Probing Temperature-Dependent Recombination Kinetics in Polymer:Fullerene Solar Cells by Electric Noise Spectroscopy
Abstract
:1. Introduction
2. Electric Noise: General Concepts
3. Results
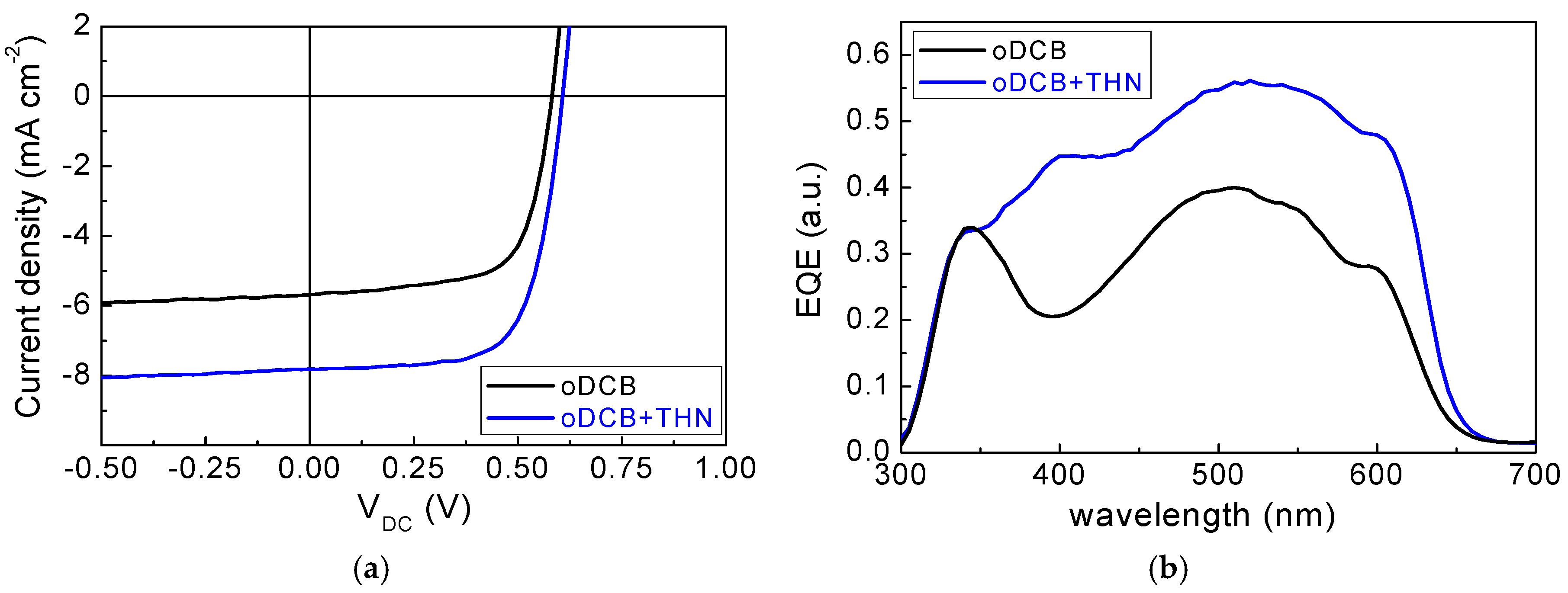
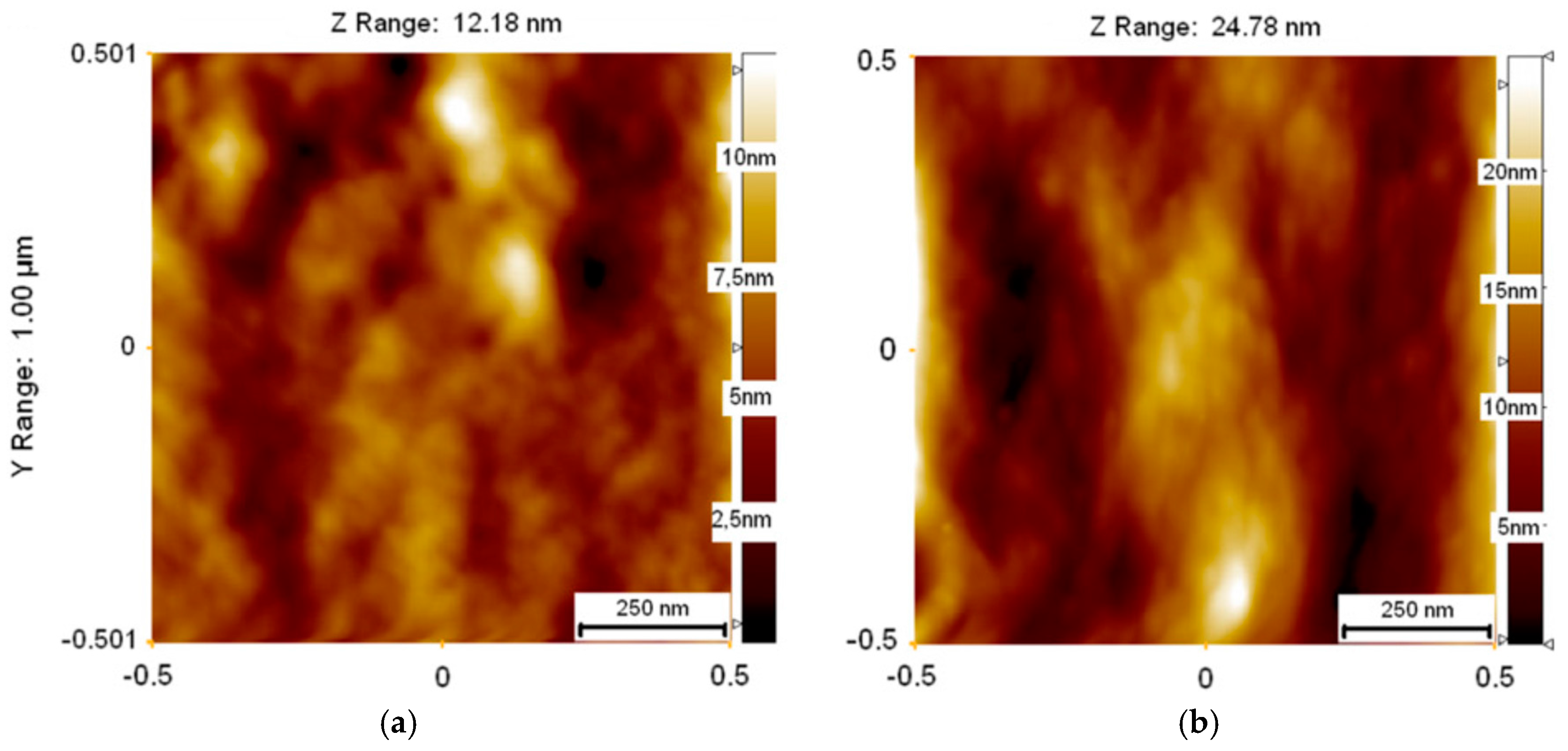
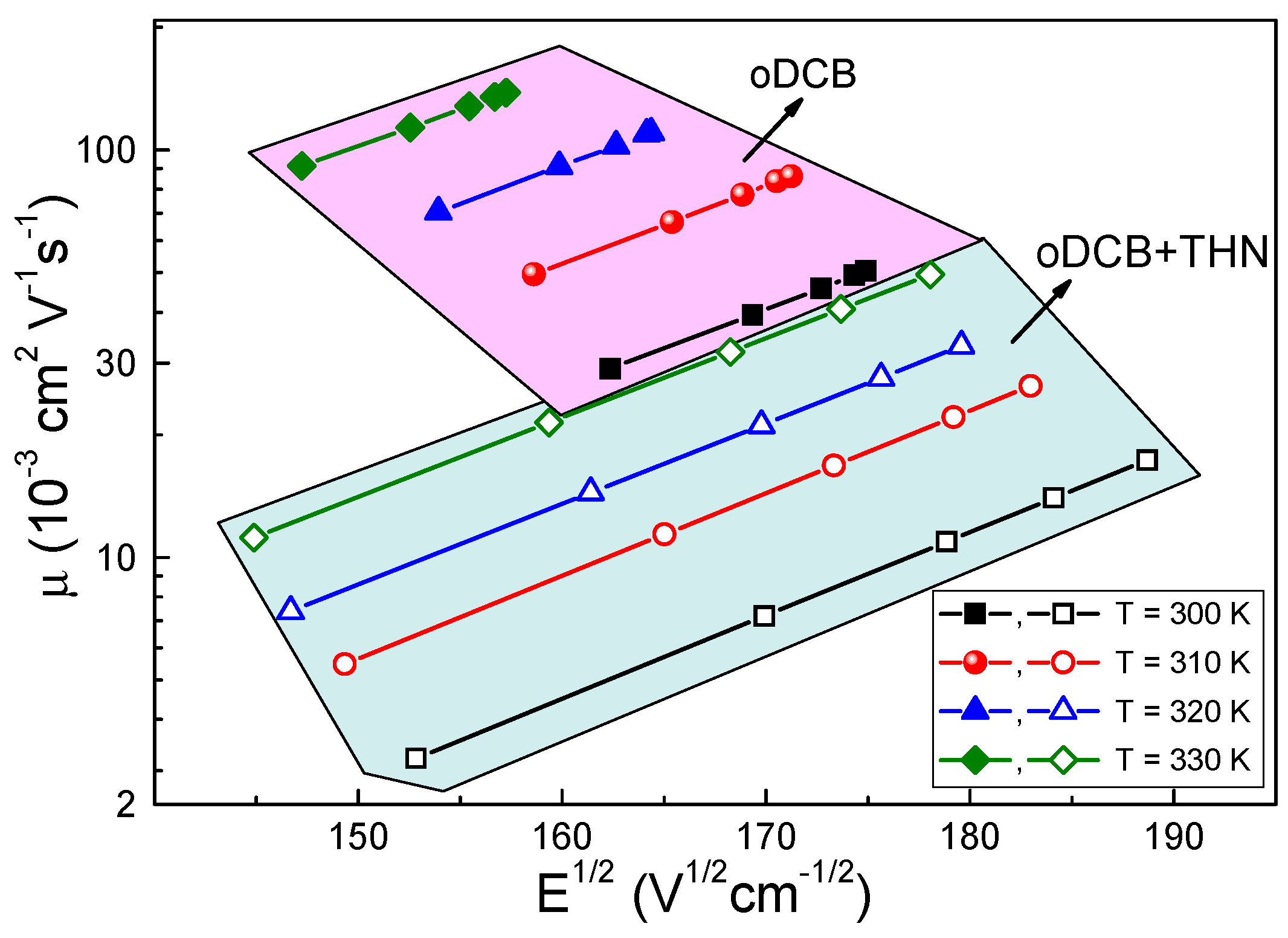
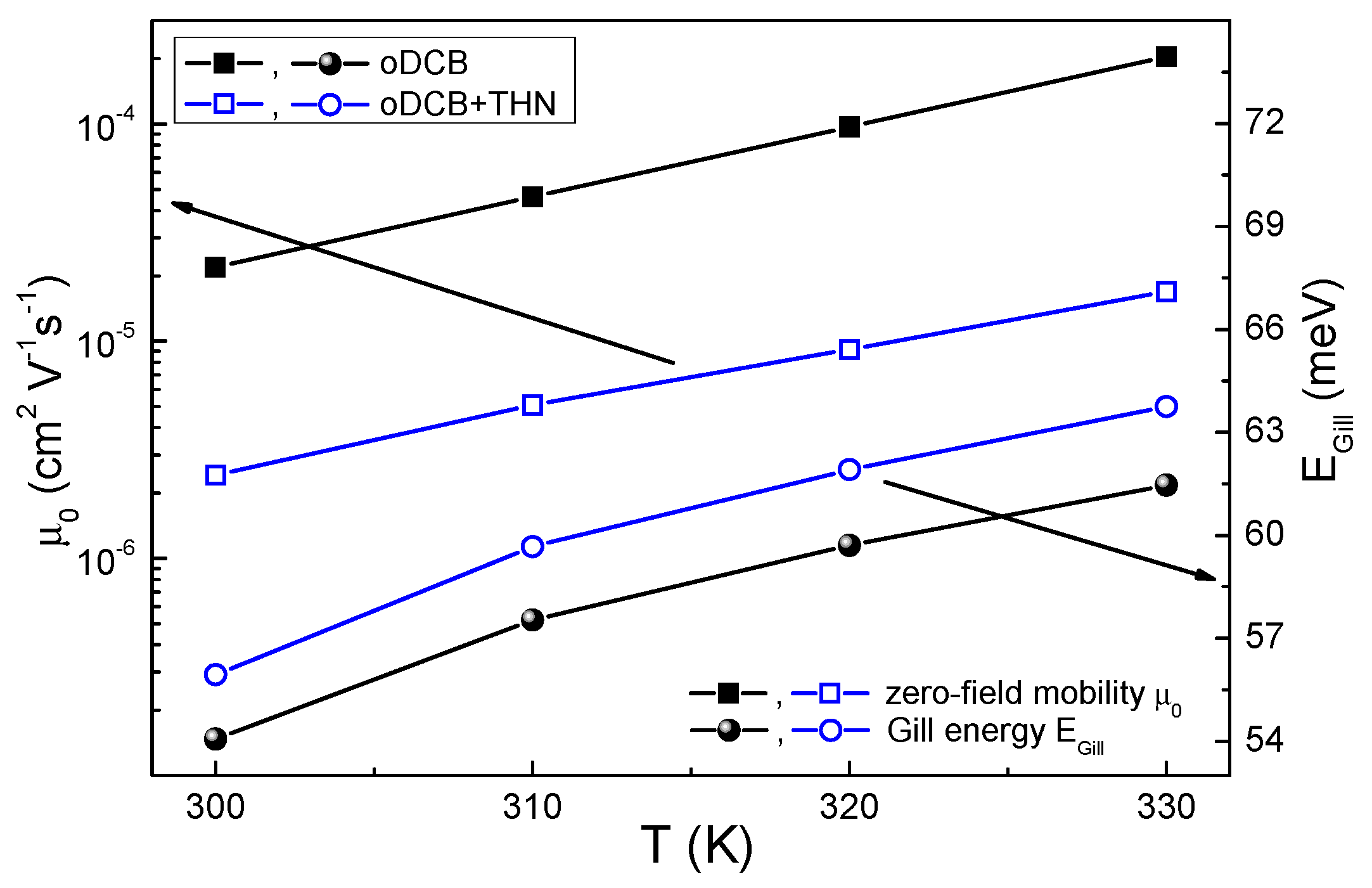
3.1. Electrical Transport and Structural Properties
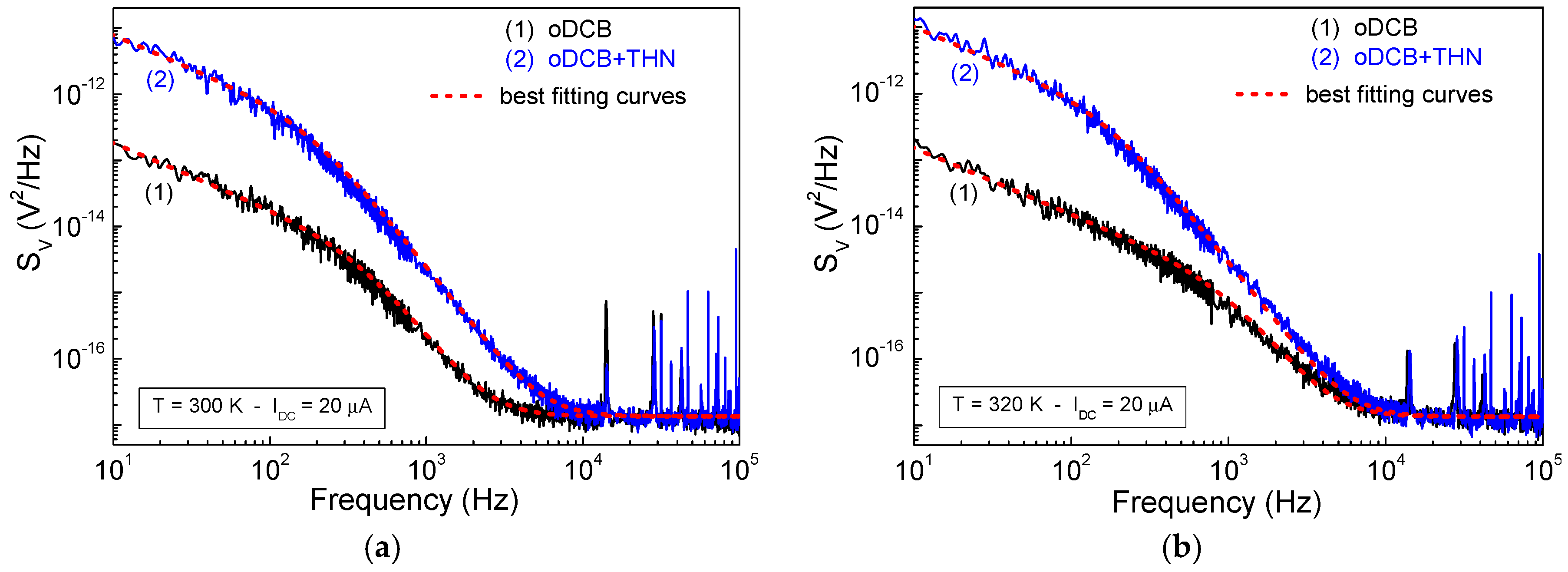
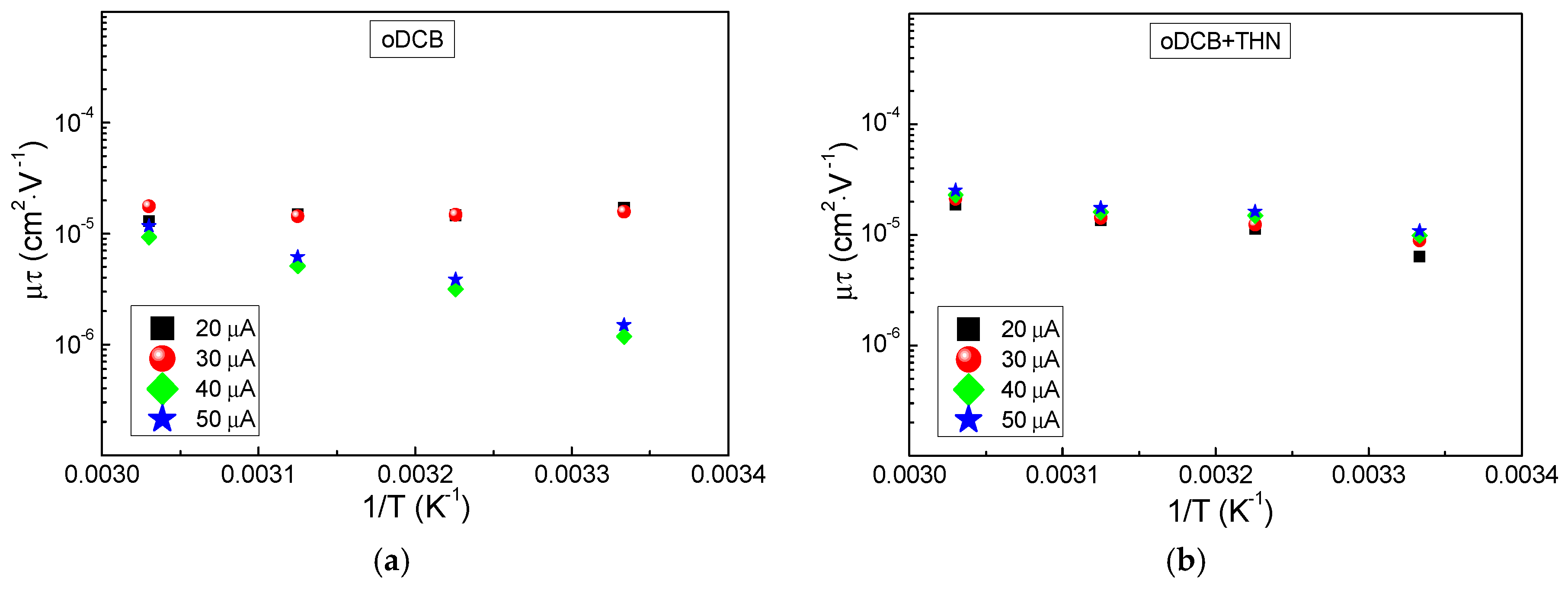
3.2. Noise Properties
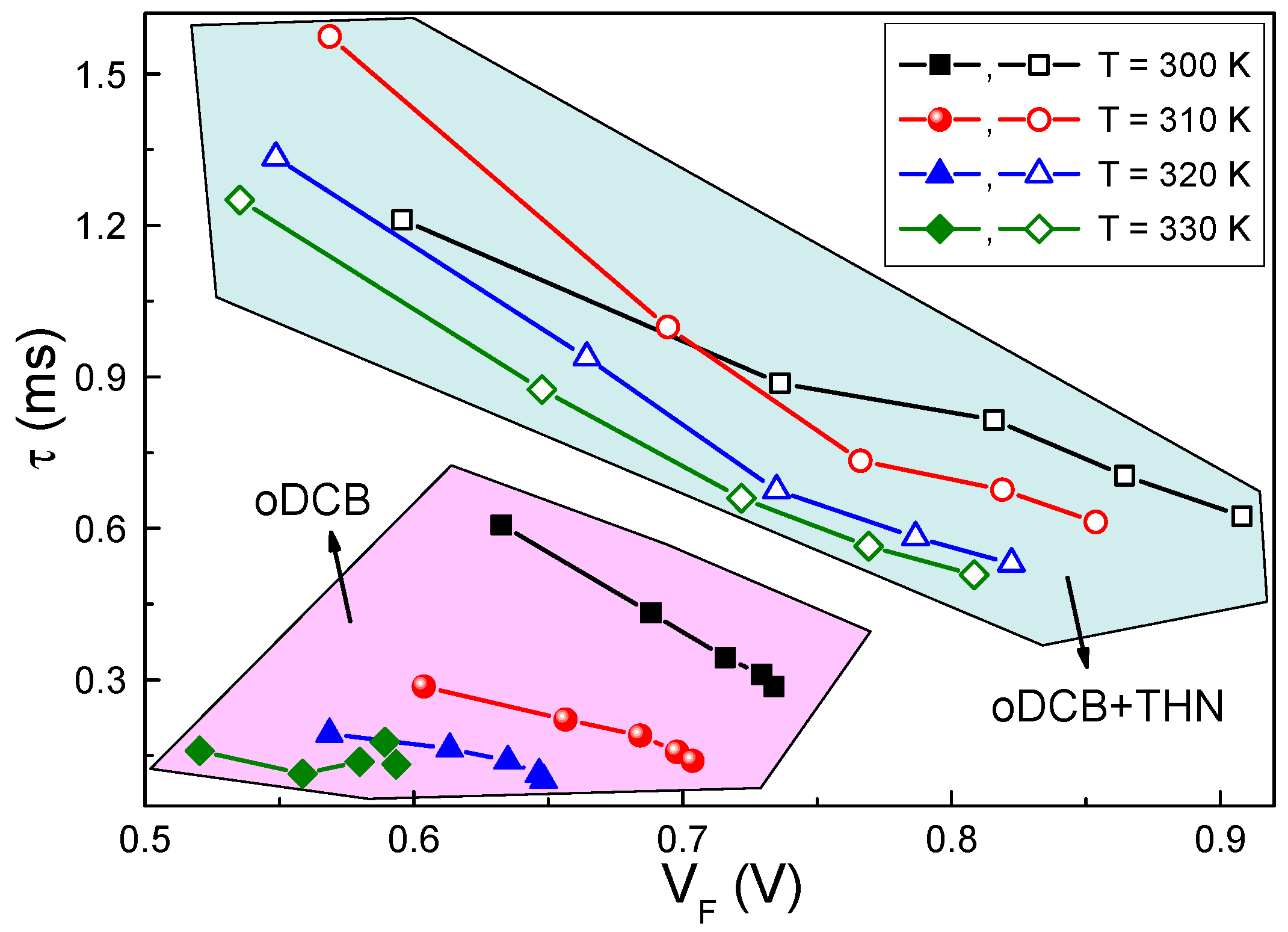
4. Discussion
4.1. Solvent Influence on the Charge Carrier Recombination Process
4.2. Solvent Influence on the Charge Carrier Extraction
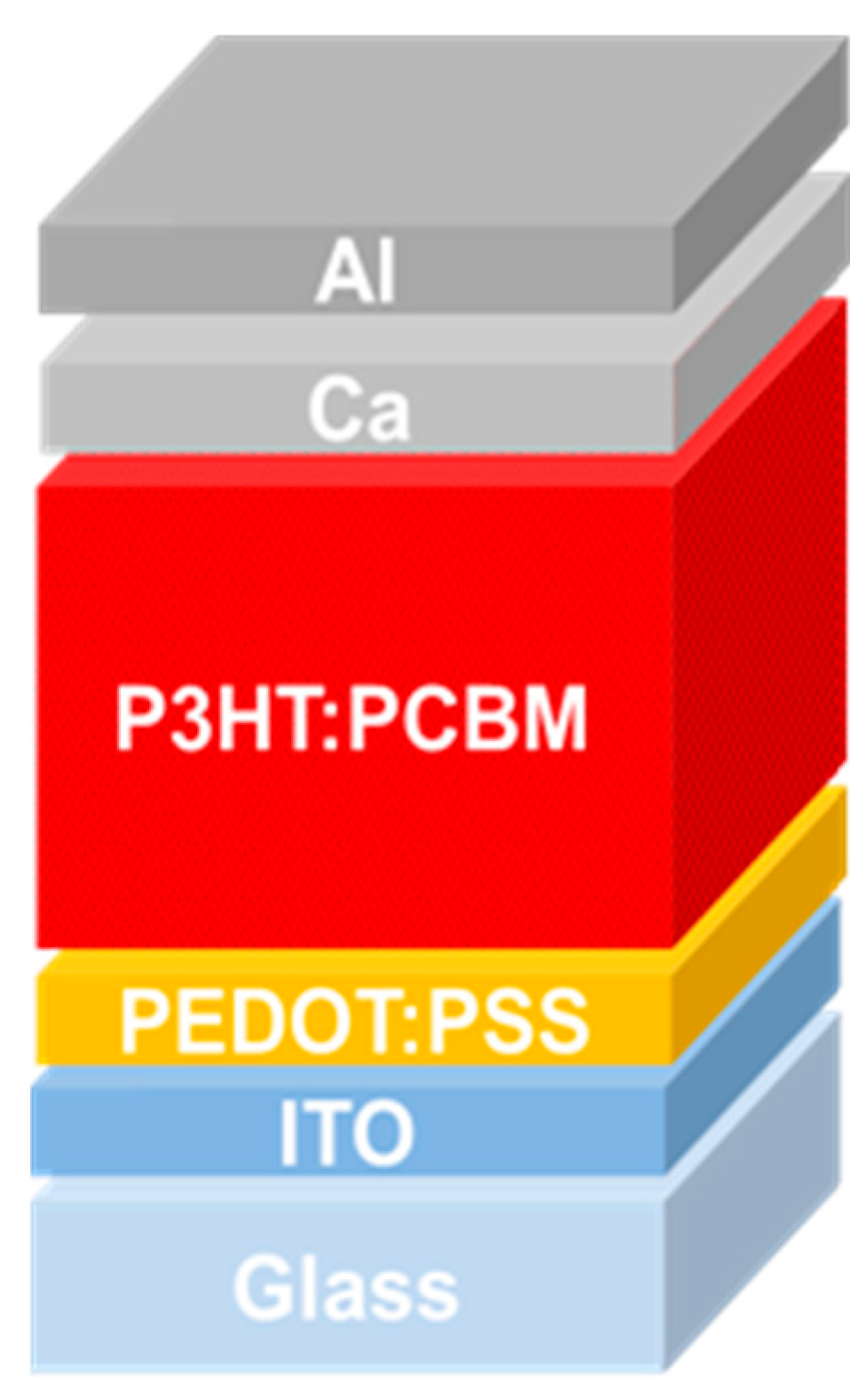
5. Materials and Methods
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhao, J.; Li, Y.; Yang, G.; Jiang, K.; Lin, H.; Ade, H.; Ma, W.; Yan, H. Efficient organic solar cells processed from hydrocarbon solvents. Nat. Energy 2016, 1, 15027. [Google Scholar] [CrossRef]
- Zhao, G.; He, Y.; Li, Y. 6.5% Efficiency of polymer solar cells based on poly(3-hexylthiophene) and indene-C60 bisadduct by device optimization. Adv. Mater. 2010, 22, 4355–4358. [Google Scholar] [CrossRef] [PubMed]
- González, D.M.; Körstgens, V.; Yao, Y.; Song, L.; Santoro, G.; Roth, S.V.; Müller-Buschbaum, P. Improved power conversion efficiency of P3HT:PCBM organic solar cells by strong spin-orbit coupling-induced delayed fluorescence. Adv. Energy Mater. 2015, 5, 1401770. [Google Scholar] [CrossRef]
- Li, M.; Gao, K.; Wan, X.; Zhang, Q.; Kan, B.; Xia, R.; Liu, F.; Yang, X.; Feng, H.; Ni, W.; et al. Solution-processed organic tandem solar cells with power conversion efficiencies > 12%. Nat. Photonics 2016, 11, 85–90. [Google Scholar] [CrossRef]
- Cowan, S.R.; Roy, A.; Heeger, A.J. Recombination in polymer-fullerene bulk heterojunction solar cells. Phys. Rev. B 2010, 82, 245207. [Google Scholar] [CrossRef]
- Hallermann, M.; Kriegel, I.; Da Como, E.; Berger, J.M.; von Hauff, E.; Feldmann, J. Charge transfer excitons in polymer/fullerene blends: The role of morphology and polymer chain conformation. Adv. Funct. Mater. 2009, 19, 3662–3668. [Google Scholar] [CrossRef]
- Collins, B.A.; Gann, E.; Guignard, L.; He, X.; McNeill, C.R.; Ade, H. Molecular miscibility of polymer–fullerene blends. J. Phys. Chem. Lett. 2010, 1, 3160–3166. [Google Scholar] [CrossRef]
- De Sio, A.; Madena, T.; Huber, R.; Parisi, J.; Neyshtadt, S.; Deschler, F.; Da Como, E.; Esposito, S.; von Hauff, E. Solvent additives for tuning the photovoltaic properties of polymer–fullerene solar cells. Sol. Energy Mater. Sol. Cells 2011, 95, 3536–3542. [Google Scholar] [CrossRef]
- Jeon, N.J.; Noh, J.H.; Kim, Y.C.; Yang, W.S.; Ryu, S.; Seok, S.I. Solvent engineering for high-performance inorganic–organic hybrid perovskite solar cells. Nat. Mater. 2014, 13, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Clarke, T.M.; Jamieson, F.C.; Durrant, J.R. Transient absorption studies of bimolecular recombination dynamics in polythiophene/fullerene blend films. J. Phys. Chem. C 2009, 113, 20934–20941. [Google Scholar] [CrossRef]
- Offermans, T.; Meskers, S.C.J.; Janssen, R.A.J. Time delayed collection field experiments on polymer:Fullerene bulk-heterojunction solar cells. J. Appl. Phys. 2006, 100, 074509. [Google Scholar] [CrossRef]
- Fallahpour, A.H.; Di Carlo, A.; Lugli, P. Sensitivity of the drift-diffusion approach in estimating the power conversion efficiency of bulk heterojunction polymer solar cells. Energies 2017, 10, 285. [Google Scholar] [CrossRef]
- Vijila, C.; Singh, S.P.; Williams, E.; Sonar, P.; Pivrikas, A.; Philippa, B.; White, R.; Naveen Kumar, E.; Gomathy Sandhya, S.; Gorelik, S.; et al. Relation between charge carrier mobility and lifetime in organic photovoltaics. J. Appl. Phys. 2013, 114, 184503. [Google Scholar] [CrossRef]
- Liu, Y.; Zojer, K.; Lassen, B.; Kjelstrup-Hansen, J.; Rubahn, H.-G.; Madsen, M. Role of the charge-transfer state in reduced Langevin recombination in organic solar cells: A theoretical study. J. Phys. Chem. C 2015, 119, 26588–26597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baumann, A.; Lorrmann, J.; Rauh, D.; Deibel, C.; Dyakonov, V. A new approach for probing the mobility and lifetime of photogenerated charge carriers in organic solar cells under real operating conditions. Adv. Mater. 2012, 24, 4381–4386. [Google Scholar] [CrossRef] [PubMed]
- Savo, B.; Barone, C.; Galdi, A.; Di Trolio, A. DC transport properties and resistance fluctuation processes in Sr2FeMoO6 polycrystalline thin films. Phys. Rev. B 2006, 73, 094447. [Google Scholar] [CrossRef]
- Barone, C.; Romeo, F.; Galdi, A.; Orgiani, P.; Maritato, L.; Guarino, A.; Nigro, A.; Pagano, S. Universal origin of unconventional 1/f noise in the weak-localization regime. Phys. Rev. B 2013, 87, 245113. [Google Scholar] [CrossRef]
- Barone, C.; Pagano, S.; Pallecchi, I.; Bellingeri, E.; Putti, M.; Ferdeghini, C. Thermal and voltage activated excess 1/f noise in FeTe0.5Se0.5 epitaxial thin films. Phys. Rev. B 2011, 83, 134523. [Google Scholar] [CrossRef]
- Barone, C.; Pagano, S.; Neitzert, H.C. Effect of concentration on low-frequency noise of multiwall carbon nanotubes in high-density polyethylene matrix. Appl. Phys. Lett. 2010, 97, 152107. [Google Scholar] [CrossRef]
- Barone, C.; Pagano, S.; Neitzert, H.C. Transport and noise spectroscopy of MWCNT/HDPE composites with different nanotube concentrations. J. Appl. Phys. 2011, 110, 113716. [Google Scholar] [CrossRef]
- Barone, C.; Landi, G.; Mauro, C.; Neitzert, H.C.; Pagano, S. Universal crossover of the charge carrier fluctuation mechanism in different polymer/carbon nanotubes composites. Appl. Phys. Lett. 2015, 107, 143106. [Google Scholar] [CrossRef]
- Landi, G.; Barone, C.; Mauro, C.; Neitzert, H.C.; Pagano, S. A noise model for the evaluation of defect states in solar cells. Sci. Rep. 2016, 6, 29685. [Google Scholar] [CrossRef] [PubMed]
- Barone, C.; Lang, F.; Mauro, C.; Landi, G.; Rappich, J.; Nickel, N.H.; Rech, B.; Pagano, S.; Neitzert, H.C. Unravelling the low-temperature metastable state in perovskite solar cells by noise spectroscopy. Sci. Rep. 2016, 6, 34675. [Google Scholar] [CrossRef] [PubMed]
- Landi, G.; Neitzert, H.C.; Barone, C.; Mauro, C.; Lang, F.; Albrecht, S.; Rech, B.; Pagano, S. Correlation between electronic defect states distribution and device performance of perovskite solar cells. Adv. Sci. 2017, in press. [Google Scholar] [CrossRef]
- Landi, G.; Barone, C.; De Sio, A.; Pagano, S.; Neitzert, H.C. Characterization of polymer:Fullerene solar cells by low-frequency noise spectroscopy. Appl. Phys. Lett. 2013, 102, 223902. [Google Scholar] [CrossRef]
- Barone, C.; Landi, G.; De Sio, A.; Neitzert, H.C.; Pagano, S. Thermal ageing of bulk heterojunction polymer solar cells investigated by electric noise analysis. Sol. Energy Mater. Sol. Cells 2014, 122, 40–45. [Google Scholar] [CrossRef]
- Kogan, S. Electronic Noise and Fluctuations in Solids, 1st ed.; Cambridge University Press: Cambridge, UK, 1996; ISBN 978-0521460347. [Google Scholar]
- Yang, X.; Loos, J.; Veenstra, S.C.; Verhees, W.J.H.; Wienk, M.M.; Kroon, J.M.; Michels, M.A.J.; Janssen, R.A.J. Nanoscale morphology of high-performance polymer solar cells. Nano Lett. 2005, 5, 579–583. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.-C.; Tseng, H.-C.; Ko, C.-J. Solvent mixtures for improving device efficiency of polymer photovoltaic devices. Appl. Phys. Lett. 2008, 92, 103316. [Google Scholar] [CrossRef]
- Li, G.; Shrotriya, V.; Huang, J.; Yao, Y.; Moriarty, T.; Emery, K.; Yang, Y. High-efficiency solution processable polymer photovoltaic cells by self-organization of polymer blends. Nat. Mater. 2005, 4, 864–868. [Google Scholar] [CrossRef]
- Yao, Y.; Hou, J.; Xu, Z.; Li, G.; Yang, Y. Effects of solvent mixtures on the nanoscale phase separation in polymer solar cells. Adv. Funct. Mater. 2008, 18, 1783–1789. [Google Scholar] [CrossRef]
- Ripolles-Sanchis, T.; Guerrero, A.; Bisquert, J.; Garcia-Belmonte, G. Diffusion-recombination determines collected current and voltage in polymer:Fullerene solar cells. J. Phys. Chem. C 2012, 116, 16925–16933. [Google Scholar] [CrossRef]
- Perrier, G.; de Bettignies, R.; Berson, S.; Lemaître, N.; Guillerez, S. Impedance spectrometry of optimized standard and inverted P3HT-PCBM organic solar cells. Sol. Energy Mater. Sol. Cells 2012, 101, 210–216. [Google Scholar] [CrossRef]
- Boix, P.P.; Guerrero, A.; Marchesi, L.F.; Garcia-Belmonte, G.; Bisquert, J. Current-voltage characteristics of bulk heterojunction organic solar cells: Connection between light and dark curves. Adv. Energy Mater. 2011, 1, 1073–1078. [Google Scholar] [CrossRef]
- Guerrero, A.; Ripolles-Sanchis, T.; Boix, P.P.; Garcia-Belmonte, G. Series resistance in organic bulk-heterojunction solar devices: Modulating carrier transport with fullerene electron traps. Org. Electron. 2012, 13, 2326–2332. [Google Scholar] [CrossRef]
- Garcia-Belmonte, G.; Munar, A.; Barea, E.M.; Bisquert, J.; Ugarte, I.; Pacios, R. Charge carrier mobility and lifetime of organic bulk heterojunctions analyzed by impedance spectroscopy. Org. Electron. 2008, 9, 847–851. [Google Scholar] [CrossRef]
- Dennler, G.; Mozer, A.J.; Juška, G.; Pivrikas, A.; Österbacka, R.; Fuchsbauer, A.; Sariciftci, N.S. Charge carrier mobility and lifetime versus composition of conjugated polymer/fullerene bulk-heterojunction solar cells. Org. Electron. 2006, 7, 229–234. [Google Scholar] [CrossRef]
- Bonavolontà, C.; Albonetti, C.; Barra, M.; Valentino, M. Electrical mobility in organic thin-film transistors determined by noise spectroscopy. J. Appl. Phys. 2011, 110, 093716. [Google Scholar] [CrossRef]
- Pivrikas, A.; Sariciftci, N.S.; Juška, G.; Österbacka, R. A review of charge transport and recombination in polymer/fullerene organic solar cells. Prog. Photovolt. Res. Appl. 2007, 15, 677–696. [Google Scholar] [CrossRef]
- Lorrmann, J.; Badada, B.H.; Inganäs, O.; Dyakonov, V.; Deibel, C. Charge carrier extraction by linearly increasing voltage: Analytic framework and ambipolar transients. J. Appl. Phys. 2010, 108, 113705. [Google Scholar] [CrossRef]
- Brus, V.V.; Lang, F.; Bundesmann, J.; Seidel, S.; Denker, A.; Rech, B.; Landi, G.; Neitzert, H.C.; Rappich, J.; Nickel, N.H. Defect dynamics in proton irradiated CH3NH3PbI3 perovskite solar cells. Adv. Electron. Mater. 2017, 3, 1600438. [Google Scholar] [CrossRef]
- Landi, G.; Fahrner, W.R.; Concilio, S.; Sessa, L.; Neitzert, H.C. Electrical hole transport properties of an ambipolar organic compound with Zn-atoms on a crystalline silicon heterostructure. IEEE J. Electron Devices Soc. 2014, 2, 179–181. [Google Scholar] [CrossRef]
- Laquai, F.; Andrienko, D.; Mauer, R.; Blom, P.W.M. Charge carrier transport and photogeneration in P3HT:PCBM photovoltaic blends. Macromol. Rapid Commun. 2015, 36, 1001–1025. [Google Scholar] [CrossRef] [PubMed]
- Landi, G.; Tunc, A.V.; De Sio, A.; Parisi, J.; Neitzert, H.-C. Hole-mobility limits for the Zn(OC)2 organic semiconductor obtained by SCLC and field-effect measurements. Phys. Status Solidi A 2016, 213, 1909–1914. [Google Scholar] [CrossRef]
- Pivrikas, A.; Ullah, M.; Sitter, H.; Sariciftci, N.S. Electric field dependent activation energy of electron transport in fullerene diodes and field effect transistors: Gill’s law. Appl. Phys. Lett. 2011, 98, 092114. [Google Scholar] [CrossRef]
- Sze, S.M.; Ng, K.K. Physics of Semiconductor Devices, 3rd ed.; Wiley-Interscience: Hoboken, NJ, USA, 2007; ISBN 978-0471143239. [Google Scholar]
- Servaites, J.D.; Ratner, M.A.; Marks, T.J. Organic solar cells: A new look at traditional models. Energy Environ. Sci. 2011, 4, 4410–4422. [Google Scholar] [CrossRef]
- Landi, G.; Barone, C.; Pagano, S.; De Sio, A.; Neitzert, H.C. Investigation of the solvent influence on polymer–fullerene solar cells by low frequency noise spectroscopy. Can. J. Phys. 2014, 92, 879–882. [Google Scholar] [CrossRef]
- Tsoi, W.C.; Spencer, S.J.; Yang, L.; Ballantyne, A.M.; Nicholson, P.G.; Turnbull, A.; Shard, A.G.; Murphy, C.E.; Bradley, D.D.C.; Nelson, J.; et al. Effect of crystallization on the electronic energy levels and thin film morphology of P3HT:PCBM blends. Macromolecules 2011, 44, 2944–2952. [Google Scholar] [CrossRef]
- Pivrikas, A.; Juška, G.; Mozer, A.J.; Scharber, M.; Arlauskas, K.; Sariciftci, N.S.; Stubb, H.; Österbacka, R. Bimolecular recombination coefficient as a sensitive testing parameter for low-mobility solar-cell materials. Phys. Rev. Lett. 2005, 94, 176806. [Google Scholar] [CrossRef] [PubMed]
- Deledalle, F.; Shakya Tuladhar, P.; Nelson, J.; Durrant, J.R.; Kirchartz, T. Understanding the apparent charge density dependence of mobility and lifetime in organic bulk heterojunction solar cells. J. Phys. Chem. C 2014, 118, 8837–8842. [Google Scholar] [CrossRef]
- Li, L.; Tang, H.; Wu, H.; Lu, G.; Yang, X. Effects of fullerene solubility on the crystallization of poly(3-hexylthiophene) and performance of photovoltaic devices. Org. Electron. 2009, 10, 1334–1344. [Google Scholar] [CrossRef]
- Barone, C.; Pagano, S.; Méchin, L.; Routoure, J.-M.; Orgiani, P.; Maritato, L. Apparent volume dependence of 1/f noise in thin film structures: Role of contacts. Rev. Sci. Instrum. 2008, 79, 053908. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Landi, G.; Barone, C.; Mauro, C.; De Sio, A.; Carapella, G.; Neitzert, H.C.; Pagano, S. Probing Temperature-Dependent Recombination Kinetics in Polymer:Fullerene Solar Cells by Electric Noise Spectroscopy. Energies 2017, 10, 1490. https://doi.org/10.3390/en10101490
Landi G, Barone C, Mauro C, De Sio A, Carapella G, Neitzert HC, Pagano S. Probing Temperature-Dependent Recombination Kinetics in Polymer:Fullerene Solar Cells by Electric Noise Spectroscopy. Energies. 2017; 10(10):1490. https://doi.org/10.3390/en10101490
Chicago/Turabian StyleLandi, Giovanni, Carlo Barone, Costantino Mauro, Antonietta De Sio, Giovanni Carapella, Heinz Christoph Neitzert, and Sergio Pagano. 2017. "Probing Temperature-Dependent Recombination Kinetics in Polymer:Fullerene Solar Cells by Electric Noise Spectroscopy" Energies 10, no. 10: 1490. https://doi.org/10.3390/en10101490





