, G; Gessa, GL. Central effects of 1, 4-butanediol are mediated by GABAB receptors via its conversion into γ-hydroxybutyric acid. Eur. J. Pharmacol 2002, 441, 157–163. [Google Scholar]
- Broughton, R; Mamelak, M. Effects of nocturnal gamma-hydroxybutyrate on sleep/waking patterns in narcolepsy-cataplexy. Can. J. Neurol. Sci 1980, 7, 23–31. [Google Scholar]
- Dauvilliers, Y; Arnulf, I; Mignot, E. Narcolepsy with cataplexy. Lancet 2007, 369, 499–511. [Google Scholar]
- Tunnicliff, G; Raess, BU. Gamma-hydroxybutyrate (orphan medical). Curr. Opin. Investig. Drugs 2002, 3, 278–283. [Google Scholar]
- Beghè, F; Campanini, MT. Safety and tolerability of gamma-hydroxybutyric acid in the treatment of alcohol-dependents patients. Alcohol 2000, 20, 223–225. [Google Scholar]
- Snead, OC, III; Gibson, KM. Gamma-hydroxybutyric acid. N. Engl. J. Med 2005, 352, 2721–2732. [Google Scholar]
- Palatini, P; Tedeschi, L; Frison, G; Padrini, R; Zordan, R; Orlando, R; Gallimberti, L; Gessa, GL; Ferrara, SD. Dose-dependent absorption and elimination of gamma-hydroxybutyric acid in healthy volunteers. Eur. J. Clin. Pharmacol 1993, 45, 353–356. [Google Scholar]
- Ferrara, SD; Zotti, S; Tedeschi, L; Frison, G; Castagna, F; Gallimberti, L; Gessa, GL; Palatini, P. Pharmacokinetics of gamma-hydroxybutyric acid in alcohol dependent patients after single and repeated oral doses. Br. J. Clin. Pharmacol 1992, 34, 231–235. [Google Scholar]
- Ferrara, SD; Tedeschi, L; Frison, G; Orlando, R; Mazzo, M; Zordan, R; Padrini, R; Palatini, P. Effect of moderate or severe liver dysfunction on the pharmacokinetics of gamma-hydroxybutyric acid. Eur. J. Clin. Pharmacol 1996, 50, 305–310. [Google Scholar]
- Davies, M. The role of GABAA receptors in mediating the effects of alcohol in the central nervous system. J. Psychiatry Neurosci 2003, 28, 263–274. [Google Scholar]
- Nie, Z; Madamba, SG; Siggins, GR. Ethanol inhibits glutamatergic neurotransmission in nucleus accumbens neurons by multiple mechanisms. J. Pharmacol. Exp. Ther 1994, 271, 1566–1573. [Google Scholar]
- Snead, OC, III; Liu, CC. GABAA receptor function in the g-hydroxybutyrate model of generalized absence seizures. Neuropharmacology 1993, 32, 401–409. [Google Scholar]
- Malcolm, RJ. GABA systems, benzodiazepines, and substance dependence. J. Clin. Psychiatry 2003, 64, 36–40. [Google Scholar]
- Leggio, L; Kenna, GA; Swift, RM. New developments for the pharmacological treatment of alcohol withdrawal syndrome. A focus on non-benzodiazepine GABAergic medications. Prog. Neuropsychopharmacol. Biol. Psychiatry 2008, 32, 1106–1117. [Google Scholar]
- Gallimberti, L; Canton, G; Gentile, N; Ferri, M; Cibin, M; Ferrara, SD; Fadda, F; Gessa, GL. Gamma-hydroxybutyric acid for treatment of alcohol withdrawal syndrome. Lancet 1989, 2, 787–789. [Google Scholar]
- Addolorato, G; Balducci, G; Capristo, E; Attilia, ML; Taggi, F; Gasbarrini, G; Ceccanti, M. Gamma-hydroxybutyric acid (GHB) in the treatment of alcohol withdrawal syndrome: a randomized comparative study versus benzodiazepine. Alcohol Clin. Exp. Res 1999, 23, 1596–1604. [Google Scholar]
- Nava, F; Premi, S; Manzato, E; Campagnola, W; Lucchini, A; Gessa, GL. Gamma-hydroxybutyrate reduces both withdrawal syndrome and hypercortisolism in severe abstinent alcoholics: an open study vs. diazepam. Amer. J. Drug Alcohol Abuse 2007, 33, 379–392. [Google Scholar]
- Nimmerrichter, AA; Walter, H; Gutierrez-Lobos, KE; Lesch, OM. Double blind controlled trial of γ-hydroxybutyrate and clomethiazole in the treatment of alcohol withdrawal. Alcohol Alcoholism 2002, 37, 67–73. [Google Scholar]
- Korninger, C; Roller, RE; Lesch, OM. Gamma-hydroxybutyric acid in the treatment of alcohol withdrawal syndrome in patients admitted to hospital. Acta. Med. Austriaca 2003, 3, 83–86. [Google Scholar]
- Agabio, R; Colombo, G; Loche, A; Lobina, C; Pani, ML; Reali, R; Gessa, GL. Gamma-hydroxybutyric acid reducing effect on ethanol intake: evidence in favour of a substitution mechanism. Alcohol Alcoholism 1998, 33, 465–474. [Google Scholar]
- Colombo, G; Agabio, R; Lobina, C; Reali, R; Fadda, F; Gessa, GL. Cross tolerance to ethanol and gamma-hydroxybutyric acid. Eur. J. Pharmacol 1995, 273, 235–238. [Google Scholar]
- Gessa, GL; Agabio, R; Carai, MA; Lobina, C; Pani, M; Reali, R; Colombo, G. Mechanism of the anti-alcohol effect of gamma-hydroxybutyric acid. Alcohol 2000, 20, 271–276. [Google Scholar]
- Biggio, G; Cibin, M; Diana, M; Fadda, F; Ferara, SD; Gallimberti, L; Gessa, GL; Mereu, GP; Rossetti, ZL; Serra, M. Suppression of voluntary ethanol intake in rats and alcoholics by gamma-hydroxybutyric acid: a non-GABAergic mechanism. Adv. Biochem. Psychopharmacol 1992, 47, 281–288. [Google Scholar]
- Gallimberti, L; Ferri, M; Ferrara, SD; Fadda, F; Gessa, GL. Gamma-hydroxybutyric acid in the treatment of alcohol dependance: a double-blind study. Alcohol. Clin. Exp. Res 1992, 16, 673–676. [Google Scholar]
- Addolorato, G; Castelli, E; Stefanini, GF; Casella, G; Caputo, F; Marsigli, L; Bernardi, M; Gasbarrini, G. An open multicentric study evaluating 4-hydroxybutyric acid sodium salt in the medium-term treatment of 179 alcohol dependent subjects. GHB Study Group. Alcohol Alcoholism 1996, 31, 341–345. [Google Scholar]
- Addolorato, G; Cibin, M; Caputo, F; Capristo, E; Gessa, GL; Stefanini, GF; Gasbarrini, G. Gamma-hydroxybutyric acid in the treatment of alcoholism: dosage fractioning utility in non-responder alcoholic patients. Drug Alcohol Depend 1998, 53, 7–10. [Google Scholar]
- Maremmani, I; La Manna, F; Tagliamone, A. Long-term therapy using GHB (sodium gamma hydroxybutyrate) for treatment-resistant chronic alcoholics. J. Psychoactive Drug 2001, 33, 135–142. [Google Scholar]
- Thai, D; Dyer, JE; Benowitz, NL; Haller, CA. Gamma-hydroxybutyrate and ethanol effects and interactions in humans. J. Clin. Psychopharmacol 2006, 26, 524–529. [Google Scholar]
- Caputo, F; Addolorato, G; Lorenzini, F; Domenicali, M; Greco, G; Del Re, A; Gasbarrini, G; Stefanini, GF; Bernardi, M. Gamma-hydroxybutyric acid versus naltrexone in maintaining alcohol abstinence: an open randomized comparative study. Drug Alcohol Depend 2003, 70, 85–91. [Google Scholar]
- Caputo, F; Addolorato, G; Stoppo, M; Francini, S; Vignoli, T; Lorenzini, F; Del Re, A; Comaschi, C; Andreone, P; Trevisani, F; Bernardi, M. Comparing and combining gamma-hydroxybutyric acid (GHB) and naltrexone in maintaining abstinence from alcohol: An open randomised comparative study. Eur. Neuropsychopharmacol 2007, 17, 781–789. [Google Scholar]
- Caputo, F; Stoppo, M; Vignoli, T; Francini, S; Lorenzini, F; Bernardi, M. Use of alcohol during the treatment of alcohol dependence with gamma-Hydroxybutyric Acid: Risk of severe events are avoided by the dose fractioning of the drug. J. Clin. Psychopharmacol 2007, 27, 418. [Google Scholar]
- Nava, F; Premi, S; Manzato, E; Lucchini, A. Comparing treatments of alcoholism on craving and bio-chemical measures of alcohol consumptions. J. Psychoactive Drug 2006, 38, 211–217. [Google Scholar]
- Caputo, F; Vignoli, T; Lorenzini, F; Ciuffoli, E; Del Re, A; Stefanini, GF; Addolorato, G; Trevisani, F; Bernardi, M. Alcoholism Treatment Study Group. Suppression of craving for γ-hydroxybutyric acid by naltrexone administration: three case reports. Clin. Neuropharmacol 2005, 28, 87–89. [Google Scholar]
- Stella, L; Addolorato, G; Rinaldi, B; Capuano, A; Berrino, L; Rossi, F; Maione, F. An open randomized study of the treatment of escitalopram alone and combined with γ-hydroxybutyric acidand naltrexone in alcoholic patients. Pharmacol. Res 2008, 57, 312–317. [Google Scholar]
- Verheul, R; van Den Brink, W; Geerlings, P. A three-patways psychological model of craving for alcohol. Alcohol Alcoholism 1999, 34, 197–222. [Google Scholar]
- Addolorato, G; Abenavoli, L; Leggio, L; Gasbarrini, G. How many cravings? Pharmacological aspects of craving treatment in alcohol addiction: a review. Neuropsychobiology 2005, 51, 59–66. [Google Scholar]
- Leggio, L; Kenna, GA; Fenton, M; Bonenfant, E; Swift, RM. Typologies of alcohol dependence from jellinek to genetics and beyond. Neuropsychol Rev 2009. [Google Scholar]
- Addolorato, G; Caputo, F; Capristo, E; Stefanini, GF; Gasbarrini, G. Gamma-hydroxybutyric acid efficacy, potential abuse, and dependence in the treatment of alcohol addiction. Alcohol 2000, 20, 217–222. [Google Scholar]
- Caputo, F; Francini, S; Stoppo, M; Lorenzini, F; Vignoli, T; Del Re, A; Comaschi, C; Leggio, L; Addolorato, G; Zoli, G; Bernardi, M. Incidence of craving for and abuse of gamma-hydroxybutyric acid (GHB) in different populations of treated alcoholics: an open comparative study. J Psychopharmacol 2009. [Google Scholar]
- Kalivas, PW; Volkow, ND. The neural basis of addiction: A pathology of motivation and choice. Am. J. Psychiatry 2005, 162, 1403–1413. [Google Scholar]
- Rosen, MI; Pearsall, HR; Woods, SW; Kosten, TR. Effects of gamma-hydroxybutyric acid (GHB) in opioid-dependent patients. J. Subst. Abuse Treat 1997, 14, 149–154. [Google Scholar]
- Anderson, SM; Pierce, RC. Cocaine-induced alterations in dopamine receptor signalling: implications for reinforcement and reinstatement. Pharmacol. Ther 2005, 106, 389–403. [Google Scholar]
- Volkow, ND; Fowler, JS; Wang, GJ; Swanson, JM. Dopamine in drug abuse and addiction: results from imaging studies and treatment implications. Mol. Psychiatry 2004, 9, 557–569. [Google Scholar]
- Xi, ZX; Ramamoorthy, S; Shen, H; Lake, R; Samuvel, DJ; Kalivas, PW. GABA transmission in the nucleus accumbens is altered after withdrawal from repeated cocaine. J. Neurosci 2003, 23, 3498–3505. [Google Scholar]
- Jayaram, P; Steketee, JD. Effects of repeated cocaine on medial prefrontal cortical GABAB receptor modulation of neurotransmission in the mesocorticolimbic dopamine system. J. Neurochem 2004, 90, 839–847. [Google Scholar]
- Martin, TJ; Kahn, WR; Xiao, R; Childers, SR. Differential regional effects of methadone maintenance compared to heroin dependence on mu-opioid receptor desensitization in rat brain. Synapse 2007, 61, 176–184. [Google Scholar]
- Shi, J; Zhao, LY; Copersino, ML; Fang, YX; Chen, Y; Tian, J; Deng, Y; Shuai, Y; Jin, J; Lu, L. PET imaging of dopamine transporter and drug craving during methadone maintenance treatment and after prolonged abstinence in heroin users. Eur. J. Pharmacol 2007, 28, 160–166. [Google Scholar]
- Johnson, BA; Swift, RM; Addolorato, G; Ciraulo, DA; Myrick, H. Safety and efficacy of GABAergic medications for treating alcoholism. Alcohol. Clin. Exp. Res 2005, 29, 248–254. [Google Scholar]
- Tambour, S; Quertemont, E. Preclinical and clinical pharmacology of alcohol dependence. Fundam. Clin. Pharmacol 2007, 21, 9–28. [Google Scholar]
- Addolorato, G; Caputo, F; Leggio, L; Vignoli, T; Abenavoli, L; Lorenzini, F; Bernardi, M; Gasbarrini, G. Gamma hydroxybutyric acid (GHB) withdrawal does not occur at therapeutic dosage. Drug. Alcohol. Depend 2005, 77, 209. [Google Scholar]
- Nicholson, KL; Blaster, RL. GHB: a new and novel drug of abuse. Drug Alcohol Depend 2000, 63, 1–22. [Google Scholar]
- Ricaurte, GA; McCann, UD. Recognition and management of complications of new recreational drug use. Lancet 2005, 365, 2137–2145. [Google Scholar]
- Knudsen, K; Greter, J; Verdicchio, M. High mortality rates among GHB abusers in Western Sweden. Clin. Toxicol 2008, 46, 187–192. [Google Scholar]
- Caputo, F; Addolorato, G; Trevisani, F; Bernardi, M. Gamma-hydroxybutyrate as a treatment for alcoholism. Lancet 2005, 366, 981–982. [Google Scholar]
- Addolorato, G; Leggio, L; Ferrulli, A; Caputo, F; Gasbarrini, A. The therapeutic potential of gamma-hydroxybutyric acid for alcohol dependence: balancing the risks and benefits. A focus on clinical data. Expert. Opin. Investig. Drug 2009, 18, 1–12. [Google Scholar]
- Caputo, F; Bernardi, M; Zoli, G. Treatment of alcohol use disorders. Lancet 2009, 373, 1519. [Google Scholar]



© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Caputo, F.; Vignoli, T.; Maremmani, I.; Bernardi, M.; Zoli, G. Gamma Hydroxybutyric Acid (GHB) for the Treatment of Alcohol Dependence: A Review. Int. J. Environ. Res. Public Health 2009, 6, 1917-1929. https://doi.org/10.3390/ijerph6061917
Caputo F, Vignoli T, Maremmani I, Bernardi M, Zoli G. Gamma Hydroxybutyric Acid (GHB) for the Treatment of Alcohol Dependence: A Review. International Journal of Environmental Research and Public Health. 2009; 6(6):1917-1929. https://doi.org/10.3390/ijerph6061917
Chicago/Turabian StyleCaputo, Fabio, Teo Vignoli, Icro Maremmani, Mauro Bernardi, and Giorgio Zoli. 2009. "Gamma Hydroxybutyric Acid (GHB) for the Treatment of Alcohol Dependence: A Review" International Journal of Environmental Research and Public Health 6, no. 6: 1917-1929. https://doi.org/10.3390/ijerph6061917