Urinary Arsenic Metabolites of Subjects Exposed to Elevated Arsenic Present in Coal in Shaanxi Province, China
Abstract
:1. Introduction
2. Experimental Section
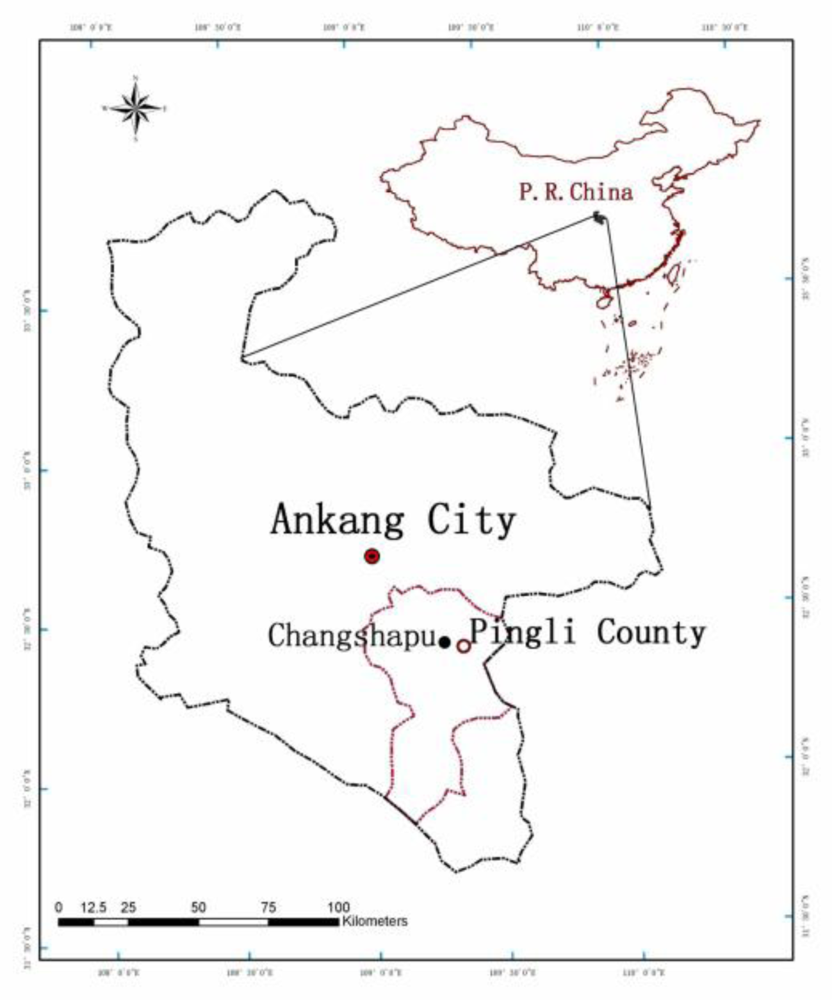
2.1. Study Area
2.2. Study Population
2.3. Sample Collection
2.4. Reagents and Standards
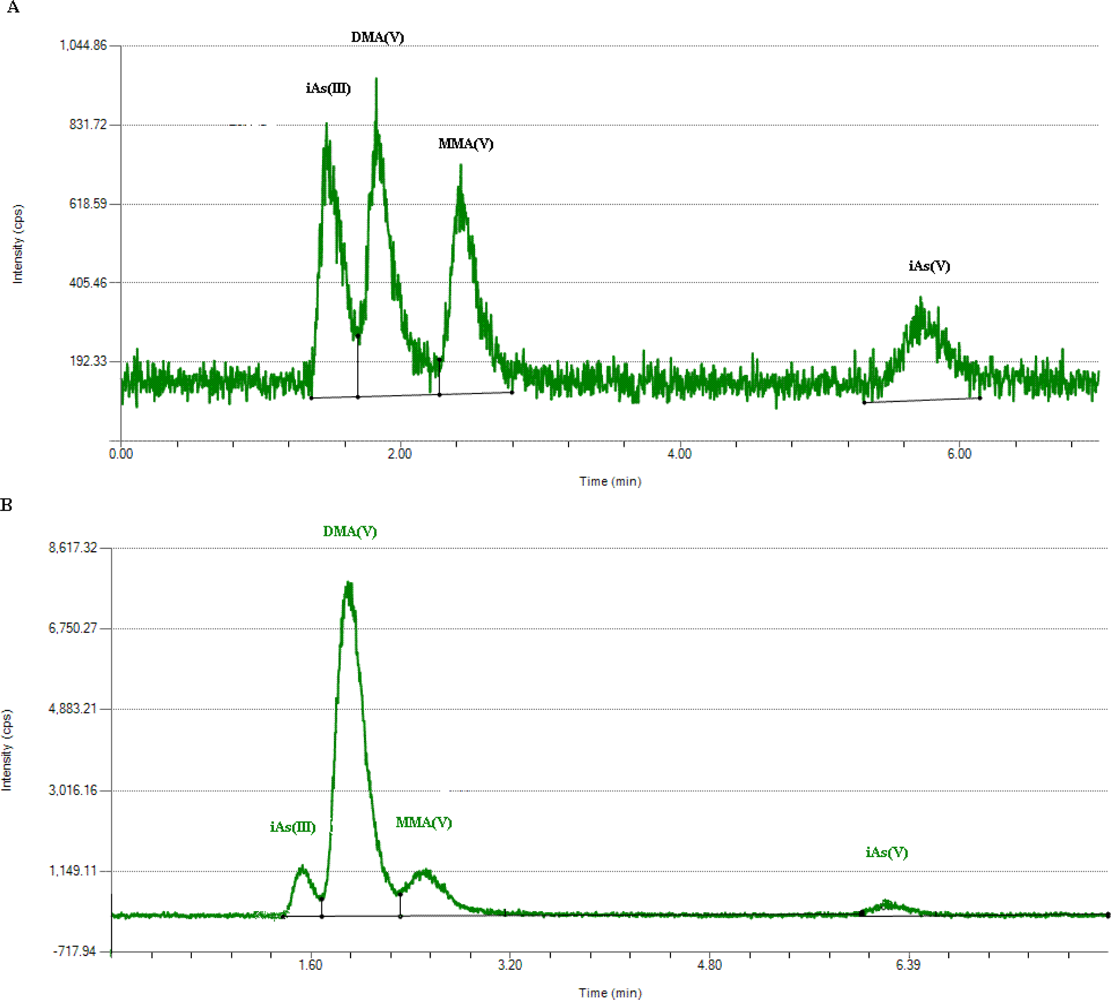
2.5. Determination of Arsenic Metabolites
2.6. Creatinine in Urine
2.7. Statistical Analysis
3. Results and Discussion
3.1. Urinary Arsenic Metabolites of the Study Population
3.2. Urinary Arsenic Metabolites between Subjects with and without Skin Lesions
3.3. Urinary Arsenic Metabolites among Men and Women
3.4. Relationship between Age and Urinary Arsenic Metabolites
3.5. Profiles of Urinary Arsenic Metabolites
3.6. Arsenic Metabolism and Arsenic-Induced Skin Lesions
3.7. Differences of Arsenic Metabolism between Men and Women
3.8. Arsenic Excretion and Metabolism in Different Age
3.9. Limitations to the Present Study
4. Conclusions
Acknowledgments
- Conflict of InterestThe authors declare no conflict of interest.
References and Notes
- Lindberg, AL; Rahman, M; Persson, LA; Vahter, M. The risk of arsenic induced skin lesions in Bangladeshi men and women is affected by arsenic metabolism and the age at first exposure. Toxicol. Appl. Pharmacol 2008, 230, 9–16. [Google Scholar]
- Ahsan, H; Chen, Y; Kibriya, MG; Slavkovich, V; Parvez, F; Jasmine, F; Gamble, MV; Graziano, JH. Arsenic metabolism, genetic susceptibility, and risk of premalignant skin lesions in Bangladesh. Cancer Epidemiol. Biomarkers Prev 2007, 16, 1270–1278. [Google Scholar]
- Hsueh, YM; Huang, YL; Huang, CC; Wu, WL; Chen, HM; Yang, MH; Lue, LC; Chen, CJ. Urinary levels of inorganic and organic arsenic metabolites among residents in an arseniasis-hyperendemic area in Taiwan. J. Toxicol. Environ. Health Part A 1998, 54, 431–444. [Google Scholar]
- Walton, FS; Harmon, AW; Paul, DS; Drobna, Z; Patel, YM; Styblo, M. Inhibition of insulin-dependent glucose uptake by trivalent arsenicals: Possible mechanism of arsenic-induced diabetes. Toxicol. Appl. Pharmacol 2004, 198, 424–433. [Google Scholar]
- Mushak, P; Crocetti, AF. Risk and revisionism in arsenic cancer risk assessment. Environ. Health Perspect 1995, 103, 684–689. [Google Scholar]
- Vahter, M; Concha, G. Role of metabolism in arsenic toxicity. Pharmacol. Toxicol 2001, 89, 1–5. [Google Scholar]
- Petrick, JS; Ayala-Fierro, F; Cullen, WR; Carter, DE; Vasken Aposhian, H. Monomethylarsonous acid (MMA(III)) is more toxic than arsenite in Chang human hepatocytes. Toxicol. Appl. Pharmacol 2000, 163, 203–207. [Google Scholar]
- Mandal, BK; Ogra, Y; Suzuki, KT. Identification of dimethylarsinous and monomethylarsonous acids in human urine of the arsenic-affected areas in West Bengal, India. Chem. Res. Toxicol 2001, 14, 371–378. [Google Scholar]
- Hopenhayn-Rich, C; Biggs, ML; Kalman, DA; Moore, LE; Smith, AH. Arsenic methylation patterns before and after changing from high to lower concentrations of arsenic in drinking water. Environ. Health Perspect 1996, 104, 1200–1207. [Google Scholar]
- Meza, MM; Kopplin, MJ; Burgess, JL; Gandolfi, AJ. Arsenic drinking water exposure and urinary excretion among adults in the Yaqui Valley, Sonora, Mexico. Environ. Res 2004, 96, 119–126. [Google Scholar]
- Del Razo, LM; Garcia-Vargas, GG; Vargas, H; Albores, A; Gonsebatt, ME; Montero, R; Ostrosky-Wegman, P; Kelsh, M; Cebrian, ME. Altered profile of urinary arsenic metabolites in adults with chronic arsenicism. A pilot study. Arch. Toxicol 1997, 71, 211–217. [Google Scholar]
- Sun, G; Xu, Y; Li, X; Jin, Y; Li, B; Sun, X. Urinary arsenic metabolites in children and adults exposed to arsenic in drinking water in Inner Mongolia, China. Environ. Health Perspect 2007, 115, 648–652. [Google Scholar]
- Li, Y. Relation between arsenic in environment and coal burning-born endemic arsenism in the south of Shaanxi province. Chin J Endemiol 2004, 23, 562–565. (in Chinese). [Google Scholar]
- Chen, P; Tang, XY. Arsenic in coal of China. Coal Geol Chin 2002, 14, 18–24. (in Chinese). [Google Scholar]
- Yudovich, YE. Arsenic in coal: A review. Int. J. Coal Geol 2005, 61, 141–196. [Google Scholar]
- Ministry of Health. Hygienic Standards for the Design of Industrial Enterprises, TJ 36–1979; Ministry of Health of the People’s Republic of China: Beijing, China, 1979. [Google Scholar]
- Ministry of Health. Maximum Levels of Contaminants in Foods, GB 2762-2005; Ministry of Health of the People’s Republic of China: Beijing, China, 2005. [Google Scholar]
- Ministry of Health. Standards for Drinking Water Quality, GB 5749-2006; Ministry of Health of the People’s Republic of China: Beijing, China, 2006. [Google Scholar]
- Bai, GL; Liu, XL; Fan, ZX; Li, XQ. Epldemiologic survey on coal-burning endemic arsenism in Shaanxi province. Chin J Endemiol 2006, 25, 57–60. (in Chinese). [Google Scholar]
- Tang, L. The Transport and Transformation of Airborne Arsenic from Burning Stone-Like Coal in Shaanxi; Master dissertation; China Capital Normal University: Beijing, China, 2009. (in Chinese) [Google Scholar]
- Ministry of Health. Stnadards of Diagnosis for Endemic Arsenism, WS/T 211-2001; Ministry of Health of the People’s Republic of China: Beijing, China, 2001. [Google Scholar]
- World Medical Association Declaration Of Helsinki—Ethical Principles for Medical Research Involving Human Subjects. Available online: http://www.wma.net/en/30publications/10policies/b3/17c.pdf (accessed on 30 May 2011).
- Cornelis, R; Heinzow, B; Herber, RF; Christensen, JM; Poulsen, OM; Sabbioni, E; Templeton, DM; Thomassen, Y; Vahter, M; Vesterberg, O. Sample collection guidelines for trace elements in blood and urine. J. Trace Elem. Med. Biol 1996, 10, 103–127. [Google Scholar]
- Chen, LW; Lu, X; Le, XC. Complementary chromatography separation combined with hydride generation-inductively coupled plasma mass spectrometry for arsenic speciation in human urine. Anal. Chim. Acta 2010, 675, 71–75. [Google Scholar]
- Xie, R; Johnson, W; Spayd, S; Hall, GS; Buckley, B. Arsenic speciation analysis of human urine using ion exchange chromatography coupled to inductively coupled plasma mass spectrometry. Anal. Chim. Acta 2006, 578, 186–194. [Google Scholar]
- Lin, TH; Huang, YL. Chemical speciation of arsenic in urine of patients with blackfoot disease. Biol. Trace Elem. Res 1995, 48, 251–261. [Google Scholar]
- Vahter, M. Methylation of inorganic arsenic in different mammalian species and population groups. Sci. Prog 1999, 82, 69–88. [Google Scholar]
- Vahter, M; Concha, G; Nermell, B; Nilsson, R; Dulout, F; Natarajan, AT. A unique metabolism of inorganic arsenic in native Andean women. Eur. J. Pharmacol 1995, 293, 455–462. [Google Scholar]
- Chiou, HY; Hsueh, YM; Hsieh, LL; Hsu, LI; Hsu, YH; Hsieh, FI; Wei, ML; Chen, HC; Yang, HT; Leu, LC; et al. Arsenic methylation capacity, body retention, and null genotypes of glutathione S-transferase M1 and T1 among current arsenic-exposed residents in Taiwan. Mutat. Res 1997, 386, 197–207. [Google Scholar]
- Lindberg, AL; Ekstrom, EC; Nermell, B; Rahman, M; Lonnerdal, B; Persson, LA; Vahter, M. Gender and age differences in the metabolism of inorganic arsenic in a highly exposed population in Bangladesh. Environ. Res 2008, 106, 110–120. [Google Scholar]
- Vahter, M. Genetic polymorphism in the biotransformation of inorganic arsenic and its role in toxicity. Toxicol Lett 2000, 112–113, 209–217. [Google Scholar]
- Choi, BS; Choi, SJ; Kim, DW; Huang, M; Kim, NY; Park, KS; Kim, CY; Lee, HM; Yum, YN; Han, ES; et al. Effects of repeated seafood consumption on urinary excretion of arsenic species by volunteers. Arch. Environ. Contam. Toxicol 2010, 58, 222–229. [Google Scholar]
- Hsueh, YM; Chiou, HY; Huang, YL; Wu, WL; Huang, CC; Yang, MH; Lue, LC; Chen, GS; Chen, CJ. Serum beta-carotene level, arsenic methylation capability, and incidence of skin cancer. Cancer Epidemiol. Biomarkers Prev 1997, 6, 589–596. [Google Scholar]
- Vahter, M. Mechanisms of arsenic biotransformation. Toxicology 2002, 181–182, 211–217. [Google Scholar]
- Thomas, DJ; Styblo, M; Lin, S. The cellular metabolism and systemic toxicity of arsenic. Toxicol. Appl. Pharmacol 2001, 176, 127–144. [Google Scholar]
- Styblo, M; Drobna, Z; Jaspers, I; Lin, S; Thomas, DJ. The role of biomethylation in toxicity and carcinogenicity of arsenic: A research update. Environ. Health Perspect 2002, 110, 767–771. [Google Scholar]
- Tseng, CH. Arsenic methylation, urinary arsenic metabolites and human diseases: Current perspective. J. Environ. Sci. Health, Part C: Environ. Carcinog. Ecotoxicol. Rev 2007, 25, 1–22. [Google Scholar]
- Gong, Z. Unstable trivalent arsenic metabolites, monomethylarsonous acid and dimethylarsinous acid. J. Anal. At. Spectrom 2001, 16, 1409–1413. [Google Scholar]
- Chung, CJ; Huang, CJ; Pu, YS; Su, CT; Huang, YK; Chen, YT; Hsueh, YM. Urinary 8-hydroxydeoxyguanosine and urothelial carcinoma risk in low arsenic exposure area. Toxicol. Appl. Pharmacol 2008, 226, 14–21. [Google Scholar]
- Gamble, MV; Liu, X; Ahsan, H; Pilsner, R; Ilievski, V; Slavkovich, V; Parvez, F; Levy, D; Factor-Litvak, P; Graziano, JH. Folate, homocysteine, and arsenic metabolism in arsenic-exposed individuals in Bangladesh. Environ. Health Perspect 2005, 113, 1683–1688. [Google Scholar]
- Shraim, A; Cui, X; Li, S; Ng, JC; Wang, J; Jin, Y; Liu, Y; Guo, L; Li, D; Wang, S; et al. Arsenic speciation in the urine and hair of individuals exposed to airborne arsenic through coal-burning in Guizhou, PR China. Toxicol. Lett 2003, 137, 35–48. [Google Scholar]
- Zeisel, SH. Betaine supplementation and blood lipids: Fact or artifact? Nutr. Rev 2006, 64, 77–79. [Google Scholar]
- Chiuve, SE; Giovannucci, EL; Hankinson, SE; Zeisel, SH; Dougherty, LW; Willett, WC; Rimm, EB. The association between betaine and choline intakes and the plasma concentrations of homocysteine in women. Am. J. Clin. Nutr 2007, 86, 1073–1081. [Google Scholar]
- Chen, YC; Su, HJ; Guo, YL; Hsueh, YM; Smith, TJ; Ryan, LM; Lee, MS; Christiani, DC. Arsenic methylation and bladder cancer risk in Taiwan. Canc. Causes Contr 2003, 14, 303–310. [Google Scholar]
- Lindberg, AL; Sohel, N; Rahman, M; Persson, LA; Vahter, M. Impact of smoking and chewing tobacco on arsenic-induced skin lesions. Environ. Health Perspect 2010, 118, 533–538. [Google Scholar]
- Vahter, M; Akesson, A; Liden, C; Ceccatelli, S; Berglund, M. Gender differences in the disposition and toxicity of metals. Environ. Res 2007, 104, 85–95. [Google Scholar]
- Chen, CJ; Hsu, LI; Wang, CH; Shih, WL; Hsu, YH; Tseng, MP; Lin, YC; Chou, WL; Chen, CY; Lee, CY; et al. Biomarkers of exposure, effect, and susceptibility of arsenic-induced health hazards in Taiwan. Toxicol. Appl. Pharmacol 2005, 206, 198–206. [Google Scholar]
- Guha Mazumder, DN; Haque, R; Ghosh, N; De, BK; Santra, A; Chakraborty, D; Smith, AH. Arsenic levels in drinking water and the prevalence of skin lesions in West Bengal, India. Int. J. Epidemiol 1998, 27, 871–877. [Google Scholar]
- Watanabe, C; Inaoka, T; Kadono, T; Nagano, M; Nakamura, S; Ushijima, K; Murayama, N; Miyazaki, K; Ohtsuka, R. Males in rural Bangladeshi communities are more susceptible to chronic arsenic poisoning than females: Analyses based on urinary arsenic. Environ. Health Perspect 2001, 109, 1265–1270. [Google Scholar]
- Huang, YK; Huang, YL; Hsueh, YM; Yang, MH; Wu, MM; Chen, SY; Hsu, LI; Chen, CJ. Arsenic exposure, urinary arsenic speciation, and the incidence of urothelial carcinoma: A twelve-year follow-up study. Canc. Causes Contr 2008, 19, 829–839. [Google Scholar]
- Tseng, CH. A review on environmental factors regulating arsenic methylation in humans. Toxicol. Appl. Pharmacol 2009, 235, 338–350. [Google Scholar]
- Cascio, C; Raab, A; Jenkins, RO; Feldmann, J; Meharg, AA; Haris, PI. The impact of a rice based diet on urinary arsenic. J. Environ. Monit 2011, 13, 257–265. [Google Scholar]
- Navas-Acien, A; Umans, JG; Howard, BV; Goessler, W; Francesconi, KA; Crainiceanu, CM; Silbergeld, EK; Guallar, E. Urine arsenic concentrations and species excretion patterns in American Indian communities over a 10-year period: The Strong Heart Study. Environ. Health Perspect 2009, 117, 1428–1433. [Google Scholar]




| HPLC parameters | |
| Column | Anion Exchange, Hamilton PRP-X100 (15 × 4.1 mm i.d.) |
| Mobile phase | 20 mM Ammonium Phosphate (dibasic); pH 6.0 |
| Column temperature | Ambient |
| Flow rate | 1.2 mL/min |
| Sample injection volume | 20 μL |
| Runtime | 8 min |
| ICP Operating parameters | |
| Plasma power | 1,350 w |
| Auxiliary gas flow | 1.2 L/min |
| Plasma gas flow | 15 L/min |
| Nebulizer gas flow | 1 l L/min |
| DRC gas flow | 0.45 L/min |
| RPq | 0.5 |
| Mass spectroscopy acquisition | |
| Monitored signal | m/z 91 |
| Dwell time | 250 ms |
| Scan mode | Peak hopping |
| Sweeps/reading | 1 |
| Readings/replicate | 1,200 |
| Replicates | 3 |
| Metabolites and Methylation index | Total subjects (n = 57) GM (95% CI) | Subjects without skin lesions (n = 21) GM (95% CI) | Subjects with skin lesion (n = 36) GM (95% CI) |
|---|---|---|---|
| TAs (μg/g Cr) | 31.97 (34.35–47.54) | 26.0 (18.68–63.9) | 36.09 ** (35.1–49.82) |
| iAsIII (μg/g Cr) | 2.97 (3.33–5.05) | 2.33 (1.83–2.97) | 3.41 ** (2.7–4.3) |
| MMAV(μg/g Cr) | 4.69 (3.99–5.51) | 3.47 (2.78–4.35) | 5.59 *** (4.55–6.85) |
| DMAV (μg/g Cr) | 23.41 (23.49–32.57) | 19.67 (15.29–25.29) | 25.91 * (21.29–31.54) |
| iAsV (μg/g Cr) | 0.9 (0.71–1.14) | 0.57 (0.40–0.79) | 1.18 *** (0.88–1.59) |
| Percent iAsIII (%) | 9.17 (8.31–10.12) | 8.98 (8–10.08) | 9.28 (8.02–10.73) |
| Percent MMAV (%) | 14.50 (13.4–15.67) | 13.36 (11.53–15.49) | 15.21 (13.87–16.65) |
| Percent DMAV (%) | 72.38 (70.57–74.24) | 75.65 (73.49–77.87) | 70.54 *** (68.15–73.03) |
| Percent iAsV (%) | 2.78 (2.34–3.31) | 2.18 (1.72–2.76) | 3.21 ** (2.54–4.06) |
| PMI | 1.32 (1.14–1.53) | 1.37 (1.11–1.69) | 1.29 (1.05–1.59) |
| SMI | 4.99 (4.54–5.49) | 5.66 (4.77–6.72) | 4.64 ** (4.15–5.18) |
| Metablates and Methylation index | Men (n = 33) | Women (n = 24) | ||
|---|---|---|---|---|
| Men without skin lesions (n = 9) GM (95% CI) | Men with skin lesions (n = 24) GM (95% CI) | Women without skin lesions (n = 12) GM (95% CI) | Women with skin lesions (n = 12) GM (95% CI) | |
| TAs (μg/g Cr) | 19.77 (13.27–29.46) | 32.3 c (25.53–40.86) | 31.92 ** (23.94–42.58) | 47.5 a,b (33.73–66.89) |
| iAsIII (μg/g Cr) | 1.97 (1.32–2.94) | 3.24 b (2.38–4. 4) | 2.65 (1.91–3.69) | 3.78 (2.56–5.57) |
| MMAV(μg/g Cr) | 3.12 (1.98–4.92) | 5.16 c (3.95–6.74) | 3.76 (2.89–4.89) | 6.55 a (4.67–9.17) |
| DMAV(μg/g Cr) | 14.4 (9.78–21.19) | 22.14 c (17.64–27.8) | 24.85 ** (18.31–33.73) | 35.49 ** (25.02–50.33) |
| iAsV(μg/g Cr) | 0.4 (0.21–0.76) | 1.11 a (0.76–1.6) | 0.74 * (0.52–1.05) | 1.34 b (0.77–2.35) |
| Percent iAsIII (%) | 9.96 (9.01–11.02) | 10.03 (8.32–12.09) | 8.31 * (6.87–10.05) | 7.95 (7.95–6.28) |
| Percent MMAV(%) | 15.80 (13.23–18.87) | 15.98 α (14.21–17.96) | 11.78 ** (9.53–14.57) | 13.78 (11.84–15.97) |
| Percent DMAV(%) | 72.81 (69.76–76.00) | 68.55 c,α (66.14–71.06) | 77.85 ** (75.19–80.61) | 74.70 ** (69.51–80.28) |
| Percent iAsV(%) | 2.01 (1.33–3.04) | 3.42 c,β (2.58–4.54) | 2.31 (1.66–3.21) | 2.83 (1.75–4.56) |
| PMI | 1.48 (1.15–1.90) | 1.20 (0.92–1.58) | 1.29 (0.91–1.83) | 1.49 (1.07–2.07) |
| SMI | 4.61 (3.73–5.69) | 4.29 α (3.76–4.90) | 6.61 ** (5.20–8.39) | 5.42 ** (4.45–6.61) |
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Gao, J.; Yu, J.; Yang, L. Urinary Arsenic Metabolites of Subjects Exposed to Elevated Arsenic Present in Coal in Shaanxi Province, China. Int. J. Environ. Res. Public Health 2011, 8, 1991-2008. https://doi.org/10.3390/ijerph8061991
Gao J, Yu J, Yang L. Urinary Arsenic Metabolites of Subjects Exposed to Elevated Arsenic Present in Coal in Shaanxi Province, China. International Journal of Environmental Research and Public Health. 2011; 8(6):1991-2008. https://doi.org/10.3390/ijerph8061991
Chicago/Turabian StyleGao, Jianwei, Jiangping Yu, and Linsheng Yang. 2011. "Urinary Arsenic Metabolites of Subjects Exposed to Elevated Arsenic Present in Coal in Shaanxi Province, China" International Journal of Environmental Research and Public Health 8, no. 6: 1991-2008. https://doi.org/10.3390/ijerph8061991





