Screening of Oomycete Fungi for Their Potential Role in Reducing the Biting Midge (Diptera: Ceratopogonidae) Larval Populations in Hervey Bay, Queensland, Australia
Abstract
:1. Introduction
2. Experimental Section
2.1. Sampling Sites
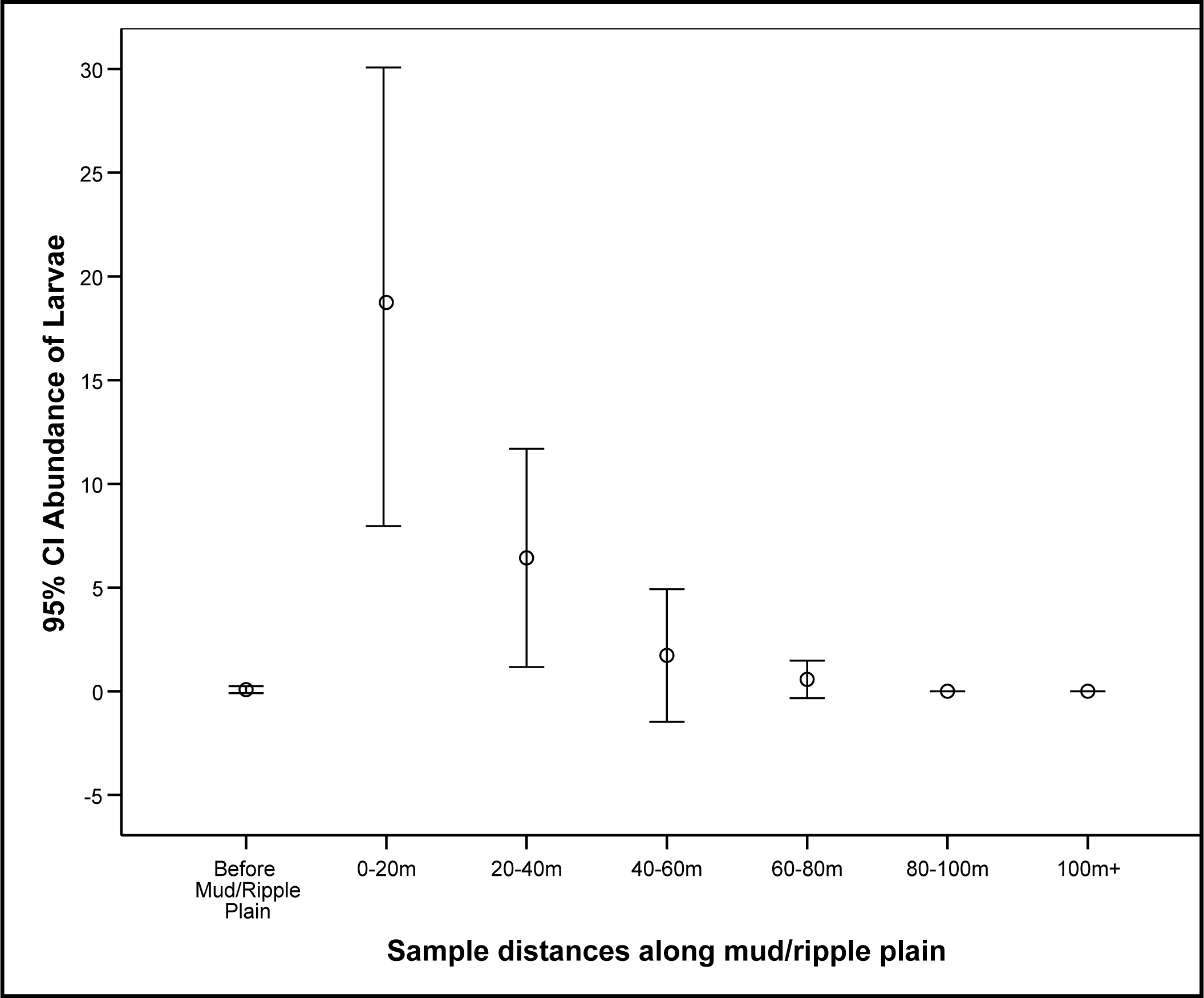
2.2. Larval Sampling and Extraction
2.3. Larval Identification
2.4. Fungal Isolations and Identifications
2.5. Insecticidal Bioassay
2.6. Statistical Analysis
3. Results
3.1. Detection of Larvae
3.2. Fungal Isolations and Identifications
3.3. Bioassays
4. Discussion and Conclusions
Acknowledgments
References
- Unkles, SE; Marriott, C; Kinghorn, JR; Panter, C; Blackwell, A. Efficacy of the Entomopathogenic Fungus, Culicinomyces clavisporus against larvae of the Biting Midge, Culicoides nubeculosus (Diptera: Ceratopogonidae). Biocontrol Sci. Techn 2004, 14, 397–401. [Google Scholar]
- Shivas, M; Whelan, P; Webb, C. The characterization of emergence sites of the biting midge Culicoides ornatus (Diptera: Ceratopogonidae) in mangroves near Darwin, NT, Australia. Arbovirus Res. Aust 1997, 7, 275–279. [Google Scholar]
- Shivas, M; Whelan, P; Webb, C. The larval biology of Culicoides ornatus (Ceratopogonidae: Diptera) in mangroves near Darwin, Northern Territory, Australia. Supp. Bull. Mosquito Contr. Assoc. Aust 1998, 10, 23–25. [Google Scholar]
- Reye, EJ. The Problems of Biting Midges (Diptera: Ceratopogonidae) in Queensland. Entomol. Soc. Queensland 1964, 3, 1–6. [Google Scholar]
- Wheland, P. Biting Midges or “Sandflies” in the NT. North. Territ. Dis. Control Bull 2003, 10, 1. [Google Scholar]
- Kettle, DS; Reye, EJ; Edwards, PB. Distribution of Culicoides Molestus (Skuse) (Diptera: Ceratopogonidae) in man-made canals in South-eastern Queensland. Aust. J. Mar. Fresh. Res 1979, 30, 653–660. [Google Scholar]
- Wright, PJ; Easton, CS. Natural incidence of Lagenidium giganteum Couch (Oomycetes: Lagenidiales) infecting the biting midge Culicoides molestus (Skuse) (Diptera: Ceratopogonidae). Aust. J. Entomol 1996, 35, 131–134. [Google Scholar]
- Edwards, PB. Seasonal changes in Larval Populations of Culicoides subimmaculatus Lee and Reye (Diptera: Ceratopogonidae), with observations on the influence of tides on larval Ecology. Aust. J. Mar. Fresh. Res 1989, 40, 69–78. [Google Scholar]
- Mellor, PS; Boorman, J; Baylis, M. Culicoides Biting Midges: Their Role as Arbovirus Vectors. Annu. Rev. Entomol 2000, 45, 307–340. [Google Scholar]
- Drolet, BS; Campbell, CL; Stuart, MA; Wilson, WC. Vector competence of Culicoides sonorensis (Diptera: Ceratopogonidae) for vesicular stomatitits virus. J. Med. Entomol 2005, 42, 409–418. [Google Scholar]
- Breard, E; Hamblin, C; Hammoumi, S; Sailleau, C; Dauphin, G; Zientara, S. The epidemiology and diagnosis of bluetongue with particular reference to Corsica. Vet. Res. Sci 2004, 77, 1–8. [Google Scholar]
- Schmidtmann, ET; Bobian, BJ; Belden, RP. Soil chemistries define aquatic habitats with immature populations of the Culicoides variipennis complex (Diptera: Ceratopogonidae). J. Med. Entomol 2000, 37, 58–61. [Google Scholar]
- Jones, RH; Roughton, RD; Foster, NM; Bando, BM. Culicoides, The Vector of Epizootic Hemorrhagic Disease in White-tailed Deer in Kentucky in 1971. J. Wildlife Dis 1977, 13, 2–8. [Google Scholar]
- Garvin, MC; Greiner, EC. Ecology of Culicoides (Diptera: Ceratopogonidae) in South Central Florida and experimental Culicoides vectors of the avian hematozoan Haemoproteus Danilewskyi Kruse. J. Wildlife Dis 2003, 30, 170–178. [Google Scholar]
- Shivas, MA. Hervey Bay Biting Midge Review 2001—Previous Studies and Research Priorities; Report to Hervey Bay City Council: Queensland, Australia, 2001; p. 41.
- Tweed Heads Shire Council. Mosquitoes & biting midges (sandflies) in the Tweed Shire. Available online: http://www.tweed.nsw.gov.au/YourEnvironment/eh_1_mosquit.htm (accessed on 20 April 2011).
- Perich, MJ; Strickman, D; Wirtz, RA; Stockwell, JL; Burge, R; Hunt, G; Lawyer, PG. Field Evaluation of Four Repellents against Leptoconops americanus (Diptera: Ceratopogonidae) Biting Midges. J. Med. Entomol 1995, 32, 306–309. [Google Scholar]
- Webb, CE; Russell, RC. Living with Mosquitoes in the Lower Hunter & Mid North Coast Region of NSW; Premiers Department: Sydney, Australia 2005. , 2nd ed Available online: http://www.hnehealth.nsw.gov.au/__data/assets/pdf_file/0020/63470/Living_With_Mosquitoes_-_2nd_Edition_-_DECEMBER_2009.pdf (accessed on 20 April 2011).
- Royle, A. A new tool for the control of mosquitoes, biting midges and flies. Wing Beats Fla. Mosq. Control Assoc 2004, 15, 18–19. [Google Scholar]
- Wheland, P. Biting midges or “sandflies” in the NT. North. Territ. Dis. Control Bull 2003, 10, 1–12. [Google Scholar]
- Mishra, SK; Keller, JE; Miller, JR; Heisey, RM; Nair, MG; Putnam, AR. Insecticidal and nematicidal properties of microbial metabolites. J. Ind. Microbiol 1987, 2, 267–276. [Google Scholar]
- Strasser, H; Vey, A; Butt, TM. Are there any risks in using entomopathogenic fungi for pest control, with particular reference to the bioactive metabolites of Metarhizium, Tolypocladium and Beauveria species? Biocontrol Sci. Techn 2000, 10, 717–735. [Google Scholar]
- Lacey, IA; Frutos, R; Kaya, HK; Vail, P. Insect pathogens as biological control agents: Do they have a future? Biol. Control 2001, 21, 230–248. [Google Scholar]
- Scholte, EJ; Knols, BGJ; Takken, W. Autodissemination of the entomopathogenic fungus Metarhizium anisopliae amongst adults of the malaria vector Anopheles gambiae. Malaria J 2004, 3, 45. [Google Scholar]
- Jackson, MA. Optimizing nutritional conditions for the liquid culture production of effective fungal biological control agents. J. Ind. Microbiol. Biot 1997, 19, 180–187. [Google Scholar]
- Sah, PA; Pell, JK. Entomopathogenic fungi as biological control agents. Appl. Microbiol. Biot 2003, 61, 431–423. [Google Scholar]
- Peterson, EE; Semon, MJ; Kerwin, JL; Brower, JM. Regulation of attachment, germination, and apressorium formation by zoospores of Lagenidium giganteum and related Oomycetes by chitin, chitosan, and catecholamines. Protoplasma 1997, 197, 96–110. [Google Scholar]
- Newell, SY; Fell, JW. Competition among mangrove Oomycetes, and between Oomycetes and other microbes. Aquat. Microb. Ecol 1997, 12, 21–28. [Google Scholar]
- Pegg, KG; Foresberg, LI. Phytophthora in Queensland Mangroves. Wetlands 1982, 1, 2–3. [Google Scholar]
- Brey, PT; Remaudiere, G. Recognition and isolation of Lagenidium giganteum Couch. Bull. Soc. Vec. Ecol 1985, 10, 90–97. [Google Scholar]
- Deacon, J. Fungal Biology, 4th ed; Blackwell Publishing: Carlton Vic, Australia, 2006; pp. 16–47. [Google Scholar]
- Goudie, A. Geomophological Techniques; George Allen and Unwin: London, UK, 1981. [Google Scholar]
- Hribar, LJ. A review of methods for recovering biting midge larvae (Diptera: Ceratopogonidae) from substrate samples. J. Agr. Entomol 1990, 7, 71–76. [Google Scholar]
- Drenth, A; Sendall, B. Practical Guide to Detection and Identification of Phytophthora, Version 10; CRC for Tropical Plant Protection: Brisbane Australia, 2001. [Google Scholar]
- Boggs, S. Principles of Sedimentology and Stratigraphy, 2nd ed; Prentice Hall: Upper Saddle River, NJ, USA, 1995; pp. 79–91. [Google Scholar]
- Eckert, JW. A selective antibiotic medium for isolation of Phytophthora and Pythium from plant roots. Phytopathology 1962, 52, 771–777. [Google Scholar]
- Principles and Applications of Soil Microbiology, 2nd ed; Sylvia, DM; Hartel, P; Fuhrmann, J; Zuberer, D (Eds.) Prentice Hall: Upper Saddle River, NJ, USA, 2005; p. 638.
- Gerrettson-Cornell, L; Simpson, J. Three new marine Phytophthora species from New South Wales. Mycotaxon 1984, 19, 453–470. [Google Scholar]
- Frankland, JC; Latter, PM; Poskitt, JM. A Laboratory Guide to Soil Microbiology: Some General Principles and Practice; Medewood Research and Development Paper Number 115; Institute of Terrestrial Ecology, Merlewood Research Station, Grange-over-Sands: Cumbria, UK, 1995. [Google Scholar]
- Sweeney, AW. The insect pathogenic fungus Culicinomyces in mosquitoes and other hosts. Aust. J. Zool 1975, 23, 59–64. [Google Scholar]
- Sur, B; Bihari, V; Sharma, A; Joshi, AK. Studies on physiology, zoospore morphology and entomopathogenic potential of the aquatic Oomycetes: Lagenidium giganteum. Mycopathologia 2001, 154, 51–54. [Google Scholar]
- Gibbs, JN. A study of the epiphytic growth habit of Fomes annosus. Ann. Bot 1967, 31, 755–774. [Google Scholar]
- Leaño, EM; Jones, EBG; Vrijmoed, LLP. Why Halophytophthora species are so well adapted to mangroves. Fungal Divers 2000, 5, 131–151. [Google Scholar]
- Neller, DA; Howie, J. The Public Health Impact of Biting Midge in Hervey Bay; Report Prepared for Hervey Bay City Council: Hervey Bay, Queensland, Australia, 2003. [Google Scholar]
- Neller, R. The Public Health Impact of Biting Midge in Hervey Bay; Report Prepared for Hervey Bay City Council: Hervey Bay, Queensland, Australia, 2003. [Google Scholar]
- Nakagiri, A. Ecology and biodiversity of Halophytophthora species. Fungal Divers 2000, 5, 153–164. [Google Scholar]
- Fungal Ecology, 1st ed; Dix, NJ; Webster, J (Eds.) Chapman and Hall: London, UK, 1995.
- Intertidal Ecology; Raffaelli, D; Hawkins, S (Eds.) Chapman and Hall: London, UK, 1996.




| Location | Transect | Type of location | Substrate type | Vegetation | Mictyris Crab | Larvae |
|---|---|---|---|---|---|---|
| River Heads | 007 | Open Beach (West) | Mud-flat | Minimal mangrove fringe | Present in sandy area | 0 |
| 019 | Open Beach (East) | Sand/mud zone, followed by mud-flat to water’s edge | Dense mangrove forest | Present throughout inner zone of mangrove forest | 44 | |
| 020 | Open Beach (West) | Short sandy-flat rapidly changing into a mud-flat | Moderately dense mangrove fringe | Present in sandy area | 196 | |
| 021 | Open Beach (West) | Short sandy-flat rapidly changing into a mud-flat | Moderately dense mangrove fringe | Present in sandy area | 75 | |
| Urangan | 001 | Open Beach | Short coarse sand-flat moving into rocky plain | None | Absent | 0 |
| 002 | Open Beach | Short coarse sand-flat moving into rocky plain | None | Absent | 0 | |
| 003 | Open Beach | Sandy/Mud-flat | Moderately dense mangrove fringe | Minimal within mangrove fringe | 0 | |
| 018 | Tidal Creek | Sandy tidal creek beach fore-dune | Mangroves along edge of creek | Dense throughout transect | 19 | |
| Eli Creek | 004 | Open Beach | Rocky shore followed by extensive sandy flat | None | Absent | 1 |
| 006 | Estuarine | Deep mud | Dense mangroves surrounding site | Absent | 0 | |
| 008 | Open Beach | Coarse sandy beach with large ripple plain | None | Absent | 0 | |
| 009 | Open Beach | Sand-flat with mud increasing in presence | Scattered mangroves throughout mud-flats | Absent | 9 | |
| 010 | Estuarine | Mud-flats with moderate sand present | Moderately dense mangrove presence throughout mud-flats | Extensive, close to start of mud-flat, scattered throughout | 110 | |
| 011 | Estuarine | Mud-flats with moderate sand present | Scattered mangroves throughout mud-flats | Extensive, close to start of mud-flat, scattered throughout | 102 | |
| 012 | Estuarine | Mud-flat | Dense mangrove forest throughout mud-flats | Scattered | 25 | |
| 013 | Estuarine | Steep short mud-bank | Scattered mangroves present | Scattered throughout | 15 | |
| 014 | Estuarine | Steep short mud-bank | Moderately dense mangroves present | Scattered throughout | 55 | |
| 015 | Estuarine | Island within Eli Creek, mud-flats | Moderately dense mangroves present | Absent | 133 | |
| 016 | Estuarine | Large meander bend flanked by Eli Creek, mud-banks | Four distinct zones of mangrove succession, dense mangrove presence | Some present | 304 | |
| 017 | Estuarine | Transition of sand to mud-flats | Mangroves changing in density throughout transect | Extensive, close to start of mud-flat, scattered throughout | 1202 | |
| Beelbi | 005 | Estuarine | Sandy mud | Mangrove fringe | Absent | 0 |
| Location | Transect | Isolate numbers |
|---|---|---|
| River Heads | 019 | USC-019-A2; USC-019-A3; USC-019-A4; USC-019-C1; USC-019-C2; USC-019-C3 |
| 020 | USC-020-A3; USC-020-C1; USC-020-C2 | |
| 021 | USC-021-A1; USC-021-A3; USC-021-B2; USC-021-C1; USC-021-C2; USC-021-C4 | |
| Urangan | 001 | USC-001-1; USC-001-2 |
| Eli Creek | 004 | USC-004-1 |
| 013 | USC-013-A1; USC-013-A2 | |
| 014 | USC-014-1 | |
| 016 | USC-016-C1; USC-016-C2; USC-016-B1; USC-016-B2 | |
| Beelbi Creek | 005 | USC-005-1 |
| Variables | Fungi isolated | Fungi not isolated | ||
|---|---|---|---|---|
| Median | Range | Median | Range | |
| Salinity (ppt) | 36.43 | 29.34–39.01 | 29.88 | 16.93–39.01 |
| Temperature (°C) | 20.45 | 19.2–23.4 | 24.10 | 19.1–26.8 |
| pH | 7.91 | 7.72–8.11 | 7.6 | 7.47–8.11 |
| Isolate code | Effect on the larvae |
|---|---|
| Control (no fungal inoculum present in the larval growth environment) | Larvae alive and active |
| Reference strain-73864(Pythium prolatum)* | Dead pupae, surrounded by mycelia, no invasion or outgrowth visible. Larvae alive |
| Reference strain-50182(Halophytophthora batemanensis)* | Dead pupae, surrounded by mycelia, no invasion or outgrowth visible. Larvae alive |
| Reference strain-76023 (Phytophthora gonapodyides)* | Dead pupae with visible mycelia growth (day 3), microscopic examination shows pupae consumed by fungi with significant fungal outgrowth from exoskeleton around the thoracic region. Larvae alive |
| Halophytophthora isolate-USC-005-1 | Pupae alive with mycelia growth attached. Larvae alive. |
| Halophytophthora isolate-USC-019-C3 | Pupae alive with mycelia growth attached. Larvae alive. |
| Halophytophthora isolate-USC-021-C4 | Two dead pupae, mycelia growth around thoracic segments of both pupae. Larvae alive. |
| Halophytophthora | Total | ||||
|---|---|---|---|---|---|
| Absent | Present | ||||
| Larval sites showing Presence/Absence of larvae | Absent | Count | 11 | 5 | 16 |
| % within Presence or Absence of Larvae | 68.8% | 31.3% | 100.0% | ||
| Present | Count | 2 | 2 | 4 | |
| % within Presence or Absence of Larvae | 50.0% | 50.0% | 100.0% | ||
| Total | Count | 13 | 7 | 20 | |
| % within Presence or Absence of Larvae | 65.0% | 35.0% | 100.0% | ||
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Stephen, K.; Kurtböke, D.I. Screening of Oomycete Fungi for Their Potential Role in Reducing the Biting Midge (Diptera: Ceratopogonidae) Larval Populations in Hervey Bay, Queensland, Australia. Int. J. Environ. Res. Public Health 2011, 8, 1560-1574. https://doi.org/10.3390/ijerph8051560
Stephen K, Kurtböke DI. Screening of Oomycete Fungi for Their Potential Role in Reducing the Biting Midge (Diptera: Ceratopogonidae) Larval Populations in Hervey Bay, Queensland, Australia. International Journal of Environmental Research and Public Health. 2011; 8(5):1560-1574. https://doi.org/10.3390/ijerph8051560
Chicago/Turabian StyleStephen, Kirsty, and D. Ipek Kurtböke. 2011. "Screening of Oomycete Fungi for Their Potential Role in Reducing the Biting Midge (Diptera: Ceratopogonidae) Larval Populations in Hervey Bay, Queensland, Australia" International Journal of Environmental Research and Public Health 8, no. 5: 1560-1574. https://doi.org/10.3390/ijerph8051560





