Toxic Metals (Pb and Cd) and Their Respective Antagonists (Ca and Zn) in Infant Formulas and Milk Marketed in Brasilia, Brazil
Abstract
:1. Introduction
2. Experimental
2.1. Apparatus
2.2. Chemicals and Samples
2.3. Procedure
2.4. Statistical Analysis
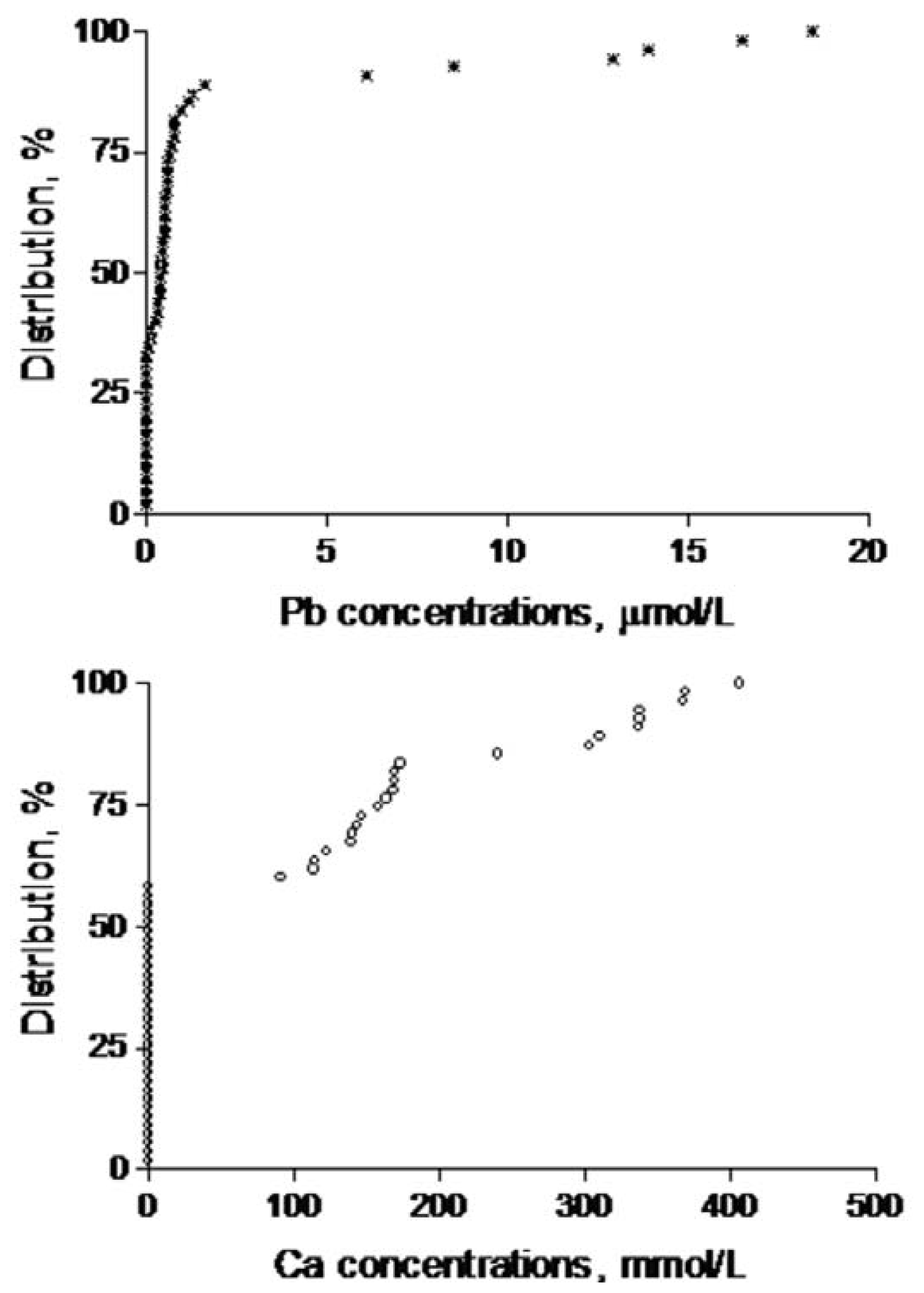
3. Results
4. Discussion
5. Conclusions
Acknowledgements
References
- Dórea, JG; Donangelo, CM. Early (in uterus and infant) exposure to mercury and lead. Clin. Nutr 2006, 25, 369–376. [Google Scholar]
- Parkpian, P; Leong, ST; Laortanakul, P; Thunthaisong, N. Regional monitoring of lead and cadmium contamination in a tropical grazing land site, Thailand. Environ. Monit. Assess 2003, 85, 157–173. [Google Scholar]
- Concon, JM. Food Toxicology, Contaminants and Additives; Marcel Dekker: New York, NY, USA, 1988; pp. 1–695. [Google Scholar]
- Creaser, C. Food Contaminants: Sources and Surveillance; Woodhead Publishing, Limited: Cambridge, UK, 1991; pp. 1–204. [Google Scholar]
- USEPA (US Environmental Protection Agency) , Study of Pb, Cu, Zn and Cd Contamination of Food Chains of Man; National Technical Information Service (NTIS): Jefferson City, MO, USA, 1972.
- Quarterman, J; Morrison, JN; Humphries, WR. The influence of high dietary calcium and phosphate on lead uptake and release. Environ. Res 1978, 17, 60–67. [Google Scholar]
- Mahaffey, KR. Environmental lead toxicity: nutrition as a component of intervention. Environ. Health Perspect 1990, 89, 75–78. [Google Scholar]
- Fullmer, CS. Intestinal calcium and lead absorption: effects of dietary lead and calcium. Environ. Res 1991, 54, 159–169. [Google Scholar]
- Bogden, JD; Oleske, JM; Louria, DB. Lead poisoning—one approach to a problem that won’t go away. Environ. Health Perspect 1997, 105, 1284–1286. [Google Scholar]
- Johnson, NE; Tenuta, K. Diets and lead blood levels of children who practice pica. Environ. Res 1979, 18, 369–376. [Google Scholar]
- Hernandez-Avila, M; Gonzalez-Cossio, T; Palazuelos, E; Romieu, I; Aro, A; Fishbein, E; Peterson, KE; Hu, H. Dietary and environmental determinations of blood and bone lead levels in lactating postpartum women living in Mexico City. Environ. Health Perspect 1996, 104, 1076–1082. [Google Scholar]
- Hauser, G; Vienna, A; Wolfsperger, M; Goessler, W. Milk consumption, smoking and lead concentration in human hair. Coll. Antropol 1999, 23, 433–436. [Google Scholar]
- Reeves, PG; Chaney, RL. Mineral status of female rats affects the absorption and organ distribution of dietary cadmium derived from edible sunflower kernels (Helianthus annuus L.). Environ. Res 2001, 85, 215–25. [Google Scholar]
- Reeves, PG; Chaney, RL. Nutritional status affects the absorption and whole-body and organ retention of cadmium in rats fed rice-based diets. Environ. Sci. Technol 2002, 36, 2684–2692. [Google Scholar]
- Miller, JN; Miller, JM. Statistics for Analytical Chemistry, 4th ed; Prentice Hall: New York, NY, USA, 2001. [Google Scholar]
- Waeny, JCC. Repetitividade e Reprodutividade I; IPT (Instituto de Pesquisas Tecnológicas): São Paulo, Brazil, 1980. [Google Scholar]
- Gitlow, H; Gitlow, S; Oppenheim, A; Oppenheim, R. Richard, D, Ed.; Tools and Methods for the Improvement of Quality; Irwin Inc: Homewood, IL, USA, 1989. [Google Scholar]
- Brazilian Ministry of Health, Princípios gerais para o estabelecimento de níveis máximos de contaminantes químicos em alimentos. In Ordinance no. 685/98; August 1998.
- General Standard for Contaminants and Toxins in Food and Feed; Codex Alimentarius Commision: Rome, Italy, 1995; pp. 1–44.
- Dórea, JG. Zinc in human milk. Nut. Res 2000, 20, 1645–1688. [Google Scholar]
- Dórea, JG; Cabral, MS; Wright, MGM; Rodrigues, KH. Osmolalities of bottle- and breast-milk fed to poor urban Brazilian infants. Ann. Trop. Paediatr 1988, 8, 181–183. [Google Scholar]
- Santos, EE; Lauria, DC; Porto da Silveira, CL. Assessment of daily intake of trace elements due to consumption of foodstuffs by adult inhabitants of Rio de Janeiro City. Sci. Total Environ 2004, 327, 69–79. [Google Scholar]
- Okada, IA; Sakuma, AM; Maio, FD; Docidauskas, S; Zenebon, O. Evaluation of lead and cadmium levels in milk due to environmental contamination in the paraíba valley region of southeastern brazil. Rev. Saude Publ 1997, 31, 140–143. [Google Scholar]
- Soares, VA; Kus, MMM; Peixoto, ALC; Carrocci, JS; Salazar, RFS; Izario Filho, HJ. Determination of nutritional and toxic elements in pasteurized bovine milk from Vale do Paraíba region (Brazil). Food Control 2010, 21, 45–49. [Google Scholar]
- Silva, CM; Alcoforado, ES; Amaral, RS; Santos Júnior, JA; Menezes, RSC; Bezerra, JD; Santos, DC; Lima, LE; Filho, CAS; Honorato, EV; Ribeiro, FCA; Tabosa, JN. Stable lead in milk and derivatives. Food Anal. Method 2010, 3, 85–89. [Google Scholar]
- Azcue, JPM; Pfeifer, WC; Donangelo, CM; Fiszman, M; Malm, O. Heavy metals in foods from the Paraiba do Sul Valley, Brazil. J. Food Comp. Anal 1988, 1, 250–258. [Google Scholar]
- Cabrera, C; Lorenzo, ML; Lopez, MC. Lead and cadmium contamination in dairy products and its repercussion on total dietary intake. J. Agric. Food Chem 1995, 43, 1605–1609. [Google Scholar]
- Belgaied, JE. Release of heavy metals from Tunisian traditional earthenware. Food Chem. Toxicol 2003, 41, 95–98. [Google Scholar]
- Kazi, TG; Jalbani, N; Baig, JA; Kandhro, GA; Afridi, HI; Arain, MB; Jamali, MK; Shah, AQ. Determination of toxic elements in infant formulae by using electrothermal atomic absorption spectrometer. Food Chem. Toxicol 2009, 47, 1425–1429. [Google Scholar]
- Licata, P; Trombetta, D; Cristani, M; Giofrè, F; Martino, D; Calo, M; Naccari, F. Levels of “toxic” and “essential” metals in samples of bovine milk from various dairy farms in Calábria, Italy. Environ. Int 2004, 30, 1–6. [Google Scholar]
- D’Ilio, S; Petrucci, F; D’Amato, M; Di Gregorio, M; Senofonte, O; Violante, N. Method validation for determination of arsenic, cadmium, chromium and lead in milk by means od dynamic reaction inductively coupled plasma mass spectrometry. Anal. Chim. Acta 2008, 624, 59–67. [Google Scholar]
- Tripathi, RM; Raghunath, R; Krishnamoorthy, TM. Dietary intake of heavy metals in Bombay city, India. Sci. Total Environ 1997, 208, 149–159. [Google Scholar]
- Rubio, MR; Sigrist, ME; Encinas, T; Baroni, EE; Coronel, JE; Boggio, JC; Beldomenico, HR. Cadmium and lead levels in cow’s milk from milking region in Santa Fe, Argentina. Bull. Environ. Contam. Toxicol 1998, 60, 164–167. [Google Scholar]
- Tokuşoglu, O; Aycan, S; Akalin, S; Koçak, S; Ersoy, N. Simultaneous differential pulse determination of cadmium, lead, and cooper in milk and dairy products. J. Agric. Food Chem 2004, 52, 1795–1799. [Google Scholar]
- Ay, U; Karayunlu, S. Modification in direct analysis method: metal levels in raw milk at the region of izmit bt graphite furnace atomic absorption spectrophotometer. Int. J. Food Sci. Technol 2008, 43, 326–329. [Google Scholar]
- Cerknenik, V; Doganoc, DK; Jan, J. Evidence of some trace elements, organochlorine pesticides and PCBs in Slovenian cow’s milk. Food Technol. Biotechnol 2000, 38, 155–160. [Google Scholar]
- Martino, FAR; Sanchez, MLF; Sanz-Medel, A. The potential of double focusing-ICP-MS for studying elemental distribution patterns in whole milk, skimmed milk and milk whey of different milks. Anal. Chim. Acta 2001, 442, 191–200. [Google Scholar]
- Dórea, JG. Concentration of zinc and copper and metallocalorie ratio in bottle-milks as prepared by poor urban families. Ann. Trop. Paediat 1992, 12, 7–11. [Google Scholar]
- Ikem, A; Nwankwoala, A; Odueyungbo, S; Nyavor, K; Egiebor, N. Levels of 26 elements in infant formula from USA, UK, and Nigeria by microwave digestion and ICP-OES. Food Chem 2002, 77, 439–447. [Google Scholar]
- Baranowska, I; Barchanska, H; Pyrsz, A. Distribution of pesticides and heavy metals in trophic chain. Chemosphere 2005, 60, 1590–1599. [Google Scholar]
- Brotanek, JM; Schroer, D; Valentyn, L; Tomany-Korman, S; Flores, G. Reasons for prolonged bottle-feeding and iron deficiency among Mexican-American toddlers: an ethnographic study. Acad. Pediatr 2009, 9, 17–25. [Google Scholar]
- Satarug, S; Garrett, SH; Sens, MA; Sens, DA. Cadmium, environmental exposure and health outcomes. Environ. Health Perspect 2010, 118, 182–190. [Google Scholar]



| Metals | [Zn2+]/μg/L | [Pb2+]/μg/L | [Cd2+]/μg/L | [Ca2+]/mg/L |
|---|---|---|---|---|
| Method LOD and LOQ | 1.89; 6.32 | 2.71; 9.04 | 0.62; 2.08 | 0.206; 0.687 |
| Linearity range | 31 a 244 | 5.3 a 42 | 9.8 a 77 | 0.69 a 208 |
| Performance Parameters | ||||
| Recovery | 92–93% | 91–111% | 95–100% | 90–110% |
| Precision | 2–5% | 3–9% | 2–3% | 1–2% |
| Accuracy | 0.001–0.19 | 0.9–1.6 | 0.02–0.63 | 0.01–0.08 |
| Milk sample | ([Zn2+] ± s )a mg/g | ([Pb2+] ± s ) a mg/kg | ([Cd2+] ±s ) a mg/kg | ([Ca2+] ± s) a mg/g |
|---|---|---|---|---|
| Ninho +1 (L1) b | 0.0647 ± 0.0035 | 3.7084 ± 0.5819 | --------- | 5.719 ± 0.128 |
| Nestogeno2 (L2) b | 0.0535 ± 0.0019 | 2.7971 ± 1.0983 | --------- | 4.430 ± 0.094 |
| Ninho +3 (L3) | 0.0528 ± 0.0028 | 1.7148 ± 0.7589 | 0.0103±0.0000 | 5.459 ± 0.241 |
| Nestogeno 1 (L4) b | 0.0559 ± 0.0011 | 2.5986 ± 1.2346 | --------- | 4.461 ± 0.296 |
| NAN A.R (L5) b | 0.0505 ± 0.0095 | 1.2271 ± 0.0327 | --------- | 5.432 ± 0.333 |
| NAN sem lactose(L6) b | 0.0568 ± 0.0005 | --------- | --------- | 6.750 ± 0.303 |
| NAN 2 (L7) b | 0.0550 ± 0.0030 | --------- | --------- | 6.378 ± 0.339 |
| Bebelac 1 (L8) b | 0.0712 ± 0.0120 | --------- | --------- | 6.605 ± 0.371 |
| Aptamil 1 (L9) b | 0.1200 ± 0.0079 | --------- | --------- | 5.597 ± 0.224 |
| Bebelac 2 (L10) b | 0.0424 ± 0.0020 | --------- | --------- | 4.792 ± 0.279 |
| Nestogeno Plus (L11) b | 0.0298 ± 0.0060 | 3.3199 ± 1.5270 | --------- | 6.147 ± 0.440 |
| Aptamil 2 (L12) b | 0.0205 ± 0.0023 | --------- | --------- | 3.573 ± 0.200 |
| Elegê kids (L13) b | 0.1111 ± 0.0206 | 0.3251 ± 0.2041 | --------- | 6.605 ± 0.301 |
| Ninho (L14) b | 0.0285 ± 0.0029 | 0.1586 ± 0.0704 | --------- | 6.584 ± 0.460 |
| Glória integral (L15) | 0.0241 ± 0.0020 | 0.2559 ± 0.1492 | --------- | 13.150 ± 0.182 |
| Itambé Desnatado (L16) | 0.0901 ± 0.0109 | --------- | --------- | 11.785 ± 0.027 |
| Ninho +6 (L17) b | 0.0237 ± 0.0006 | 0.1964 ± 0.0000 | --------- | 9.370 ± 0.337 |
| Molico desnatado (L18) | 0.0242 ± 0.0014 | --------- | --------- | 12.078 ± 0.352 |
| Glória desnatado (L19) | 0.0310 ± 0.0073 | --------- | --------- | 13.107 ± 0.470 |
| Itambé integral (L20) | 0.0286 ± 0.0003 | 0.1516 ± 0.0670 | --------- | 15.825 ± 0.395 |
| Elegê integral (L21) | 0.0284 ± 0.0061 | 0.1235 ± 0.0858 | --------- | 13.146 ± 0.222 |
| Leo (L22) | 0.0348 ± 0.0039 | --------- | --------- | 14.322 ± 0.529 |
| Carrefour (L23) | 0.0276 ± 0.0029 | --------- | --------- | 14.377 ± 0.796 |
| Milk sample | ([Zn2+] ± s ) a mg/L | ([Pb2+] ± s ) a mg/L | ([Cd2+] ± s ) a mg/L | ([Ca2+] ± s ) a g/L |
|---|---|---|---|---|
| Parmalat integral (L24) | 11.6525 ± 1.2498 | --------- | --------- | 3.617 ± 0.236 |
| Parmalat calcio plus (L25) | 13.0846 ± 0.4000 | --------- | --------- | 1.681 ± 0.100 |
| Parmalat Premium int. (L26) | 3.3773 ± 0.2244 | 0.1375 ± 0.0090 | --------- | 2.577 ± 0.182 |
| Parmalat Premium desn. (L27) | 3.3624 ± 0.5214 | 0.1593 ± 0.0065 | --------- | 1.365 ± 0.070 |
| Parmalat extra Premium (L28) | 4.4896 ± 0.6655 | 0.0618 ± 0.0208 | --------- | 2.423 ± 0.178 |
| Dietlat cálcio plus (L29) | 3.1960 ± 0.0046 | 0.0112 ± 0.0002 | 0.0720 ± 0.0000 | 4.019 ± 0.286 |
| Dietlat desnatado (L30) | 6.8179 ± 0.7231 | 0.2441 ± 0.0929 | 0.0330 ± 0.0000 | 2.118 ± 0.114 |
| Parmalat Zymil (L31) | 5.7937 ± 1.7356 | 0.0927 ± 0.0163 | 0.0171 ± 0.0023 | 2.119 ± 0.072 |
| Batavo sensy (L32) | 4.0326 ± 0.1260 | 0.0766 ± 0.0251 | 0.0573 ± 0.0300 | 1.926 ± 0.065 |
| Batavo cálcio light (L33) | 4.0046 ± 0.2861 | 0.1211 ± 0.0736 | 0.0173 ± 0.0018 | 1.541 ± 0.076 |
| Batavo ferro (L34) | 4.3945 ± 0.2825 | --------- | 0.0083 ± 0.0043 | 1.092 ± 0.083 |
| Carrefour semidesnatado (L35) | 3.0400 ± 0.1791 | 0.0809 ± 0.0313 | --------- | 1.086 ± 0.074 |
| Batavo semidesnatado (L36) | 3.3878 ± 0.1851 | 0.1059 ± 0.0254 | 0.0017 ± 0.0000 | 1.595 ± 0.084 |
| Piracanjuba desnatado (L37) | 2.8128 ± 0.7274 | 0.1111 ± 0.0281 | 0.0071 ± 0.0000 | 0.919 ± 0.086 |
| Compleite integral (L38) | 1.8559 ± 0.3373 | 0.0178 ± 0.0004 | 0.0571 ± 0.0011 | 1.020 ± 0.082 |
| Compleite desnatado (L39) | 3.1868 ± 0.4768 | 0.0981 ± 0.0210 | 0.0058 ± 0.0000 | 2.826 ± 0.175 |
| Batavo integral (L40) | 2.6017 ± 0.1867 | 0.0630 ± 0.0085 | --------- | 1.383 ± 0.084 |
| Leitbom desnatado (L41) | 1.8538 ± 0.2670 | 0.1237 ± 0.0056 | --------- | 1.682 ± 0.092 |
| Parmalat semidesnatado (L42) | 2.6025 ± 0.1138 | 0.1050 ± 0.0075 | 0.0186 ± 0.0000 | 1.449 ± 0.106 |
| Itambé semidesnatado (L43) | 3.4107 ± 0.3615 | 0.0569 ± 0.0055 | 0.0412 ± 0.0000 | 1.105 ± 0.091 |
| Italac light (L44) | 1.6272 ± 0.8564 | --------- | 0.0060 ± 0.0024 | 1.145 ± 0.028 |
| Carrefour integra (L45) | 2.1363 ± 0.0491 | --------- | 0.0060 ± 0.0044 | 0.660 ± 0.056 |
| Piracanjuba integral (L46) | 2.8251 ± 0.1588 | 0.1089 ± 0.0725 | 0.0157 ± 0.0069 | 0.998 ± 0.028 |
| Marajoara desnatado (L47) | 1.7179 ± 0.3872 | 0.0866 ± 0.0243 | 0.0321 ± 0.0000 | 0.443 ± 0.033 |
| Carrefour desnatado (L48) | 2.0203 ± 0.1637 | 0.0752 ± 0.0213 | 0.0441 ± 0.0000 | 0.244 ± 0.005 |
| Batavo desnatado (L49) | 3.2939 ± 0.3178 | 0.0879 ± 0.0346 | 0.0129 ± 0.0044 | 0.631 ± 0.012 |
| Parmalat ferro (L50) | 11.2455 ± 0.9657 | 0.0231 ± 0.0048 | 0.0141 ± 0.0033 | 0.969 ± 0.058 |
| Leitbom integral (L51) | 1.9374 ± 0.6540 | 0.1034 ± 0.0320 | 0.0771 ± 0.0052 | 0.610 ± 0,035 |
| Manacá light (L52) | 6.1996 ± 0.5509 | --------- | 0.0091 ± 0.0016 | 0.503 ± 0.027 |
| Paulista semidesnatado (L53) | 10.4534 ± 0.0050 | --------- | 0.0040 ± 0.0004 | 0.405 ± 0.036 |
| Extra integral (L54) | 2.5893 ± 0.3633 | 0.1271 ± 0.0714 | 0.0121 ± 0.0049 | 0.712 ± 0.038 |
| Escolha econômica desn. (L55) | 2.8792 ± 0.9558 | --------- | --------- | 0.281 ± 0.025 |
| Component | Variance (%) | Cumulative variance (%) |
|---|---|---|
| 1 | 47.84 | 47.84 |
| 2 | 23.12 | 70.96 |
| 3 | 19.02 | 89.97 |
| 4 | 10.03 | 100.00 |
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Castro, C.S.P.D.; Arruda, A.F.; Cunha, L.R.D.; SouzaDe, J.R.; Braga, J.W.B.; Dórea, J.G. Toxic Metals (Pb and Cd) and Their Respective Antagonists (Ca and Zn) in Infant Formulas and Milk Marketed in Brasilia, Brazil. Int. J. Environ. Res. Public Health 2010, 7, 4062-4077. https://doi.org/10.3390/ijerph7114062
Castro CSPD, Arruda AF, Cunha LRD, SouzaDe JR, Braga JWB, Dórea JG. Toxic Metals (Pb and Cd) and Their Respective Antagonists (Ca and Zn) in Infant Formulas and Milk Marketed in Brasilia, Brazil. International Journal of Environmental Research and Public Health. 2010; 7(11):4062-4077. https://doi.org/10.3390/ijerph7114062
Chicago/Turabian StyleCastro, Clarissa S. P. De, Andréa F. Arruda, Leandro R. Da Cunha, Jurandir R. SouzaDe, Jez W. B. Braga, and José G. Dórea. 2010. "Toxic Metals (Pb and Cd) and Their Respective Antagonists (Ca and Zn) in Infant Formulas and Milk Marketed in Brasilia, Brazil" International Journal of Environmental Research and Public Health 7, no. 11: 4062-4077. https://doi.org/10.3390/ijerph7114062






