2.1. Effects of Biochar Amendment on Methyl Isothiocyanate (MITC) Emissions
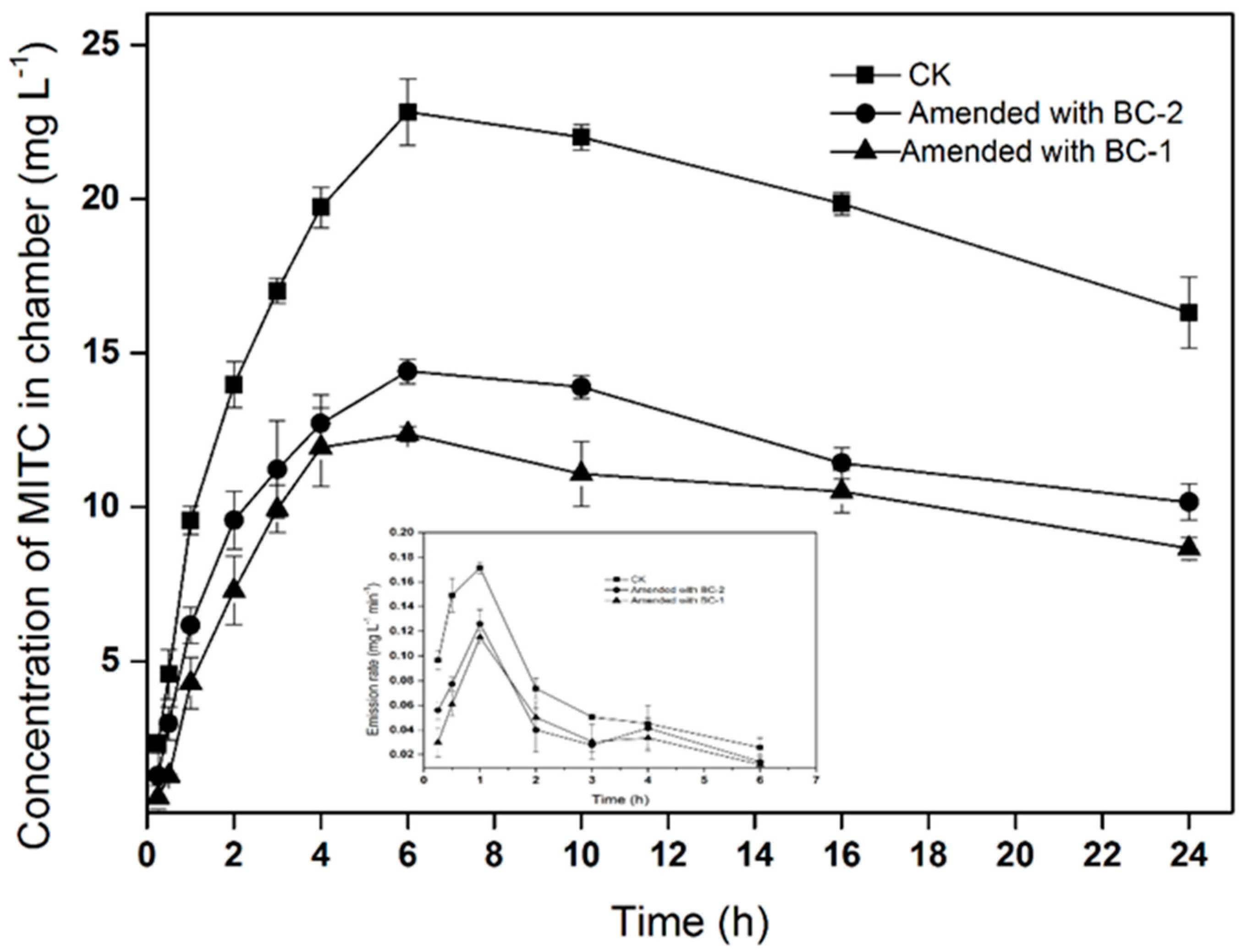
MITC emission losses were markedly reduced in soil amended with biochar (
Figure 1). The maximum air concentrations of the MITC in the chambers were 22.8, 12.3, and 14.4 mg·L
−1 in the CK (soil without biochar), BC-1, and BC-2 treatments, respectively. Compared with fumigation without biochar, BC-1 and BC-2 reduced the total fumigant emission losses by 46.1% and 36.8%, respectively. Correspondingly, the emission rates of MITC decreased in soil treated with BC-1 and BC-2. In the three treatments, the emissions flux initially increased with time, exhibiting a peak flux at 1 h after injection, before subsequently declining with time. The maximum emissions fluxes of MITC were 0.17, 0.11, and 0.12 mg·L
−1·min
−1 in the CK, BC-1 and BC-2 treatments, respectively. The results indicated that BC-1 or BC-2 amendments in the soil surface could significantly reduce MITC emissions in the air.
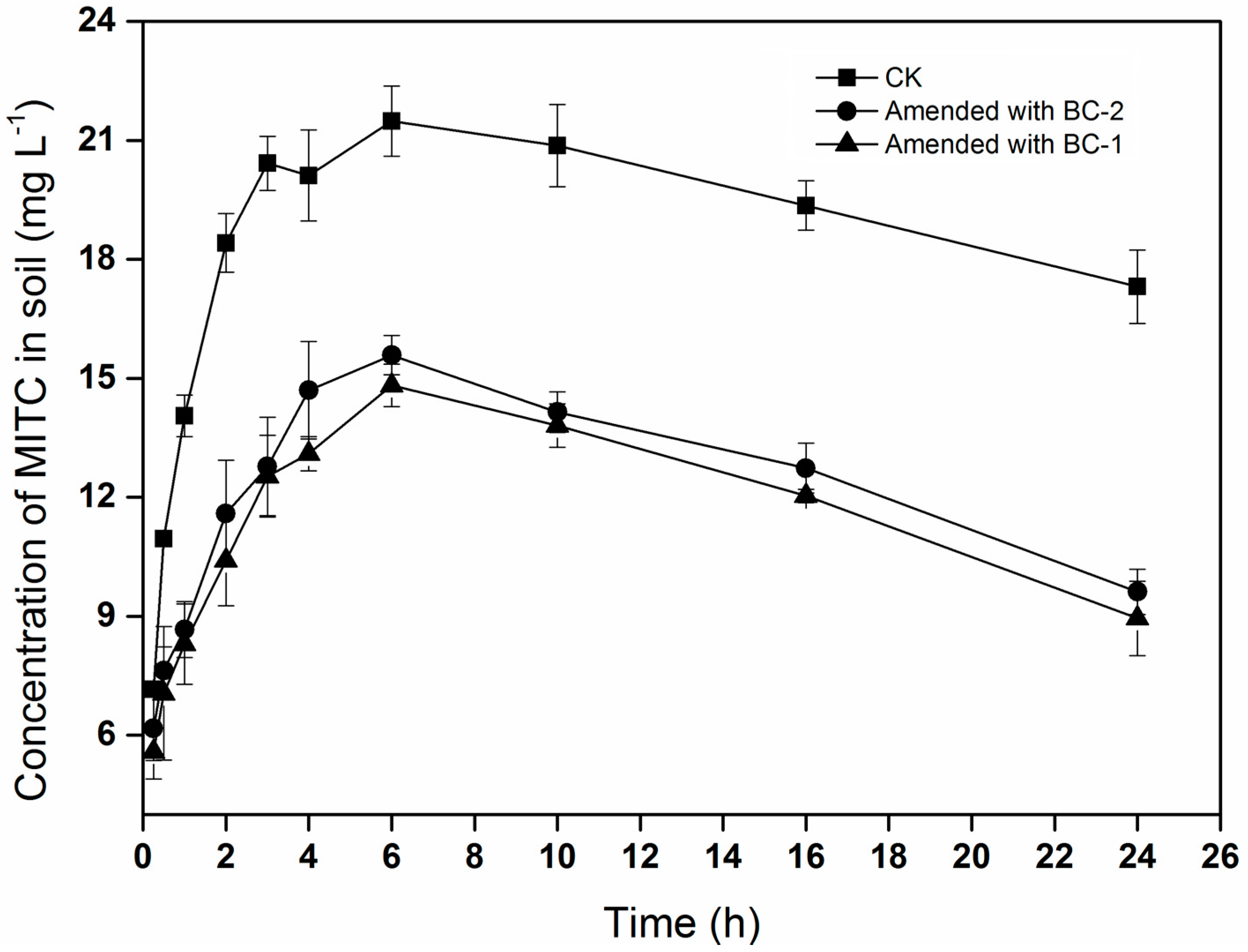
The trend of MITC concentrations in the soil was similar to that in the chamber air, increasing initially and then declining with time (
Figure 2). The concentrations of MITC in the soil treated with biochar (BC-1 or BC-2) were significantly lower than in CK. The maximum concentrations of MITC were 21.5, 14.8, and 15.6 mg·L
−1 in the CK, BC-1, and BC-2 treatments, respectively. These results showed that biochar amendments could reduce the concentrations of MITC in the soil, potentially reducing its efficacy for controlling soil-borne pests.
2.2. Effects of Biochar Amendment on the Efficacy of MITC against Soil-Borne Pests
The efficacies of MITC with respect to root-knot nematodes (
Meloidogyne spp.), weed seeds (
Abutilon theophrasti and
Digitaria sanguinalis) and key soil-borne fungi (
Fusarium spp. and
Phytophthora spp.) in soil amended with BC-1 or BC-2 are listed in
Table 1 and
Table 2, respectively. As shown in
Table 1, the corrected mortality of nematodes following fumigation of unamended soil did not differ from that in soil amended with BC-1 at rates of 0.1% to 5%; however, the corrected mortality of nematodes was significantly lower in soil amended with 10% BC-1. When the BC-1 amendment rates were less than 2% and 1%, there was no significant difference in the efficacies of MITC on control of
Digitaria sanguinalis and
Abutilon theophrasti, respectively. Compared with samples without biochar, the efficacy of MITC against
Phytophthora spp. and
Fusarium spp. was reduced significantly when the BC-1 amendment rates were greater than 2% and 1%, respectively. The above results indicated that BC-1 amendment rates less than or equal to 1% did not have negative effects on MITC’s control of soil-borne pests.
The effects of BC-2 on MITC’s efficacy against soil-borne pests were similar to BC-1 (
Table 2). The efficacy of MITC against root-knot nematode (
Meloidogyne spp.) and weed seeds (
Abutilon theophrasti and
Digitaria sanguinalis) was inhibited significantly after BC-2 amendment at rates above 1% in soil. However, the efficacy against
Fusarium spp. and
Phytophthora spp. was not reduced by BC-2 amendment rates at or below 1% compared with unamended soil. An amendment rate of less than or equal to 0.5% in BC-2 did not have negative effects on MITC’s control of soil-borne pests.
To obtain a sufficient control effect, a larger amount of DZ is required in soil amended with biochar. The efficacy of MITC against soil-borne pests was investigated in soil amended with 1% BC-1 or 0.5% BC-2, respectively (
Table 3 and
Table 4). The results indicated that the tested biochars (BC-1 or BC-2) have a negative impact on the biological activity of MITC on most soil-borne pests at a lower application rate of DZ (for example, 25 or 50 mg·kg
−1). For BC-1, to achieve the comprehensive prevention of all pests in amended soil, an amount of DZ greater than or equal to 125 mg·kg
−1 was required. For BC-2, a DZ application rate greater than or equal to 100 mg·kg
−1 did not have negative effects on MITC’s control of soil-borne pests. Consequently, increased doses of DZ were able to offset decreases in the efficacy of MITC in soils amended with biochar. The pest control efficacy of MITC in Daxing soil is higher than that in Tongzhou soil (for example,
Table 3 and
Table 4). This may be because Tongzhou soil has a history of previous applications of Metham sodium (MS) or DZ (history), while Daxing soil does not (nonhistory). Many studies show that accelerated MITC degradation in history soils resulted in a significant reduction in
Verticillium dahliae,
Sclerotium rolfsii and
Fusarium spp. mortality compared to exposure in nonhistory soils [
17,
18].
2.3. Effects of Biochar Amendment on MITC Degradation and Adsorption
The degradation parameters of MITC in soil, biochar alone, and soil amended with biochar (BC-1 or BC-2) are listed in
Table 5. The results show that MITC degradation is slower in BC-1 alone and soil amended with BC-1 (at 1%) than in soil alone. Compared with the control, the degradation rate of MITC was 6.2 times slower in soil amended with 1% BC-1, and the degradation rate was as much as 14 times slower in pure BC-1. It is clear that BC-1 can significantly inhibit the degradation of MITC. In contrast, BC-2 is able to drastically accelerate the degradation of MITC. The MITC degradation rates (k) in soil amended with 1% BC-2 or BC-2 alone increased 4.1 times and 11.3 times over those in soil alone, respectively. We also observed that the MITC degradation was significantly accelerated after amending the soil with biochar that possessed a high degradation capacity. The above results indicated that biochar has the ability to degrade MITC and that the degradation capacity differs with the type of biochar.
Previous studies have shown that the major degradation pathway for MITC is reaction with hydroxyl (˙OH) radicals [
19]. In addition, there are many organic radicals existing in biochar [
20,
21], and these free radicals could induce ˙OH generation. Fang et al. [
22] reported that the proposed mechanism of ˙OH generation was for free radicals in biochar to transfer electrons to O
2 to produce the superoxide radical anion and hydrogen peroxide, which reacts further with free radicals to produce ˙OH. This indicates that the ˙OH generated on biochar may contribute to MITC degradation. Moreover, Chen et al. suggested that the H/C (hydrogen atom/carbon atom ratio) atomic ratio of organic components in biochar can be used to characterize their aromaticity and polarity [
23]: the higher the H/C value, the lower the aromaticity. Therefore, higher H/C values mean that more functional groups are able to generate ˙OH and more MITC could be degraded. For example, BC-2 biochar had a higher H/C value (0.25), and the MITC degradation rate in BC-2 was greater.
Soil amended with BC-1 inhibited MITC degradation, possibly due to BC-1’s high adsorption capacity for MITC. To clarify the mechanism by which biochar inhibits MITC degradation, we investigated the biochar adsorption kinetics for MITC at different temperatures. The parameters of MITC adsorption by biochar are listed in
Table 6. The
r2 values of all treatments were at least 0.82, and the calculated amounts of MITC adsorption at the equilibrium time (
qe) approached the values measured in the experiment, indicating that the observed adsorption of MITC to biochar provides a good fit to Bangham models [
24]. At 30 °C, the amounts of MITC adsorbed onto BC-1 and BC-2 at the equilibrium time (
qe) were 57.87 mg·g
−1 and 11.97 mg·g
−1, respectively, indicating that BC-1 has a higher adsorption capacity (for MITC) than BC-2 but much lower than AC (activated charcoal, 211.91 mg·g
−1), possibly because MITC may be bound to BC-1 and is not available to degrade. In addition, BC-1 has a low H/C value, and chemical degradation was weak, so the MITC degradation rate in BC-1 or soil amended with BC-1 was lower than in unamended soil. As the temperature increased from 15 °C to 45 °C, the adsorption rates of biochar increased; however, the amount of adsorption at the equilibrium time (
qe) fell as the temperature increased. Kołohynska et al. note that the sorption capacity of biochar depends mainly on the polarity, aromaticity, surface area, and pore size distribution, etc. [
25]. The tested biochars have different surfaces and different pore structures, providing different adsorption rates and adsorption capacities. In general, the adsorption capacity of biochar for MITC increased with the SSA (specific surface area) of the biochar [
25]. For example, BC-1 had a larger SSA (382.81 m
2·g
−1) with a higher amount of adsorption (57.87 mg·g
−1), while BC-2 (SSA 36.14 m
2·g
−1) had a smaller SSA and lower amount of adsorption (11.97 mg·g
−1).
As noted above, the degradation rates of MITC were much lower in biochar (BC-1) and biochar-amended soil than in unamended soil. This occurred because MITC was adsorbed onto the biochar and thus degraded much more slowly, decreasing as the adsorption capacity increased. In contrast, BC-2 has a weaker absorbability of MITC but possesses a higher degradability; thus, the degradation rates of MITC were much faster in the biochar (BC-2) and biochar-amended soil than in unamended soil. Therefore, biochar’s good absorbability or degradability of MITC were speculated to play an important role in reducing or accelerating MITC’s degradation rate in soil amended with these types of biochar. Studies have shown that the degradation of MITC comprises both biological and chemical degradation and that biodegradation accounted for 51%–97% of the total degradation [
26,
27]. The slow degradation rate of MITC in biochar-amended soil is likely due to reduced microbial degradation. In addition, the surface of biochar contains a large number of chemical functional groups [
23], and a vast amount of free radicals may potentially accelerate the degradation of MITC via radical reaction. In summary, the dissipation of MITC in soil amended with biochar depends on the balance between the amount of adsorption and degradation and is positively correlated with the SSA and H/C values, respectively.
However, the effects of biochar on fumigant emissions are known to be complex. Studies have shown that the emission of 1,3-D was reduced after biochar was applied to the surface, due to the enhanced adsorption of 1,3-D onto the biochar [
8,
28], while the emission of chloropicrin (CP) was reduced by biochar due to the accelerated degradation of CP in soil amended with biochar [
7]. We found that biochar used in this experiment can significantly reduce the volatilization of MITC by degradation or adsorption. However, at the same time, biochar amendments also decrease the concentration of MITC in the soil, which potentially reduces its efficacy for controlling soil-borne pests. Through adsorption or degradation, biochar can minimize the concentration of MITC in the soil at different levels. In addition, the amount of reduction varies with different types of biochar. BC-1 has a high SSA (382.2 m
2·g
−1) and a small H/C value (0.01), with a
k value of 0.08 d
−1 and
qe value of 57.87 mg·g
−1 at 30 °C, meaning that it has a greater absorbability of MITC, resulting in a reduced concentration of MITC in the air and soil. On the contrary, BC-2, with a larger H/C value (0.25) and a smaller SSA (36.1 m
2·g
−1), (the
k value was 15.19 d
−1,
qe value was 11.97 mg·g
−1 at 30 °C), has a greater degradative effect on MITC and leads to a reduction of MITC both in air and soil. However, the reduction of MITC in the air and soil with the amendment of BC-1 was greater than that in BC-2 (
Figure 1 and
Figure 2), which may be due to the greater adsorption of MITC by BC-1 rather than the degradation of MITC by BC-2. It is precisely because the concentration of MITC is reduced in soil amended by biochar that its efficacy in controlling soil-borne pests is reduced. For example, 5% BC-1 or 2% BC-2 amendments significantly reduce the efficiency of
Phytophthora spp. and
Fusarium spp. Increased doses of DZ were able to offset decreases in the efficacy of MITC in soils amended with biochar. When the DZ rate was only 25 mg·kg
−1, the efficacy of MITC against
Abutilon theophrasti was reduced in soil amended with 1% BC-1, compared with unamended soil. However, the above reduction in efficacy was alleviated by increasing the application dose of DZ to 100 mg·kg
−1 (corresponding to a field rate of 360 kg·ha
−1).
Wang et al. have indicated that biochar amendment at less than 1% in soil did not have negative effects on the levels of pathogens and nematode control achieved by chloropicrin fumigation [
7], and the efficacy of Dimethyl Disulfide (DMDS) for controlling root-knot nematodes and
Fusarium spp. was not reduced when biochar was applied at a rate less than 2% and 0.5%, respectively [
29]. Another study noted that, while nematode control was adequate in the specific system studied, biochar amendment could adversely impact pest control depending on the sorption strength of the particular type of biochar [
10]. The actual impact on the efficacy will be a function of the interplay between the application rates of the pesticide and the biochar and the sorption capacity of the specific biochar for the specific pesticide. This issue should be considered when determining the desirable physical and chemical characteristics of biochar for agronomic systems. In the specific experimental system studied here, adequate pest control was achieved at a standard biochar application rate (13 Mg·ha
−1). However, different source materials and different production processes create types of biochar with different physical and chemical properties, as well as different adsorption properties or degradability. For example, biochars produced at higher temperatures have been shown to have substantially higher sorption capacities than those produced at lower temperatures. It is apparent that the potential detrimental impact of biochar amendments on pest control must be taken into account when considering the use of biochar in agriculture. In summary, the impact of biochar soil amendments on the efficacy of MITC against soil-borne pests depends on the biochar type, amendment rate, and the application dose of DZ. Because biochar can play a significant role in reducing fumigant emissions [
7,
8], it is important to select an appropriate biochar amendment that does not affect the efficacy of fumigants such as MITC.