Analysis of Porous Structure Parameters of Biomass Chars Versus Bituminous Coal and Lignite Carbonized at High Pressure and Temperature—A Chemometric Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
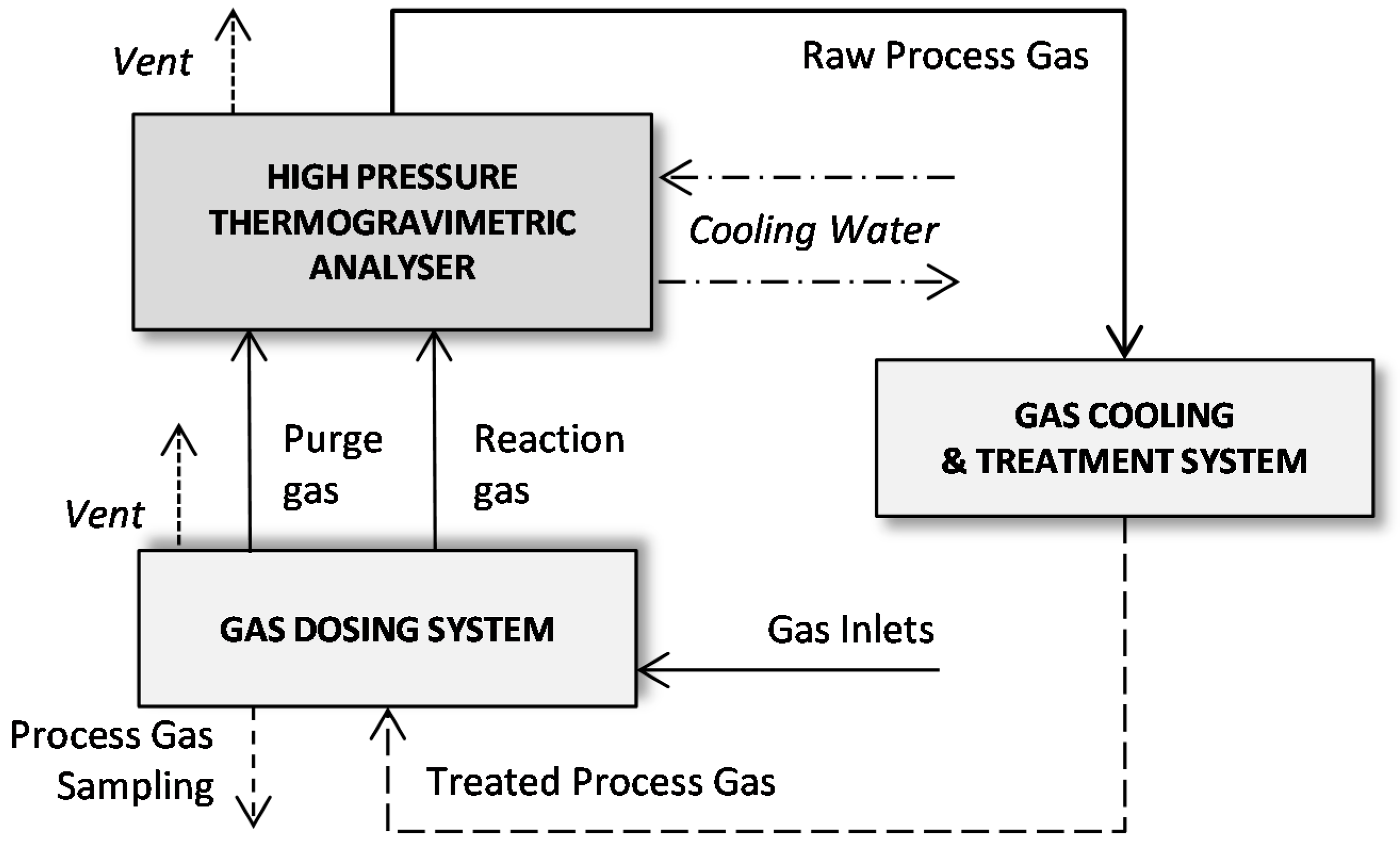
2.2. Porous Structure Characterization
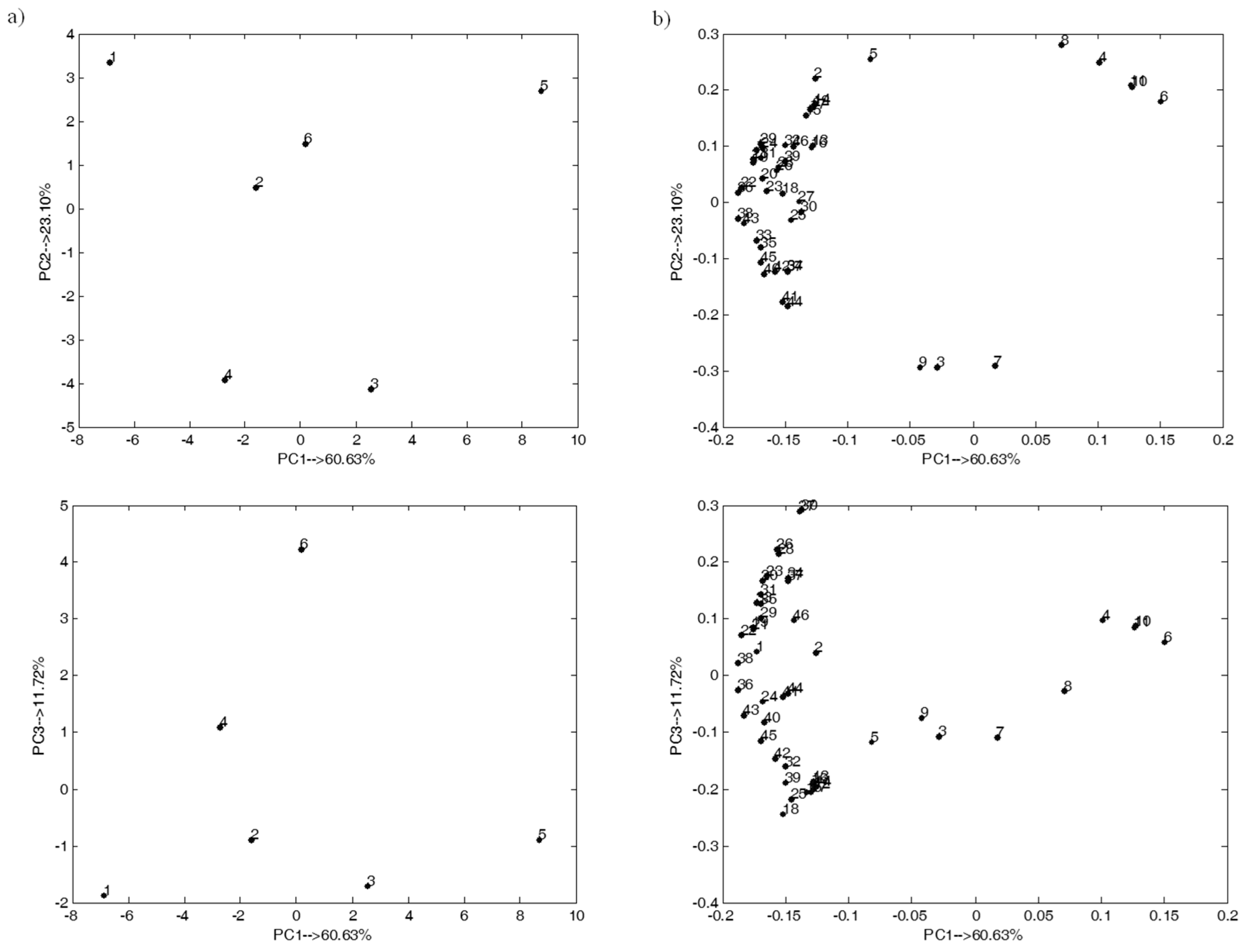
2.3. Exploratory Analysis of the Studied Multivariate Dataset
2.4. Experimental Dataset
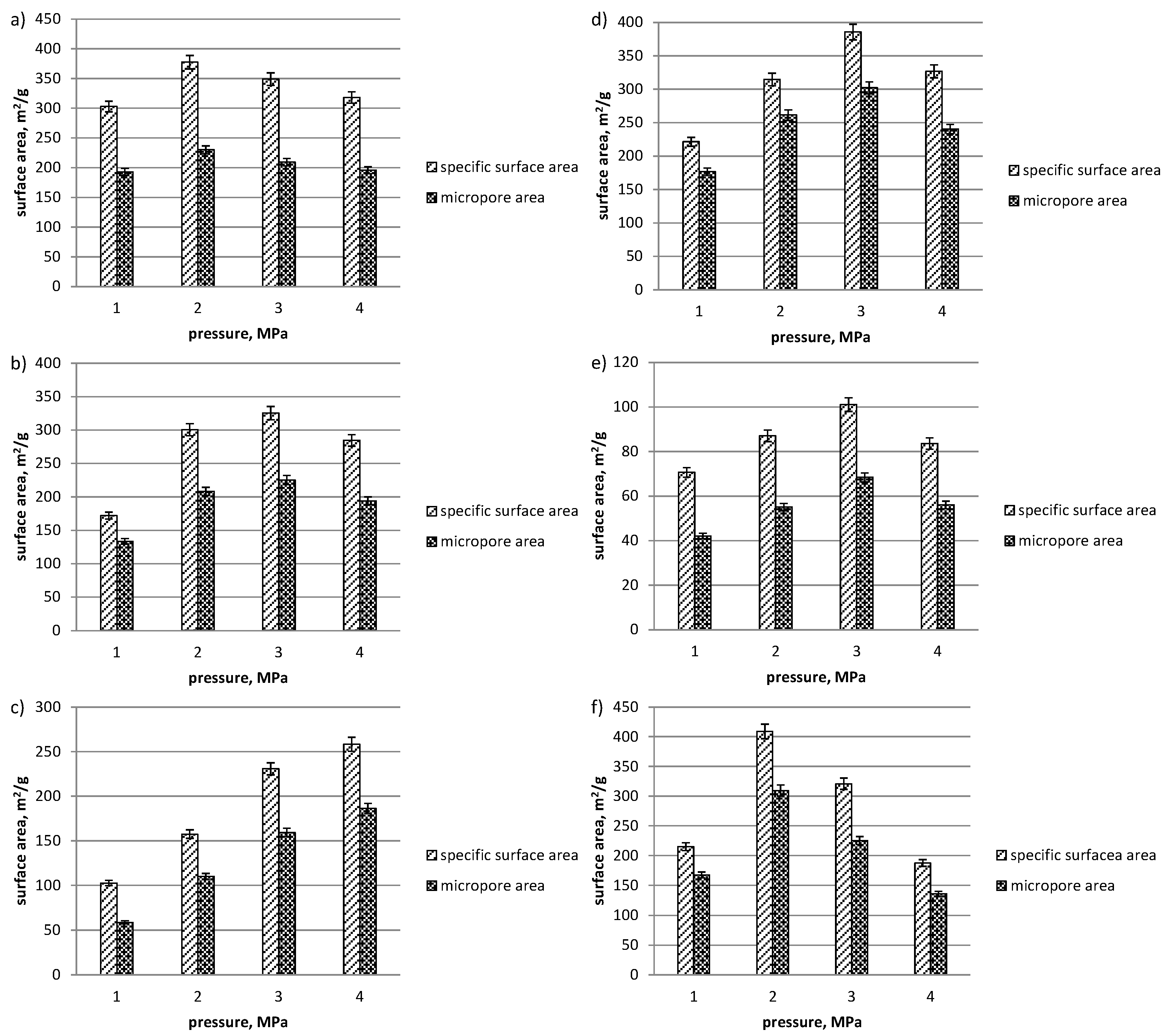
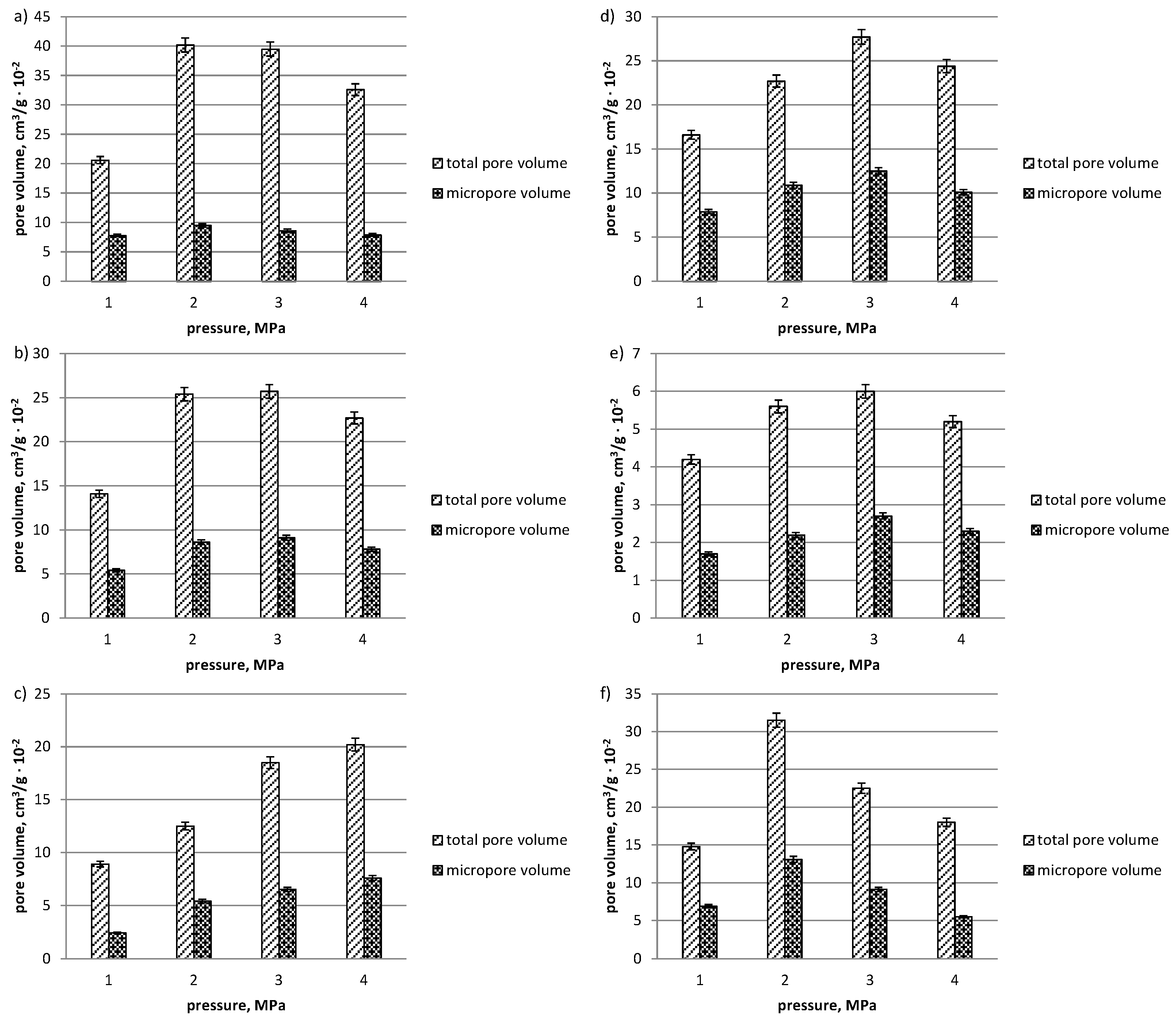
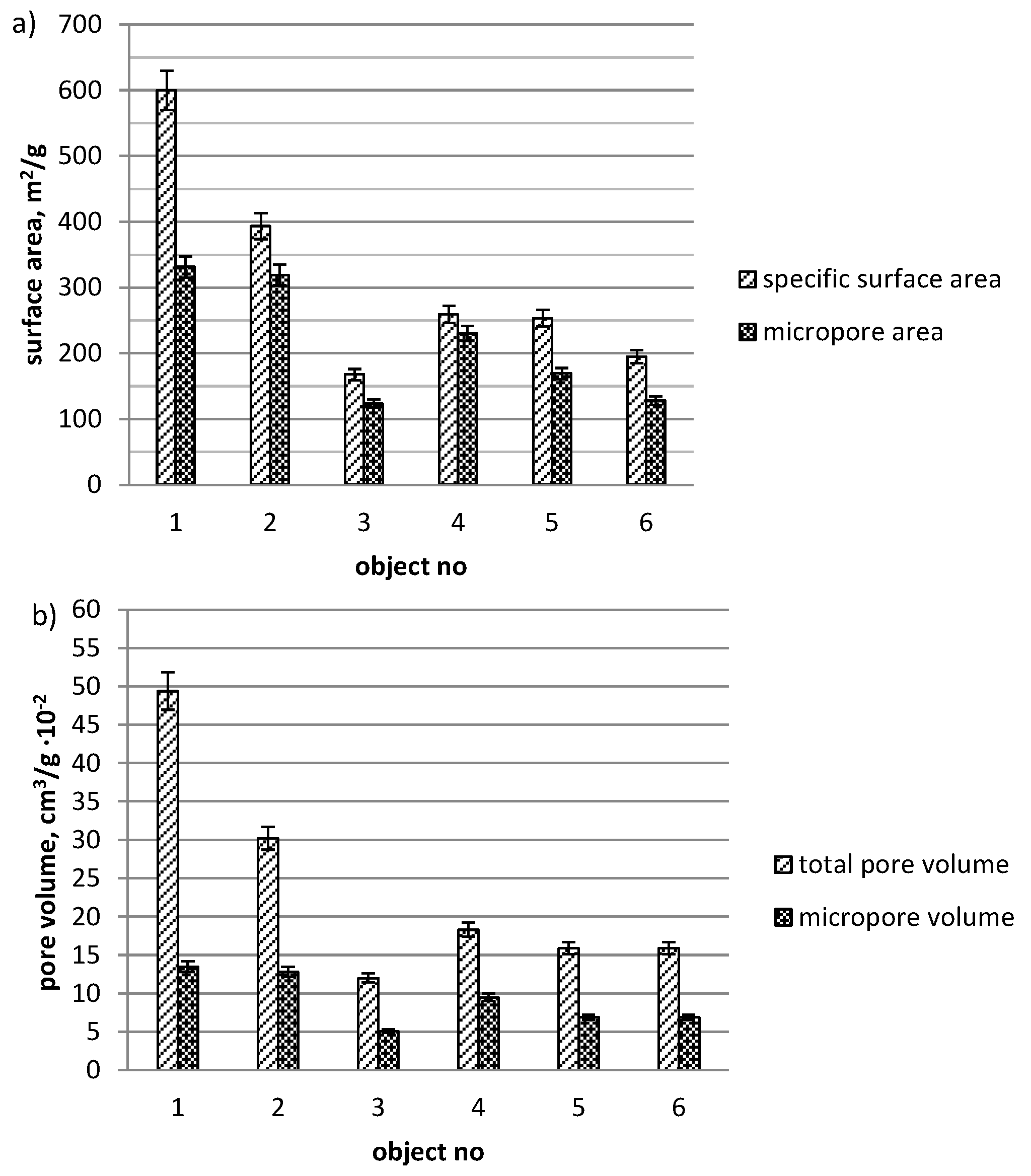
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Acevedo, B.; Barriocanal, C. The influence of the pyrolysis conditions in a rotary oven on the characteristics of the products. Fuel Process. Technol. 2015, 131, 109–116. [Google Scholar] [CrossRef]
- Fatemi, M. Effect of pressure on pyrolysis on a sub-bituminous coal in an entrained flow reactor. Prepr. Pap. Am. Chem. Soc. Div. Fuel Chem. 1987, 32, 117–124. [Google Scholar]
- Lee, C.W.; Scaroni, A.W.; Jenkins, R.G. Effect of pressure on the devolatilization and swelling behavior of a softening coal during rapid heating. Fuel 1991, 70, 957–965. [Google Scholar] [CrossRef]
- Solomon, P.R.; Fletcher, T.H. Impact of coal pyrolysis on combustion. Symp. Int. Combust. 1994, 25, 463–474. [Google Scholar] [CrossRef]
- Tremel, A.; Haselsteiner, T.; Nakonz, M.; Spliethoff, H. Coal and char properties in high temperature entrained flow gasifier. Energy 2012, 45, 176–182. [Google Scholar] [CrossRef]
- Wall, T.F.; Liu, G.; Wu, H.; Roberts, D.G.; Benfell, K.E.; Gupta, S.; Lucas, J.A.; Harris, D.J. The effects of pressure on coal reactions during pulverised coal combustion and gasification. Prog. Energy Combust. 2002, 28, 405–433. [Google Scholar] [CrossRef]
- Gale, T.K.; Bartholomew, C.H.; Fletcher, T.H. Decreases in the swelling and porosity of bituminous coals during devolatilization at high heating rates. Combust. Flame 1995, 100, 94–100. [Google Scholar] [CrossRef]
- Khan, M.R.; Jenkins, R.G. Swelling and plastic properties of coal devolatilized at elevated pressures: An examination of the influence of coal type. Fuel 1986, 65, 725–731. [Google Scholar] [CrossRef]
- Lee, C.W.; Jenkins, R.G.; Schobert, H.H. Structure and reactivity of char from elevated pressure pyrolysis of Illinois no. 6 bituminous coal. Energy Fuel 1992, 6, 40–47. [Google Scholar] [CrossRef]
- Xi, J.; Liang, J.; Sheng, X.; Shi, L.; Li, S. Characteristics of lump lignite pyrolysis and the influence of temperature on lignite swelling in underground coal gasification. Anal. Appl. Pyrolysis 2016, 117, 228–235. [Google Scholar] [CrossRef]
- Basu, P. Biomass Gasification, Pyrolysis and Torrefaction; Academic Press: Oxford, UK, 2013. [Google Scholar]
- Benfell, K.E.; Liu, G.; Roberts, D.G.; Harris, D.J.; Lucas, J.A.; Bailey, J.G. Modelling char combustion: The influence of parent coal petrography and pyrolysis pressure on the structure and intrinsic reactivity of its char. Proc. Combust. Inst. 2000, 28, 2233–2241. [Google Scholar] [CrossRef]
- Liu, T.F.; Fang, Y.T.; Wang, Y. An experimental investigation into the gasification reactivity of chars prepared at high temperatures. Fuel 2008, 87, 460–466. [Google Scholar] [CrossRef]
- Duman, G.; Uddin, M.A.; Yanik, J. The effect of char properties on gasification reactivity. Fuel Process. Technol. 2014, 18, 75–81. [Google Scholar] [CrossRef]
- Rouquerol, J.; Avnir, D.; Fairbridge, C.W.; Everett, D.H.; Haynes, J.H.; Pernicone, N.; Ramsay, J.D.F.; Sing, K.S.W.; Unger, K.K. Recommendations for the characterization of porous solids. Pure Appl. Chem. 1994, 66, 1739–1758. [Google Scholar] [CrossRef]
- Heidari, A.; Stahl, R.; Younesi, H.; Rashidi, A.; Troeger, N.; Ghoreyshi, A.A. Effect of process conditions on product yield and composition of fast pyrolysis of Eucalyptus grandis in fluidized bed reactor. J. Ind. Eng. Chem. 2014, 20, 2594–2602. [Google Scholar] [CrossRef]
- Huang, H.; Wang, Y.; Cannon, F.S. Pore structure development of in-situ pyrolyzed coals for pollution prevention in iron foundries. Fuel Process. Technol. 2009, 90, 1183–1191. [Google Scholar] [CrossRef]
- Karaman, I.; Yagmur, E.; Banford, A.; Aktas, Z. The effect of process parameters on the carbon dioxide based production of activated carbon from lignite in a rotary reactor. Fuel Process. Technol. 2014, 118, 34–41. [Google Scholar] [CrossRef]
- Peiyong, M.; Yadong, S.; Xianjun, X.; Hui, L.; Tao, L.; Yunlong, H. Effect of the Catalytic Pyrolysis Conditions on the Properties of Biological Coke. Energy Procedia 2015, 66, 289–292. [Google Scholar] [CrossRef]
- Trubetskaya, A.; Jensen, P.A.; Jensen, A.D.; Llamas, A.D.G.; Umeki, K.; Glarborgm, P. Effect of fast pyrolysis conditions on biomass solid residues at high temperatures. Fuel Process. Technol. 2016, 143, 118–129. [Google Scholar] [CrossRef] [Green Version]
- Yangsheng, Z.; Fang, Q.; Zhijun, W.; Yuan, Z.; Weiguo, L.; Qiaorong, M. Experimental investigation on correlation between permeability variation and pores structure during coal pyrolysis. Transp. Porous Media 2010, 82, 401–412. [Google Scholar] [CrossRef]
- Yu, Y.; Xu, M.; Yao, H.; Yu, D.; Qiao, Y.; Sui, J.; Liu, X.; Cao, Q. Char characteristics and particulate matter formation during Chinese bituminous coal combustion. Proc. Combust. Inst. 2007, 31, 1947–1954. [Google Scholar] [CrossRef]
- Xiumin, J.; Chuguang, Z.; Che, Y.; Dechang, L.; Jianrong, Q.; Jubin, L. Physical structure and combustion properties of super fine pulverized coal particle. Fuel 2002, 81, 793–797. [Google Scholar] [CrossRef]
- Fukuyama, H.; Terai, S. Preparing and characterizing the active carbon produced by steam and carbon dioxide as a heavy oil hydrocracking catalyst support. Catal. Today 2008, 130, 382–388. [Google Scholar] [CrossRef]
- Hsu, L.Y.; Teng, H. Influence of different chemical reagents on the preparation of activated carbons from bituminous coal. Fuel Process. Technol. 2000, 64, 155–166. [Google Scholar] [CrossRef]
- Kopac, T.; Toprak, A. Preparation of activated carbons from Zonguldak region coals by physical and chemical activations for hydrogen sorption. Int. J. Hydrog. Energy 2007, 32, 5005–5014. [Google Scholar] [CrossRef]
- Xing, B.; Huang, G.; Chen, L.; Guo, H.; Zhang, C.; Xie, W.; Chen, Z. Microwave synthesis of hierarchically porous activated carbon from lignite for high performance supercapacitors. J. Porous Mater. 2016, 23, 67–73. [Google Scholar] [CrossRef]
- Howaniec, N. The effects of pressure on coal chars porous structure development. Fuel 2016, 172, 118–123. [Google Scholar] [CrossRef]
- Howaniec, N. Development of porous structure of lignite chars at high pressure and temperature. Fuel Process. Technol. 2016, 154, 163–167. [Google Scholar] [CrossRef]
- Howaniec, N. Temperature induced development of porous structure of bituminous coal chars at high pressure. J. Sustain. Min. 2016, 15, 120–124. [Google Scholar] [CrossRef]
- Wall, T.F. ACARP Project C6051 Final Report; CRC for Black Coal Utilization: Callaghan, Australia, 1999. [Google Scholar]
- Jagiełło, J.; Thommes, M. Comparison of DFT characterization methods based on N2, Ar, CO2, and H2 adsorption applied to carbons with various pore size distribution. Carbon 2004, 42, 1225–1229. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Rouquerol, F.; Rouquerol, J.; Sing, K.S.W.; Llewellyn, P.; Maurin, G. Adsorption by Powders and Porous Solids, Principles, Methodology and Applications; Academic Press: Oxford, UK, 2014. [Google Scholar]
- De Boer, J.H.; Linsen, B.G.; van der Plas, T.H.; Zondervan, G.J. Studies on pore systems in catalysts: VII. Description of the pore dimensions of carbon blacks by the t method. J. Catal. 1965, 4, 649–653. [Google Scholar] [CrossRef]
- Joliffe, T. Principal Components Analysis; Springer: New York, NY, USA, 1986. [Google Scholar]
- Massart, D.L.; Vandeginste, B.G.M.; Buydens, L.M.C.; de Jong, S.; Lewi, P.J.; Smeyers-Verbeke, J. Handbook of Chemometrics and Qualimetrics: Part A; Elsevier: Amsterdam, The Netherlands, 1997. [Google Scholar]
- Smoliński, A.; Howaniec, N.; Stańczyk, K. A comparative experimental study of biomass, lignite and hard coal steam gasification. Renew. Energy 2011, 36, 1836–1842. [Google Scholar] [CrossRef]
- Vandeginste, B.G.M.; Massart, D.L.; Buydens, L.M.C.; de Jong, S.; Lewi, P.J.; Smeyers-Verbeke, J. Handbook of Chemometrics and Qualimetrics: Part B; Elsevier: Amsterdam, The Netherlands, 1998. [Google Scholar]
- Wold, S.; Esbensen, K.; Geladi, P. Principal Components Analysis. Chemom. Intell. Lab. Syst. 1987, 2, 37–52. [Google Scholar] [CrossRef]
| Parameter | Object 1 | Object 2 | Object 3 | Object 4 | Object 5 | Object 6 |
|---|---|---|---|---|---|---|
| Moisture, %w/w | 14.24 (1) | 7.18 (1) | 4.74 (2) | 8.69 (2) | 3.04 (1) | 9.15 (1) |
| Ash, %w/w | 12.63 (1) | 9.12 (1) | 1.51 (3) | 4.31 (3) | 4.21 (1) | 8.93 (1) |
| Volatiles, %w/w | 36.90 (1) | 47.18 (1) | 73.16 (4) | 69.89 (4) | 29.86 (1) | 30.49 (1) |
| Fixed carbon, %w/w | 36.23 (5) | 36.52 (5) | 20.59 (5) | 17.11 (5) | 62.89 (5) | 51.43 (5) |
| Sulfur, %w/w | 1.78 (6) | 1.32 (6) | 0.05 (6) | 0.12 (6) | 0.79 (6) | 0.71 (6) |
| Carbon, %w/w | 51.03 (7) | 56.82 (7) | 52.19 (8) | 45.77 (8) | 76.79 (7) | 63.72 (7) |
| Hydrogen, %w/w | 3.65 (7) | 5.00 (7) | 6.22 (8) | 5.62 (8) | 4.07 (7) | 3.82 (7) |
| Nitrogen, %w/w | 0.83 (7) | 0.54 (7) | b.d. | b.d. | 1.33 (7) | 0.67 (7) |
| Oxygen, %w/w | 16.11 (5) | 20.22 (5) | 35.29 (5) | 35.58 (5) | 9.97 (5) | 13.11 (5) |
| Heat of combustion, kJ/kg | 19,640 (9) | 23,942 (9) | 18,171 (10) | 16,920 (10) | 30,614 (9) | 25,855 (9) |
| Calorific value, kJ/kg | 18,405 (9) | 22,675 (9) | 16,697 (10) | 15,481 (10) | 29,651 (9) | 24,665 (9) |
| No. | Parameters | No. | Parameters |
|---|---|---|---|
| 1 | Moisture, %w/w | 24 | DFT method cumulative pore volume, 1 MPa |
| 2 | Ash, %w/w | 25 | Average pore diameter, 1 MPa |
| 3 | Volatiles, %w/w | 26 | Multipoint BET, 2 MPa |
| 4 | Fixed carbon, %w/w | 27 | T-method micropore surface area, 2 MPa |
| 5 | Sulfur, %w/w | 28 | DFT cumulative surface area, 2 MPa |
| 6 | Carbon, %w/w | 29 | Total pore volume, 2 MPa |
| 7 | Hydrogen, %w/w | 30 | T-method micropore volume, 2 MPa |
| 8 | Nitrogen, %w/w | 31 | DFT method cumulative pore volume, 2 MPa |
| 9 | Oxygen, %w/w | 32 | Average pore diameter, 2 MPa |
| 10 | Heat of combustion, kJ/kg | 33 | Multipoint BET, 3 MPa |
| 11 | Calorific value, kJ/kg | 34 | T-method micropore surface area, 3 MPa |
| 12 | Multipoint BET, 0.1 MPa | 35 | DFT cumulative surface area, 3 MPa |
| 13 | T-method micropore surface area, 0.1 MPa | 36 | Total pore volume, 3 MPa |
| 14 | DFT cumulative surface area, 0.1 MPa | 37 | T-method micropore volume, 3 MPa |
| 15 | Total pore volume, 0.1 MPa | 38 | DFT method cumulative pore volume, 3 MPa |
| 16 | T-method micropore volume, 0.1 MPa | 39 | Average pore diameter, 3 MPa |
| 17 | DFT method cumulative pore volume, 0.1 MPa | 40 | Multipoint BET, 4 MPa |
| 18 | Average pore diameter, 0.1 MPa | 41 | T-method micropore surface area, 4 MPa |
| 19 | Multipoint BET, 1 MPa | 42 | DFT cumulative surface area, 4 MPa |
| 20 | T-method micropore surface area, 1 MPa | 43 | Total pore volume, 4 MPa |
| 21 | DFT cumulative surface area, 1 MPa | 44 | T-method micropore volume, 4 MPa |
| 22 | Total pore volume, 1 MPa | 45 | DFT method cumulative pore volume, 4 MPa |
| 23 | T-method micropore volume, 1 MPa | 46 | Average pore diameter, 4 MPa |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smoliński, A.; Howaniec, N. Analysis of Porous Structure Parameters of Biomass Chars Versus Bituminous Coal and Lignite Carbonized at High Pressure and Temperature—A Chemometric Study. Energies 2017, 10, 1457. https://doi.org/10.3390/en10101457
Smoliński A, Howaniec N. Analysis of Porous Structure Parameters of Biomass Chars Versus Bituminous Coal and Lignite Carbonized at High Pressure and Temperature—A Chemometric Study. Energies. 2017; 10(10):1457. https://doi.org/10.3390/en10101457
Chicago/Turabian StyleSmoliński, Adam, and Natalia Howaniec. 2017. "Analysis of Porous Structure Parameters of Biomass Chars Versus Bituminous Coal and Lignite Carbonized at High Pressure and Temperature—A Chemometric Study" Energies 10, no. 10: 1457. https://doi.org/10.3390/en10101457






