Acetaldehyde Removal from Indoor Air through Chemical Absorption Using L-Cysteine
Abstract
:1. Introduction
2. Experimental Section
2.1. Bubbling Method
2.1.1. Amino acids
2.1.2. Experimental apparatus
2.1.3. PTR-MS
2.2. Bag Method
3. Results and Discussion
3.1. Inlet Concentration
3.2. Bubbling Method
3.3. Bag Method
4. Conclusions
Acknowledgments
References
- The International Programme on Chemical Safety Environmental Health Criteria Acetaldehyde; World Health Organization: Geneva, Switzerland, 1995.
- Re-evaluation of Some Organic Chemicals, Hydrazine and Hydrogen Peroxide. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; International Agency for Research on Cancer: Lyon, France, 1999; Volume 77, pp. 319–335.
- Miyamoto, K; Tohmura, S; Inoue, A. Aldehyde and volatile organic compound emissions from laminated veneer lumber (in Japanese). Mokuzai Gakkaishi 2006, 52, 113–118. [Google Scholar]
- Zhang, J; Smith, KR. Emission of carbonyl compounds from various cookstoves in China. Environ. Sci. Technol 1999, 33, 2311–2320. [Google Scholar]
- Löefroth, G; Burton, RM; Forehand, L; Hammond, SK; Seila, RL; Zweidinger, RB; Lewtas, J. Characterization of environmental tobacco smoke. Environ. Sci. Technol 1998, 23, 610–614. [Google Scholar]
- Jones, AW. Measuring and reporting the concentration of acetaldehyde in human breath. Alcohol Alcoholism 1995, 30, 271–285. [Google Scholar]
- Summarized Report of Field Survey on Actual IAQ in Japan in 2005 (in Japanese); Center for Housing Renovation and Dispute Settlement Support: Tokyo, Japan, 2005.
- Okubo, M; Yamamoto, T; Kuroki, T; Fukumoto, H. Electric air cleaner composed of nonthermal plasma reactor and electrostatic precipitator. IEEE Trans. Ind. Appl 2001, 37, 1505–1511. [Google Scholar]
- Sano, N; Nagamoto, T; Tamon, H; Suzuki, T; Okazaki, M. Removal of acetaldehyde and skatole in gas by a corona-discharge reactor. Ind. Eng. Chem. Res 1997, 36, 3783–3791. [Google Scholar]
- Lee, A; Goldstein, AH; Keywood, MD; Gao, S; Varutbangkul, V; Bahreini, R; Ng, NL; Flagan, RC; Seinfeld, JH. Gas-phase products and secondary aerosol yields from the ozonolysis of ten different terpenes. J. Geophys. Res 2006, 111, D07302. [Google Scholar]
- Mo, JH; Zhang, YP; Xu, Q; Lamson, JJ; Zhao, R. Photocatalytic purification of volatile organic compounds in indoor air: A literature review. Atmos. Environ 2009, 43, 2229–2246. [Google Scholar]
- Ollis, DF. Photocatalytic purification and remediation of contaminated air and water. C. R. Acad. Sci. Ser. III C 2000, 3, 405–411. [Google Scholar]
- Obuchi, E; Sakamoto, T; Nakano, K; Shiraishi, F. Photocatalytic decomposition of acetaldehyde over TiO2/SiO2 catalyst. Chem. Eng. Sci 1999, 54, 1525–1530. [Google Scholar]
- Dimotakis, ED; Cal, MP; Economy, J; Rood, MJ; Larson, SM. Chemically treated activated carbon cloths for removal of volatile organic carbons from gas streams: Evidence for enhanced physical adsorption. Environ. Sci. Technol 1995, 29, 1876–1880. [Google Scholar]
- El-Sayed, Y; Bandosz, TJ. A study of acetaldehyde adsorption on activated carbons. J. Colloid Interface Sci 2001, 242, 44–51. [Google Scholar]
- El-Sayed, Y; Bandosz, TJ. Acetaldehyde adsorption on nitrogen-containing activated carbons. Langmuir 2002, 18, 3213–3218. [Google Scholar]
- Hayashi, T; Kumita, M; Otani, Y. Removal of acetaldehyde vapor with impregnated activated Carbons: Effects of steric structure on impregnant and acidity. Environ. Sci. Technol 2005, 39, 5436–5441. [Google Scholar]
- Parodi, S; Flora, SD; Cavanna, M; Pino, A; Robbiano, L; Bennicelli, C; Brambilla, G. DNA-damaging activity in vivo and bacterial mutagenicity of sixteen hydrazine derivatives as related quantitatively to their carcinogenicity. Cancer Res 1981, 41, 1469–1482. [Google Scholar]
- Lindinger, W; Hansel, A; Jordan, A. On-line monitoring of volatile organic compounds at pptv levels by means of Proton-Transfer-Reaction Mass Spectrometry (PTR-MS) medical applications, food control and environmental research. Int. J. Mass Spectrom. Ion Proc 1998, 173, 191–241. [Google Scholar]
- Carey, FA; Sundberg, RJ. Advanced Organic Chemistry Part A: Structure and Mechanisms; Springer: Heidelberg, Germany, 2007. [Google Scholar]
- Friedman, M. The Chemistry and Biochemistry of the Sulfhdryl Group in Amino Acids, Peptides and Proteins; Pergamon press: London, UK, 1973; pp. 88–90. [Google Scholar]
- Salaspuro, V; Hietala, J; Kaihovaara, P; Pihlajarinne, L; Marvola, M; Salaspuro, M. Removal of acetaldehyde from saliva by a slow-release buccal tablet of L-cysteine. Int. J. Cancer 2002, 97, 361–364. [Google Scholar]

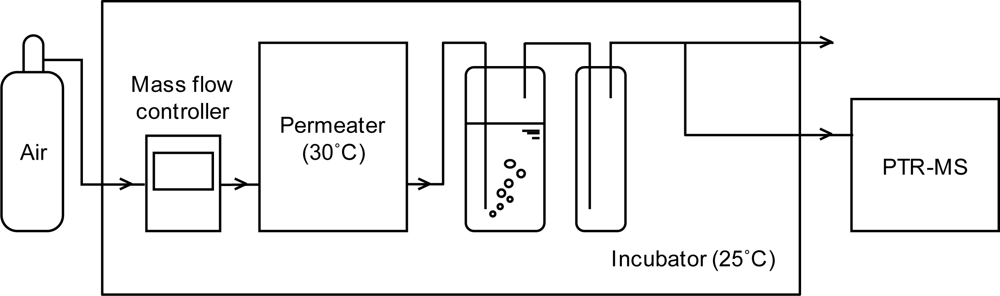

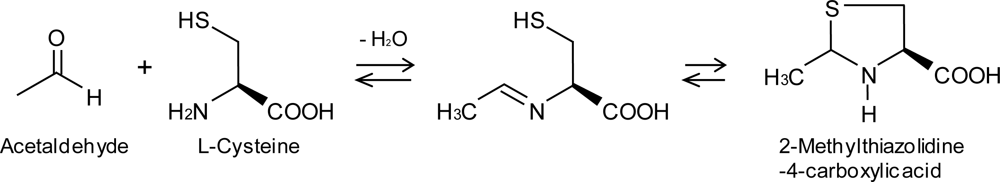
| Substance | n | Inlet concentration[ppm] |
|---|---|---|
| Water | 3 | 1.59–1.67 |
| Glycine | 3 | 1.66–1.67 |
| L-Lysine | 1 | 1.11 |
| L-Methionine | 1 | 1.02 |
| L-Cysteine | 3 | 1.58–1.59 |
| L-Cystine | 3 | 1.66–1.68 |
| Operation mode | Multiple Ion Detecting (MID) mode |
| Detected ion | m/z = 45 (acetaldehyde) |
| Reaction rate constant | 3.6 × 10−9 cm3 molecule−1 s−1 |
| Dwell time | 60 s |
| Sample solution | Removal rate [%] |
|---|---|
| Water | 55 ± 5 |
| Glycine | 52 ± 0.2 |
| l-Lysine | 64 |
| l-Methionine | 58 |
| l-Cysteine | 91 ± 0.4 |
| l-Cystine | 50 ± 1 |
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Yamashita, K.; Noguchi, M.; Mizukoshi, A.; Yanagisawa, Y. Acetaldehyde Removal from Indoor Air through Chemical Absorption Using L-Cysteine. Int. J. Environ. Res. Public Health 2010, 7, 3489-3498. https://doi.org/10.3390/ijerph7093489
Yamashita K, Noguchi M, Mizukoshi A, Yanagisawa Y. Acetaldehyde Removal from Indoor Air through Chemical Absorption Using L-Cysteine. International Journal of Environmental Research and Public Health. 2010; 7(9):3489-3498. https://doi.org/10.3390/ijerph7093489
Chicago/Turabian StyleYamashita, Kyoko, Miyuki Noguchi, Atsushi Mizukoshi, and Yukio Yanagisawa. 2010. "Acetaldehyde Removal from Indoor Air through Chemical Absorption Using L-Cysteine" International Journal of Environmental Research and Public Health 7, no. 9: 3489-3498. https://doi.org/10.3390/ijerph7093489




