1. Introduction
Organ transplantation is one of the most significant advances achieved in medicine during the latter half of the 20th century and still remains in many cases the only effective therapy for end-stage organ failure. Wherein, it is critical to maintain the supply line of organs for transplantation via organ preservation. In a logistical sense, preservation buys time, which is essential to organize staff and facilities, transport organs, and perform necessary laboratory tests and surgery [
1]. However, organ preservation is severally limited by a short preservation time.
Currently, there are nearly 2 million people living with extremity limb loss in the United States. The main causes of extremity limb loss have been attributed to vascular disease (54%)—including diabetes and peripheral arterial disease, trauma (45%), and cancer (less than 2%) [
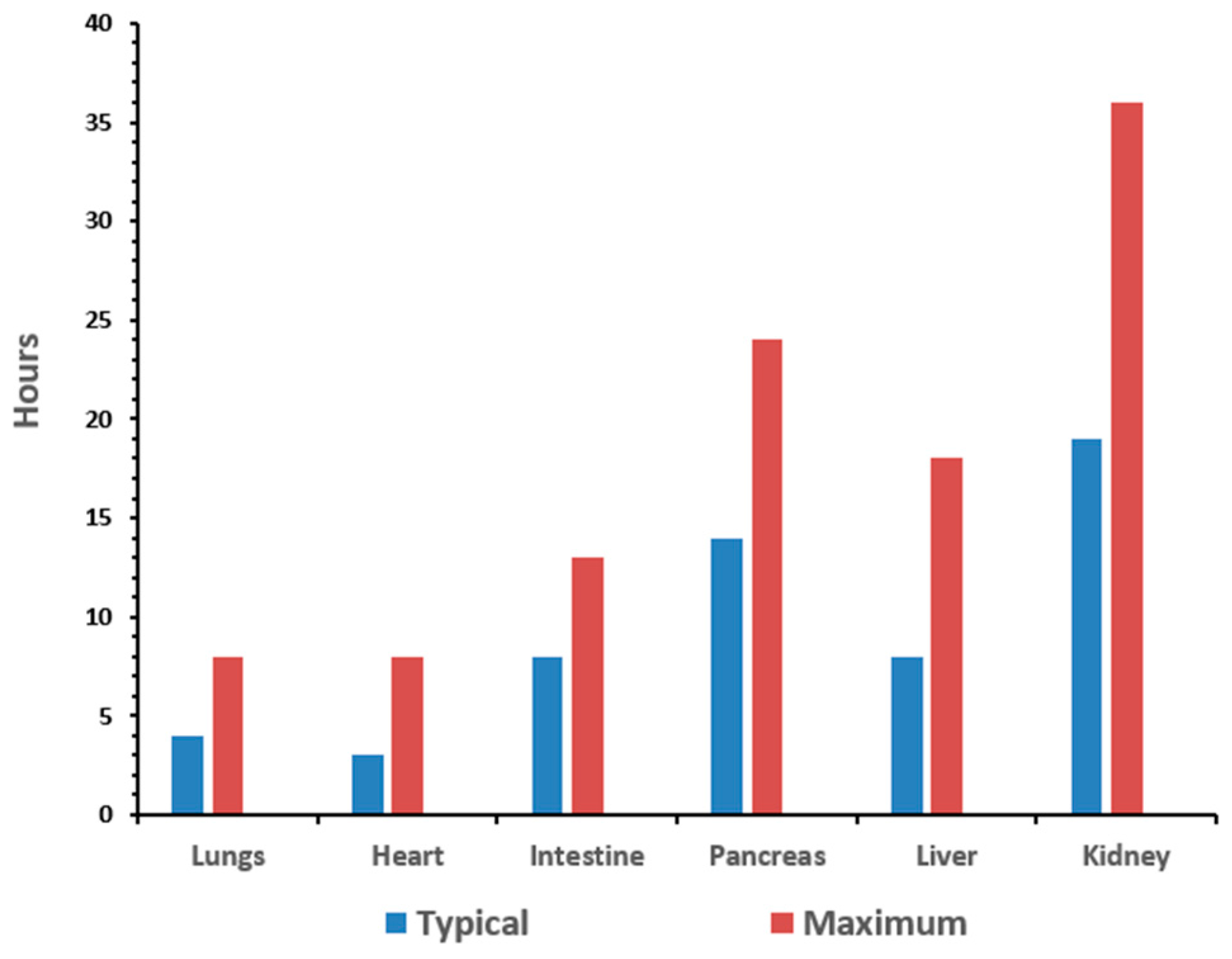
2]. The goal of organ preservation is to develop a method to monitor the biomarkers of stress induced by the procurement and preservation process in real-time in order to extend the preservation period to allow for assembly of the surgical team and the transport of the organ between facilities. The typical preservation time for organs is anywhere from 15 to 30 h [
3]. The time for the organs to remain viable depends on the organ type, as illustrated in
Figure 1.
Lactic acid is a key biomarker of stress that increases during the organ procurement process and is the main source of metabolically produced acid responsible for tissue acidosis. Therefore, when an individual experiences an extremity limb loss, the flow of nutrients and oxygen to that limb ceases, which initiates the deterioration of the limb’s tissue. This process is described as ischemia [
3]. Ischemia can be identified by the coupled and accelerated production of lactic acid and oxidation of glucose due to elevated glycolysis in the oxygen-deprived tissue [
4]. Thus, lactic acid concentration levels can serve as excellent biomarkers for monitoring tissue and organ health.
Generally, lactic acid exists as L-(+) and D-(-) enantiomers. While L-(+)-lactate is the normal intermediate in mammalian metabolism, the D-(-) enantiomer is usually produced by microorganisms, algae, and plants and is of limited utilization in humans [
5]. Determination of L-lactate concentration in blood is essential for the diagnosis of patient conditions in intensive care and during surgery. An elevated lactate level in blood is a major indicator of ischemic conditions of the respective tissue. This ischemic situation can be caused by shock trauma, respiratory insufficiency, carbon monoxide or cyanide intoxication, etc. [
6,
7]. Another cause for an altered lactate level is a disturbed lactate metabolism, which may sometimes be attributed to diabetes or absorptive abnormalities of short-chain fatty acids in the colon [
8]. In sport or space medicine, blood lactate levels during exercise can be used as an indicator of training status [
9,
10] and are now common for monitoring training and fitness of athletes or racing animals.
It is apparent that lactate is an important metabolite, and monitoring the existence or production of L- and D-lactic acid in a variety of media is of great interest. Among the various conventional analytical methods available for the determination of this analyte, colorimetric tests and chromatographic analysis are commonly used. However, these methods are complex, laborious, time-consuming, and complicated by intensive sample pre-treatment and reagent preparation [
11,
12]. Therefore, the development of an alternative method for lactic acid detection that is simple, direct, and inexpensive and enables real-time sensing with no need for sample preparation is highly desired.
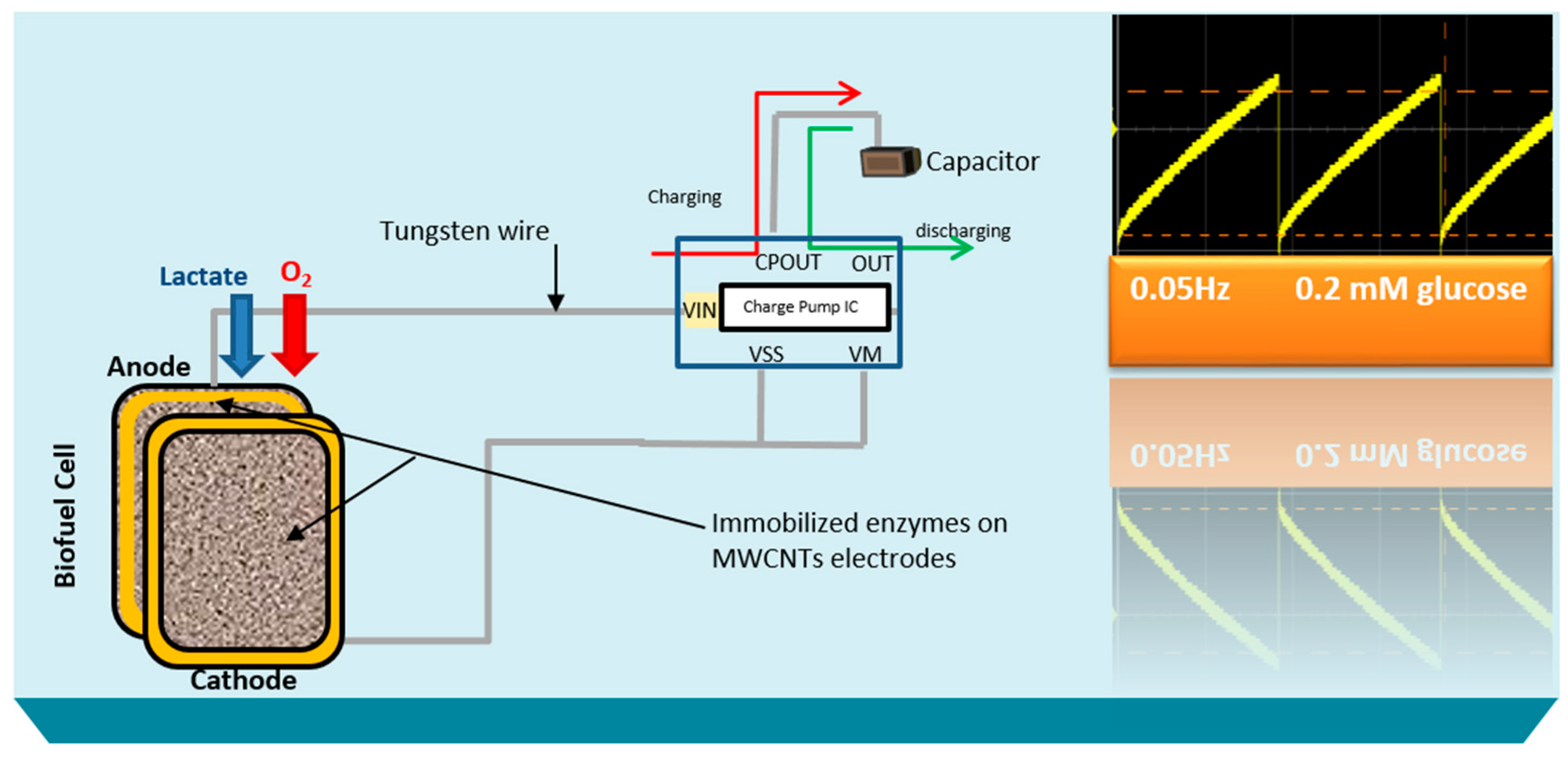
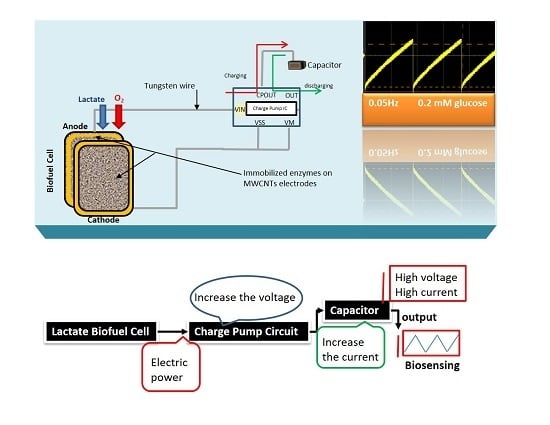
Here, the goal of our research work is to actively monitor the biodeteriotiation of the organ after the procement process by measuring lactic acid levels in the perfusate surrounding the organ using a novel self-powered biosensor. We present a self-powered electrochemical lactate biosensor that can generate its own electrical power to drive its internal circuits using carbon nanotubes. Such self-powered electrochemical biosensor comprises enzymatic biofuel cell and capacitor circuit functioning as a transducer. This enables the sensor to generate an electric power proportional to the analyte concentration and senses analyte concentration via the charging frequency of the capacitor circuit. These types of biosensors first reported by Slaughter and Kulkarni [
13] and are an improvement to traditional, battery-powered biosensors. This self-powered biosensor does not rely on external power sources, such as batteries and completely eliminate the potentiostat circuit employed in amperometric biosensors by deriving electrical power from its environment [
14,
15]. Thereby, the system continuously generates electrical power and senses analyte as long as there is a continuous supply of analytes.
3. Results and Discussion
A substrate material, such as the dense mesh network of multi-walled carbon nanotubes with high surface area is crucial to enhancing the enzyme loading and hence the electrocatlaytic activity to generate large electrical power from the constructed biofuel cell. The respective enzymes were successfully immobilized on to the PBSE-modified electrodes via the formation of amide bonds between the amino groups on the protein and the carboxyl group provided by PBSE [
13], wherein the double bond between the carbon and oxygen on PBSE is broken, leaving oxygen with a negative formal charge as the electrons move towards the more electronegative species. An unstable intermediate is formed when the nitrogen from the enzyme’s amino group donates electrons to the now electron-deficient carbon on the PBSE and subsequently develops a positive formal charge. The π electrons on the oxygen reform the double bond with the carbon atom on the PBSE [
16]. Simultaneously, the ester group also attached to the carbon breaks off, along with one of the hydrogen attached to the nitrogen, thus leaving behind a neutral, immobilized enzyme. D-lactate dehydrogenase (D-LDH) was used to catalyze the oxidation of lactic acid, and bilirubin oxidase was used to catalyze the reduction of molecular oxygen as illustrated in the redox equations of the two half reactions below:
under stressful conditions such as shock trauma in biological species, the production of lactic acid increases, so lactate dehydrogenase was selected to oxidize lactate since it does not produce toxic hydrogen peroxide byproduct, which can subsequently foul the electrode surface [
17,
18,
19]. Lactate oxidase was not employed in the bioelectrode design because it was observed that it is difficult to achieve direct electron transfer on carbon nanotube surfaces without the use of mediators [
18].
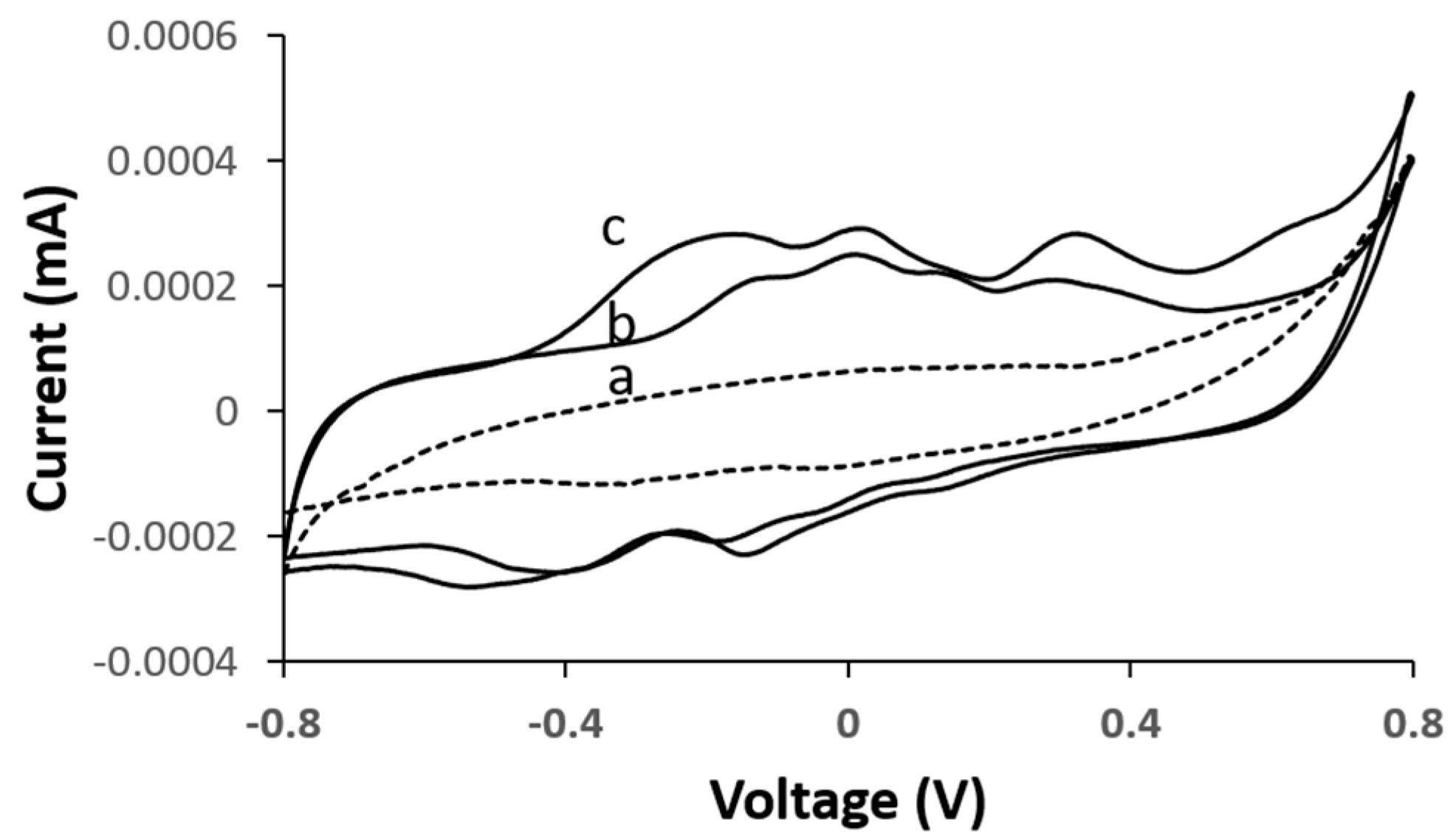
To evaluate the electrocatalytic activity of the bioanode and biocathode, cyclic voltammetry (CV) curves of the corresponding bioelectrodes were measured under their respective analytes. According to the measurements acquired in
Figure 3, with the D-LDH functionalized bioanode, the intensity in the reaction peak increased in the presence of increasing lactate concentrations—(b) 7 mM and (c) 15 mM—at an oxidation onset potential of between −0.28 and 0.46 V vs. Ag/AgCl, which is comparable to measurements previously reported [
20]. It is evident that the CV profile in the presence of lactic acid remained relatively unchanged irrespective of the lactic acid concentration level, thereby indicating that the D-LDH catalyst reacts with lactic acid and in turn is the best catalyst for the lactic acid oxidation reaction.
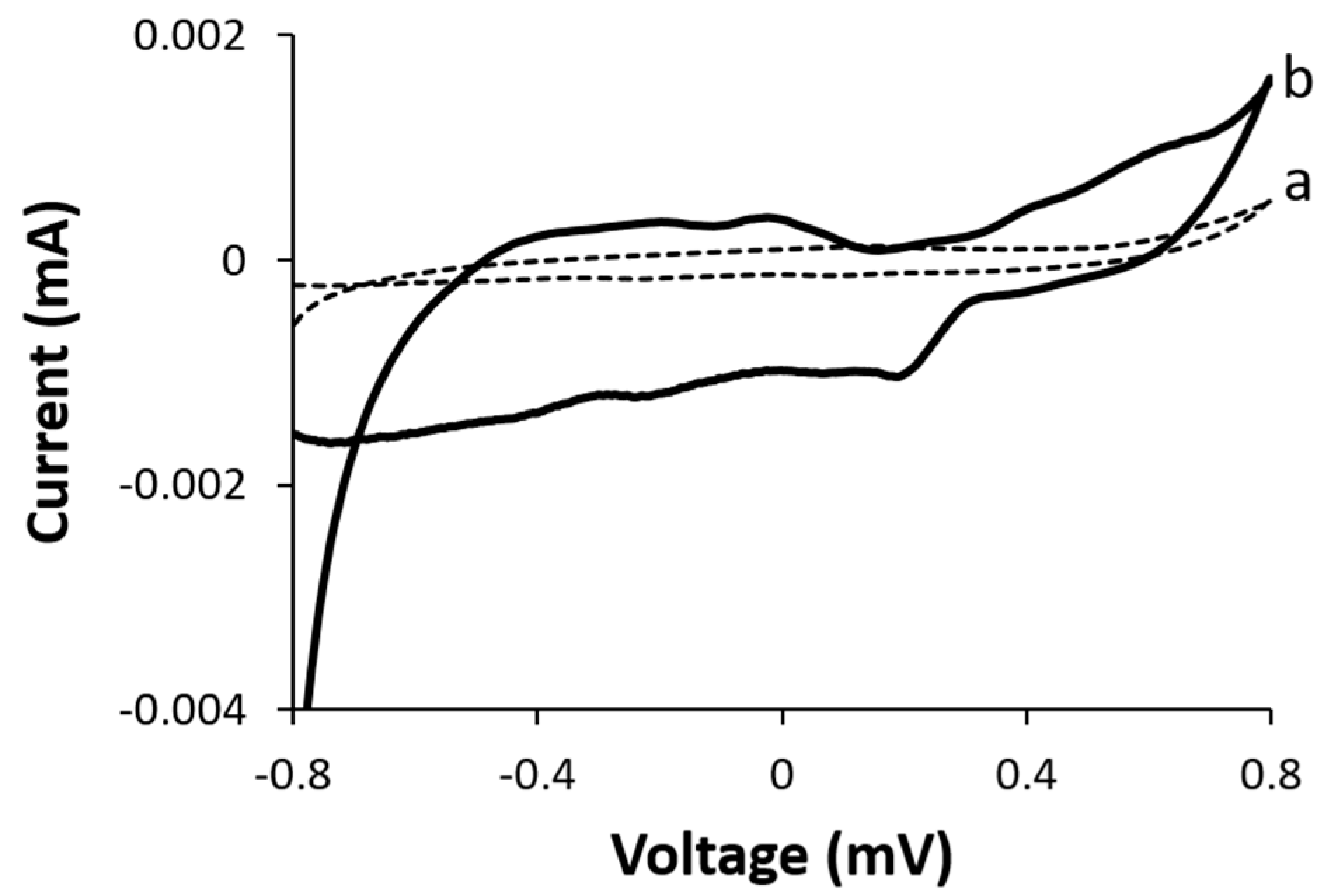
To examine the electrochemical reaction at the biocathode, we examined the effect of dissolved molecular oxygen gas on the electrocatalytic activity of the functionalized BOD biocathode.
Figure 4 represents the CV curves of the biocathode in the presence and absence of dissolved molecular oxygen. Insignificant electrocatalytic activity was observed in the absence of oxygen, whereas the onset of oxygen reduction was observed at ca. +0.32 V vs. Ag/AgCl in the presence of dissolved oxygen. The downshift in the electrocatalytic current is attributed to an oxygen reduction reaction as previously reported [
21].
These effects are ascribed to the increased active surface area achieve with the carbon nanotubes for enzyme immobilization and the formation of amide bonds between the enzymes and PBSE and the formation of π–π stacking on CNTs [
22,
23]. All of these effects enabled the electron transfer between the active center of the enzyme to the carbon nanotubes, thereby implying that both D-LDH and BOD were successfully immobilized on the dense mesh network of carbon nanotubes.
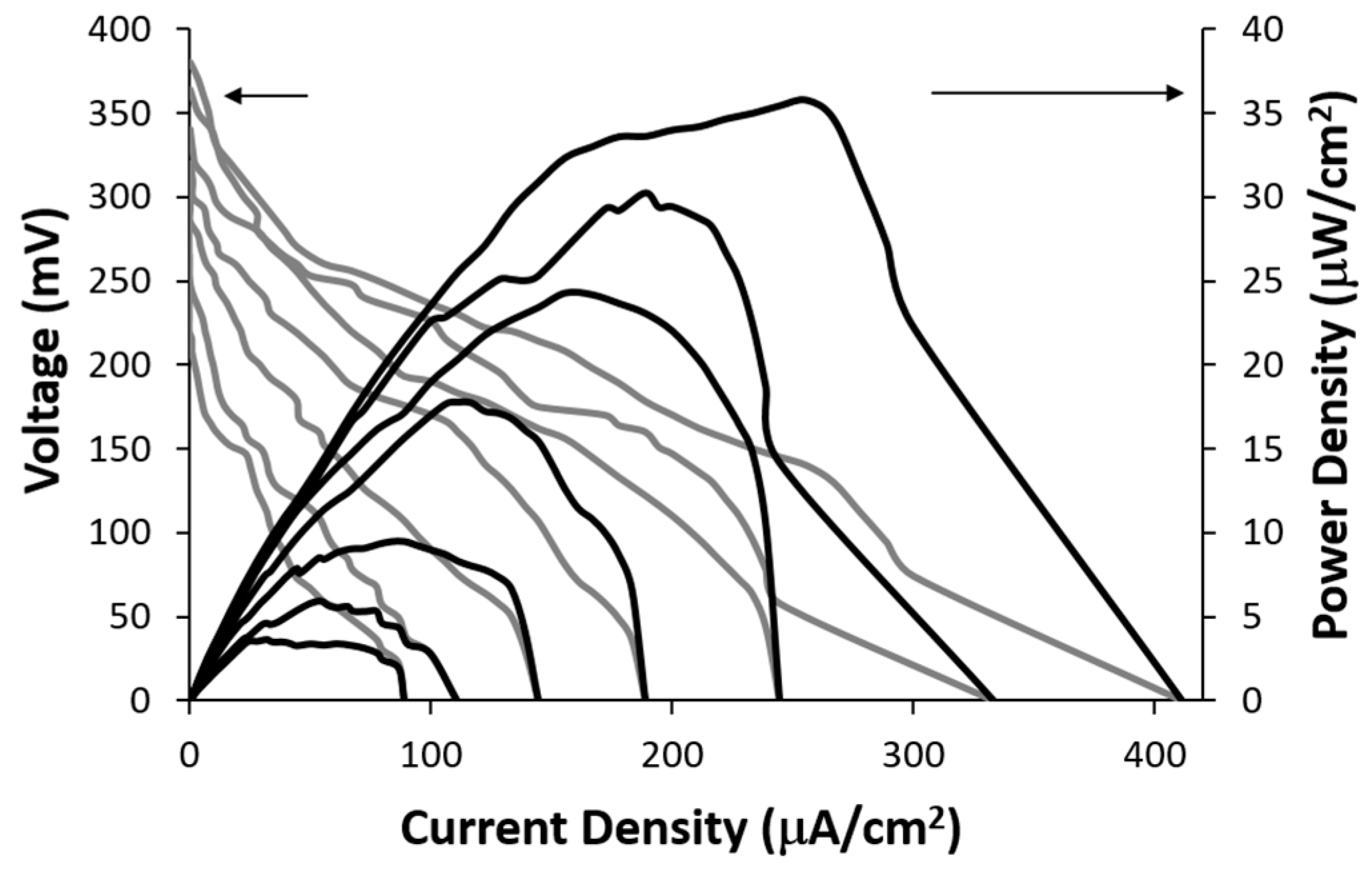
The electrical power generated by the lactate biofuel cell was determined using a series of resistance loads ranging from 1 MΩ to 0 Ω, while monitoring both potential and current as a function of time. The obtained data was subsequently employed to generate current-voltage and power-current density curves shown in
Figure 5. The open circuit voltages for 25 mM and 15 mM lactic acid solutions were 395.3 mV and 368.4 mV, respectively. The corresponding short-circuit current densities were observed to be 418.8 µA/cm² and 334.25 µA/cm², respectively.
Figure 5 shows the achievable power of 35.7 μW/cm
2 at a cell voltage of 0.14 V for 25 mM lactic acid. The lactate biofuel cell was designed for generating electrical power from low lactate concentrations of up to 25 mM for biological samples such as tears, urine and serum [
24].
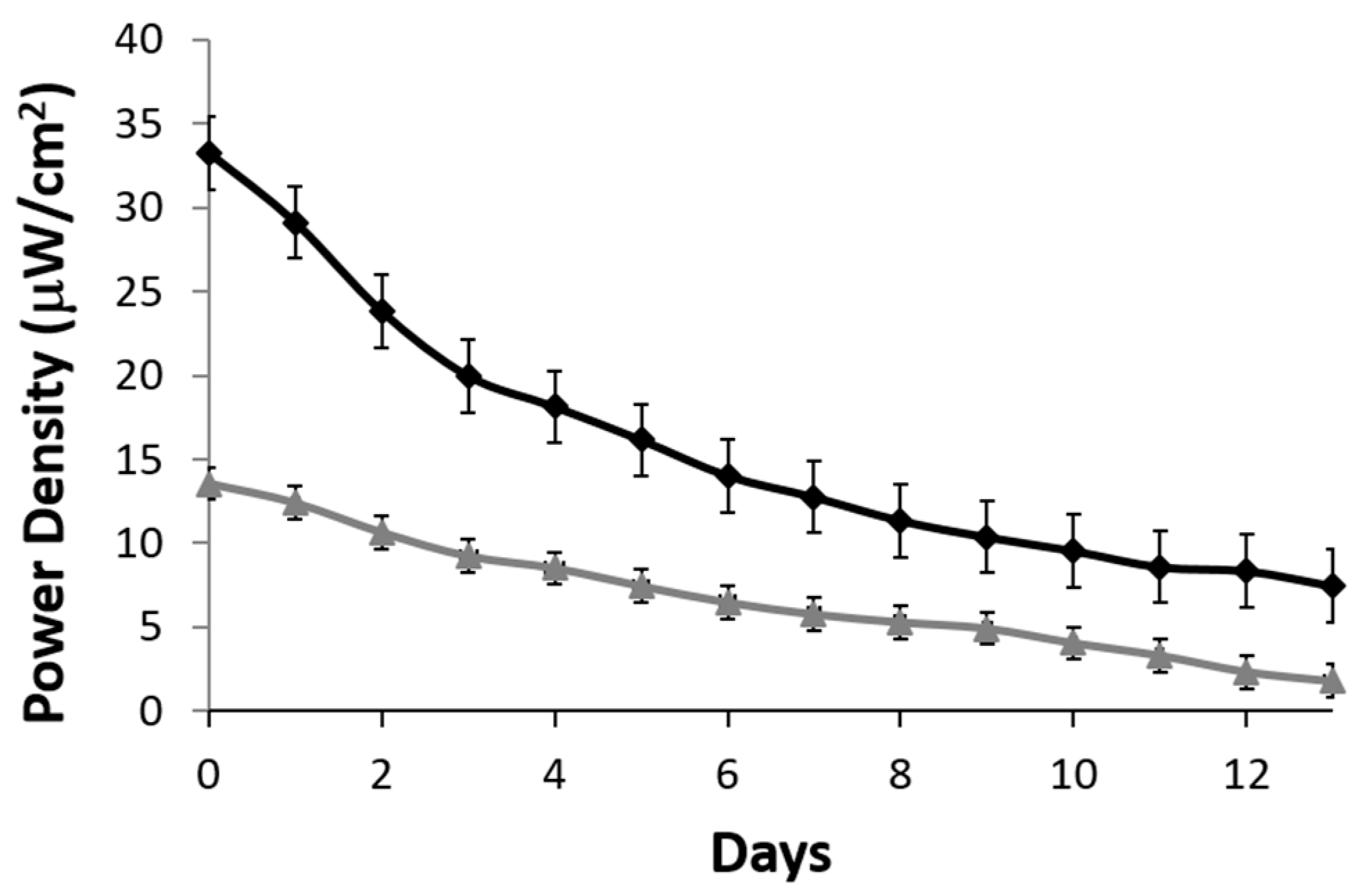
A stability profile of the lactate biofuel cell was generated over a two-week period. A resistance of 200 kΩ was used as a load. Over the 15-day period, the lactate biofuel cell’s electrical power gradually decreased from 32.3 to 14.02 μW/cm
2 when operating continuously in 25 mM lactate (black curve) for one week. This represents a 56.5% drop in the original biofuel cell activity and drops by an overall 76.9% by the end of the two-week period. On the other hand, when operating in 1 mM lactate (gray curve), a 52.2% drop in original electrical power was observed at Week 1 and an overall 86.8% was observed at the end of the two-week period as shown in
Figure 6. According to the stability profile, the system and the bioelectrodes would need to be calibrated and replaced every two weeks.
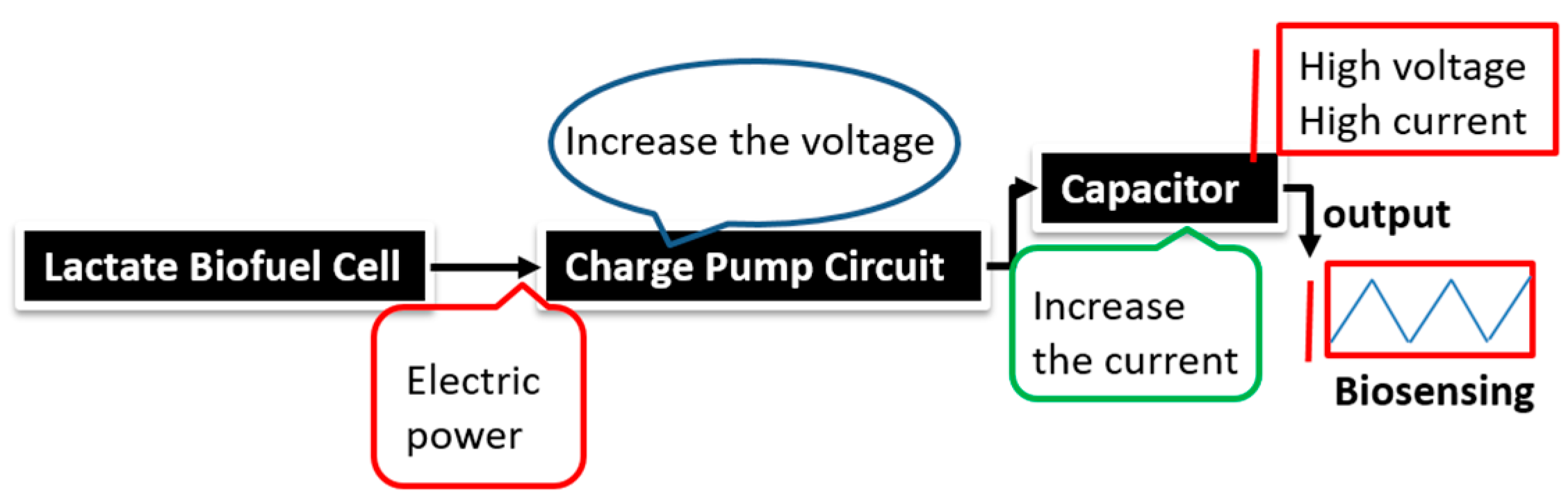
The stability profile illustrates the potential of using the electrical power produced by the lactate biofuel cell in the development of a self-powered electrochemical lactate biosensor. Hence, the electrical power generated from the lactate biofuel cell is supplied as the input voltage for the charge pump integrated circuit (IC, s882Z), which requires a minimum operating input voltage of approximately 275 mV. This nominal input voltage from the lactate biofuel cell is then amplified by the charge pump circuit to 1.8 V via the 10 pF capacitor functioning as the lactate transducer. The sensing ability of the system was determined by measuring the charging/discharging frequency of the 10 pF capacitor connected to the charge pump circuit in response to lactate concentrations. Once the capacitor is fully charged, the charge pump IC discharges the capacitor until the potential reaches 1.2 V, thereby supplying a burst of power as shown in
Figure 7.
This charging/discharging of the capacitor continues and is observed to be directly proportional to the biocatalytic oxidation of lactate at the bioanode. Therefore, by monitoring the capacitor charging/discharging frequency, the exact concentration of the analyte can be deduced. This self-powering system is less costly in terms of the circuit components used when compared to amperometric biosensors. It employs a charge pump IC and a capacitor, whereas an amperometric biosensor composed primary of operational precision amplifiers, transistors, resistors, and capacitors.
The overall self-powered lactate biosensor performance in lactic acid solution is shown in
Figure 8. The average frequency of charge/discharge cycle of the capacitor was observed for various levels of lactate analyte. Based on the results obtained, the sensitivity was calculated to be 125.88 Hz/mM-cm
2 and a linear dynamic range of 1–100 mM lactic acid was observed with a linear correlation-regression coefficient of 0.9948. These results are higher in comparison to amperometric lactic acid biosensors previously reported [
25,
26]. The linear calibration curve was determined using the following equation:
Additionally, this self-powered electrochemical lactate biosensor system demonstrates that lactic acid concentration levels above 25 mM, which is common in undiluted biological fluid, such as sweat can be easily sensed. Therefore, this self-powered electrochemical lactate biosensor is calibrated to sense a wide range of lactate concentration from 1 to 100 mM to account for both diluted and undiluted samples, thereby confirming the utility of the system for sensing a wide range of lactic acid concentrations. This includes lactic acid concentrations that are at the normal lactate level in the human body to the maximum level that can occur [
27,
28]. Interfering analyte, such as uric acid is reported in a corresponding article to exhibit no impact of the sensing of the analyte of interest [
15] using this self-powered lactate biosensor. Amperometric biosensors based on oxidase enzymes, the interfering analytes are decomposed at the same potential of ca. +700 mV as the byproduct hydrogen peroxide [
16,
17,
18], whereas, with this self-powered electrochemical lactate biosensor, the voltages generated by the lactate dehydrogenase-based biofuel cell are below +600 mV, thereby enabling lactic acid to be sensed with a high degree of selectivity.
4. Conclusions
The self-powered lactate biosensor describes a single lactate biofuel cell constructed from a D-LDH- and BOD-functionalized MWCNT bioanode and biocathode, respectively, for the non-invasive monitoring of lactic acid levels over an extended period of time. In this study, D-LDH enabled the oxidation of lactic acid, whereas BOD enabled the reduction of oxygen. The lactate biofuel cell produced 35.7 μW/cm2 when operating in 25 mM lactic acid. The charge pump and capacitor circuits enabled the excitation of the nominal electrical power generated by the lactate biofuel cell by generating a burst of power via the 10 pF capacitor from 1.2 to 1.8 V, which was then used to deduced lactic acid concentration in real time by monitoring the charging/discharging frequency of the capacitor.
The advantage of using dehydrogenase enzyme at the anode facilitated the electrocatalytic activity in the presence of lactic acid, which in turn was directly correlated with the burst of power generated by the charge pump and capacitor circuits. This sensing system’s stability along with its stable operation at physiological pH and temperature demonstrates that this self-powered lactate biosensor system could serve as a potential metabolic biosensor for monitoring undiluted and diluted lactate in biological fluids.
We demonstrated a lactate self-powered biosensor capable of non-invasive, real-time monitoring of a key metabolite of stress or trauma. Therefore, dehydrogenase enzymes have the advantage of achieving electron transfer on carbon nanotubes and of enabling the widespread sensing of other metabolites of interest. The combination of glucose dehydrogenace and D-LDH could enable the dual sensing of these two metabolites, which are elevated during injury. The realization of the complete self-powered lactate biosensor system could enable its deployment in clinical settings to simultaneously monitor key biomarkers to access organ health and thereby extend the health outcome of the organ transplants.