Laser Radiation Induces Growth and Lipid Accumulation in the Seawater Microalga Chlorella pacifica
Abstract
:1. Introduction
2. Results
2.1. Cell Growth Assay
2.2. Measurement of Total Chlorophyll
2.3. Nitrate Reductase Levels
2.4. Microscopic Analyses
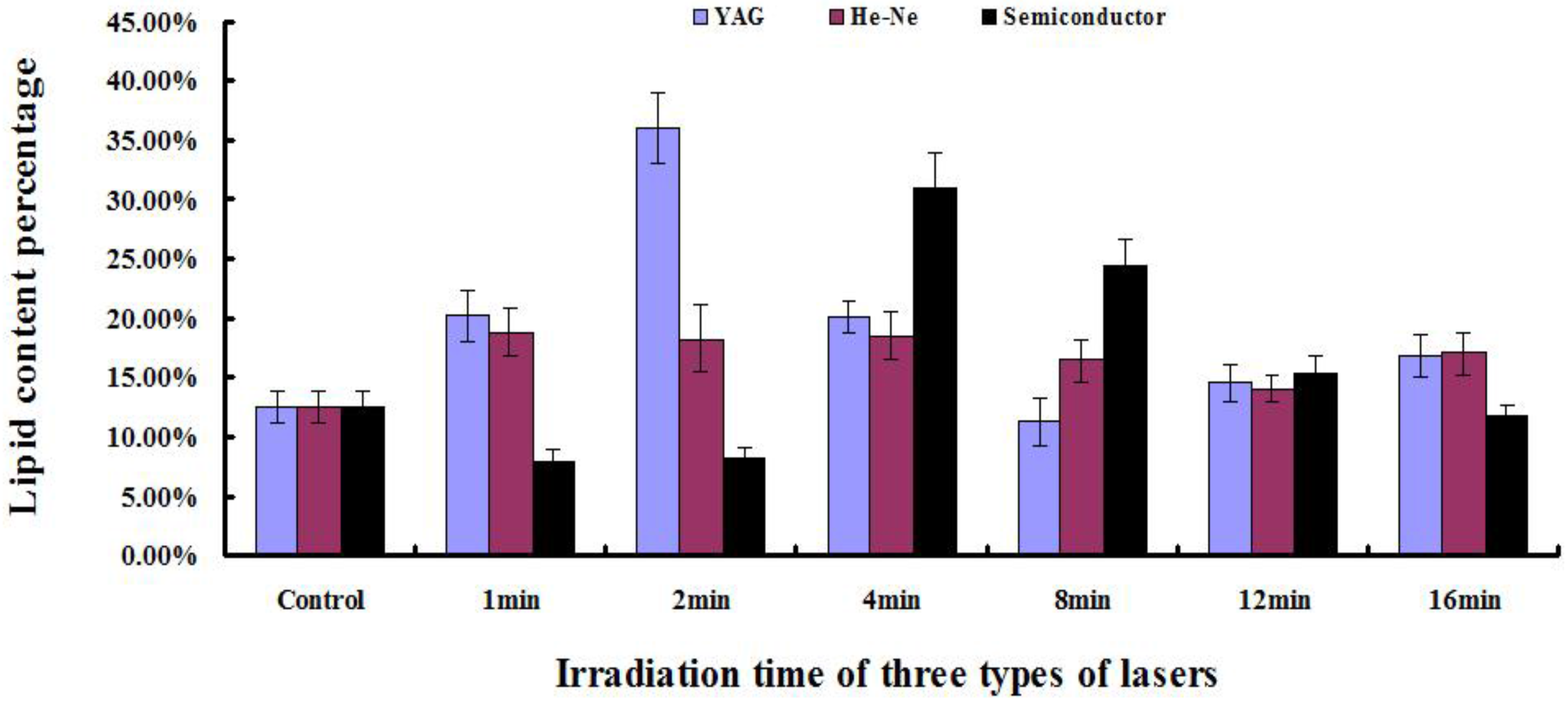
2.5. Determination of Lipid Content
2.6. Lipid Productivity
3. Discussion
4. Materials and Methods
4.1. Algal Strain and Culture Medium
4.2. Laser Irradiation
4.3. Determination of Calibration Curves
4.4. The Quantification Of Total Chlorophyll Concentration
4.5. Determination of Nitrate Reductase Activity
4.6. Microscopic Analysis
4.7. Measurements of Lipid Content with Gravimetric Methods
4.8. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Feng, Y.J.; Li, C.; Zhang, D.W. Lipid production of Chlorella vulgaris cultured in artificial wastewater medium. Bioresour. Technol. 2011, 102, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Brennan, L.; Owende, P. Biofuels from microalgae—A review of technologies for production, processing, and extractions of biofuels and co-products. Renew. Sustain. Energy Rev. 2010, 14, 557–577. [Google Scholar] [CrossRef]
- Huang, G.H.; Chen, F.; Wei, D.; Zhang, X.W.; Chen, G. Biodiesel production by microalgal biotechnology. Appl. Energy 2010, 87, 38–46. [Google Scholar] [CrossRef]
- Ahmad, A.L.; Yasin, N.H.M.; Derek, C.J.C.; Lim, J.K. Microalgae as a sustainable energy source for biodiesel production: A review. Renew. Sustain. Energy Rev. 2011, 15, 584–593. [Google Scholar] [CrossRef]
- Arenas, E.G.; Palacio, M.C.R.; Juantorena, A.U.; Fernando, S.E.L.; Sebastian, P.J. Microalgae as a potential source for biodiesel production: Techniques, methods, and other challenges. Int. J. Energy Res. 2017, 41, 761–789. [Google Scholar] [CrossRef]
- Li, Y.Q.; Horsman, M.; Wang, B.; Wu, N.; Lan, C.Q. Effects of nitrogen sources on cell growth and lipid accumulation of green alga Neochloris oleoabundans. Appl. Microbiol. Biotechnol. 2008, 81, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Converti, A.; Casazza, A.A.; Ortiz, E.Y.; Perego, P.; Del Borghi, M. Effect of temperature and nitrogen concentration on the growth and lipid content of Nannochloropsis oculata and Chlorella vulgaris for biodiesel production. Chem. Eng. Process. Process Intensif. 2009, 48, 1146–1151. [Google Scholar] [CrossRef]
- Hsieh, C.H.; Wu, W.T. Cultivation of microalgae for oil production with a cultivation strategy of urea limitation. Bioresour. Technol. 2009, 100, 3921–3926. [Google Scholar] [CrossRef] [PubMed]
- Widjaja, A.; Chien, C.C.; Ju, Y.H. Study of increasing lipid production from fresh water microalgae Chlorella vulgaris. J. Taiwan Inst. Chem. Eng. 2009, 40, 13–20. [Google Scholar] [CrossRef]
- Xin, L.; Hu, H.Y.; Ke, G.; Ying-Xue, S. Effects of different nitrogen and phosphorus concentrations on the growth, nutrient uptake, and lipid accumulation of a freshwater microalga Scenedesmus sp. Bioresour. Technol. 2010, 101, 5494–5500. [Google Scholar] [CrossRef] [PubMed]
- Khotimchenko, S.V.; Yakovleva, I.M. Lipid composition of the red alga Tichocarpus crinitus exposed to different levels of photon irradiance. Phytochemistry 2005, 66, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Weldy, C.S.; Huesemann, M.H. Lipid production by Dunaliella salina in batch culture: Effects of nitrogen limitation and light intensity. J. Undergrad. Res. 2007, 7, 115–122. [Google Scholar]
- Xin, L.; Hong-ying, H.; Yu-ping, Z. Growth and lipid accumulation properties of a freshwater microalga Scenedesmus sp. under different cultivation temperature. Bioresour. Technol. 2011, 102, 3098–3102. [Google Scholar]
- Takagi, M.; Karseno; Yoshida, T. Effect of salt concentration on intracellular accumulation of lipids and triacylglyceride in marine microalgae Dunaliella cells. J. Biosci. Bioeng. 2006, 101, 223–226. [Google Scholar] [CrossRef] [PubMed]
- Castrillo, M.; Lucas-Salas, L.M.; Rodríguez-Gil, C.; Martínez, D. High pH-induced flocculation–sedimentation and effect of supernatant reuse on growth rate and lipid productivity of Scenedesmus obliquus and Chlorella vulgaris. Bioresour. Technol. 2013, 128, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Chia, M.A.; Lombardi, A.T.; Melão, M.G.G.; Parrish, C.C. Lipid composition of Chlorella vulgaris (Trebouxiophyceae) as a function of different cadmium and phosphate concentrations. Aquat. Toxicol. 2013, 128–129, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Mata, T.M.; Martins, A.A.; Caetano, N.S. Microalgae for biodiesel production and other applications: A review. Renew. Sustain. Energy Rev. 2010, 14, 217–232. [Google Scholar] [CrossRef]
- Neenan, B.; Feinberg, D.; Hill, A.; McIntosh, R.; Terry, K. Fuels from Microalgae: Technology Status, Potential, and Research Requirements; Pub SER/SP-231-2550; Solar Energy Research Institute: Golden, CO, USA, 1986; pp. 149–158. [Google Scholar]
- Sheehan, J.; Dunahay, T.; Benemann, J.; Roessler, P. A Look Back at the U.S. Department of Energy’s Aquatic Species Program-Biodiesel from Algae; TP-580-24190, Report NREL/TP; Nation Renewable Energy Laboratory: Golden, CO, USA, 1998; pp. 580–2419. [Google Scholar]
- Chung, Y.S.; Lee, J.W.; Chung, C.H. Molecular challenges in microalgae towards cost-effective production of quality biodiesel. Renew. Sustain. Energy Rev. 2017, 74, 139–144. [Google Scholar] [CrossRef]
- Smith, V.H.; Sturm, B.S.M.; Billings, S.A. The ecology of algal biodiesel production. Trends Ecol. Evol. 2010, 25, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, A.C.; Dominguez, P.A.; Cruz, O.A.; Ivanov, R.; Carballo, C.A.; Zepeda, B.R. Laser in agriculture. Int. Agrophys. 2010, 24, 407–422. [Google Scholar]
- Yanagisawa, S. Transcription factors involved in controlling the expression of nitrate reductase genes in higher plants. Plant Sci. 2014, 229, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Bianucci, E.; Fullana, C.; Furlan, A.; Castro, S. Antioxidant defense system responses and role of nitrate reductase in the redox balance maintenance in Bradyrhizobium japonicum strains exposed to cadmium. Enzym. Microb. Technol. 2013, 53, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Kohli, R.; Bose, B.; Gupta, P.K. Induction of phr gene expression in E. coli strain KY706/pPL-1 by He–Ne laser (632.8 nm) irradiation. J. Photochem. Photobiol. B 2001, 60, 136–142. [Google Scholar] [CrossRef]
- Lu, W.Y.; Wen, J.P.; Jia, X.Q.; Sun, B.; Chen, Y.; Liu, M.H. Effect of He–Ne laser irradiation on hydrogen production by Enterobacter aerogenes. Int. J. Hydrogen Energy 2008, 33, 34–42. [Google Scholar] [CrossRef]
- Ma, F.X.; Chen, X.Y. Laser applications in seed germination, mutation breeding and gene engineering for forestry and horticulture. Phys. Beijing 2007, 36, 637–641. [Google Scholar]
- Wang, L.Q.; Yang, G.Z.; Chen, F.D. Biological effects and application in plants and animals heredity and breeding by laser mutation. Acta Laser Biol. Sin. 1996, 6, 1097–1102. [Google Scholar]
- Baer, S.; Heining, M.; Schwerna, P.; Buchholz, R.; Hübner, H. Optimization of spectral light quality for growth and product formation in different microalgae using a continuous photobioreactor. Algal Res. 2016, 14, 109–115. [Google Scholar] [CrossRef]
- De Mooij, T.; de Vries, G.; Latsos, C.; Wijffels, R.H.; Janssen, M. Impact of light color on photobioreactor productivity. Algal Res. 2016, 15, 32–42. [Google Scholar] [CrossRef]
- Wang, M.Z.; Chen, B.L.; Zhuang, H.R.; Ou, L.; Shi, Q.Q.; Wu, S.G. The effect of Nd: YAG laser on Porphyridium cruentum growth and extracellular polysaccharide production. Acta Laser Biol. Sin. 2002, 11, 6–9. [Google Scholar]
- Nandakumar, K.; Obika, H.; Shinozaki, T.; Ooie, T.; Utsumi, A.; Yano, T. Pulsed laser irradiation impact on two marine diatoms Skeletonema costatum and Chaetoceros gracilis. Water Res. 2003, 37, 2311–2316. [Google Scholar] [CrossRef]
- Katsuda, T.; Lababpour, A.; Shimahara, K.; Katoh, S. Astaxanthin production by Haematococcus pluvialis under illumination with LEDs. Enzym. Microb. Technol. 2004, 35, 81–86. [Google Scholar] [CrossRef]
- Kuwahara, S.S.; Cuello, J.L.; Myhre, G.; Pau, S. Growth of the green algae Chlamydomonas reinhardtii under red and blue lasers. Opt. Lasers Eng. 2011, 49, 434–438. [Google Scholar] [CrossRef]
- Bodnar, O.I.; Viniarska, H.B.; Vasilenko, O.V.; Grubinko, V.V. Pigment content of Chlorella vulgaris Beij. Under influence of sodium selenite and metals ions. Biotechnol. Acta 2016, 9, 71–78. [Google Scholar] [CrossRef]
- Seyfabadi, J.; Ramezanpour, Z.; Khoey, Z.A. Protein, fatty acid, and pigment content of Chlorella vulgaris under different light regimes. J. Appl. Phycol. 2011, 23, 721–726. [Google Scholar] [CrossRef]
- Lau, P.S.; Tam, N.F.Y.; Wong, Y.S. Effect of carrageenan immobilization on the physiological activities of Chlorella vulgaris. Bioresour. Technol. 1998, 63, 115–121. [Google Scholar] [CrossRef]
- Miao, X.L.; Wu, Q.Y.; Yang, C.Y. Fast pyrolysis of microalgae to produce renewable fuels. J. Anal. Appl. Pyrolysis 2004, 71, 855–863. [Google Scholar] [CrossRef]
- Tang, H.Y.; Chen, M.; Garcia, M.E.D.; Abunasser, N.; Ng, K.Y.S.; Salley, S.O. Culture of microalgae Chlorella minutissima for biodiesel feedstock production. Biotechnol. Bioeng. 2011, 108, 2280–2287. [Google Scholar] [CrossRef] [PubMed]
- Mehtani, J.; Arora, N.; Patel, A.; Jain, P.; Pruthi, P.A.; Poluri, K.M.; Pruthi, V. Augmented lipid accumulation in ethyl methyl sulphonate mutants of oleaginous microalga for biodiesel production. Bioresour. Technol. 2017, 242, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Kreslavski, V.D.; Fomina, I.R.; Los, D.A.; Carpentier, R.; Kuznetsov, V.V.; Allakhverdiev, S.I. Red and near infra-red signaling: Hypothesis and perspectives. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 190–203. [Google Scholar] [CrossRef]
- Zhang, C.; Zheng, S.; Meng, F.P. Study on Chlorella pacifica cultivation based municipal sewage sludge. Chin. J. Environ. Eng. 2010, 4, 1186–1190. [Google Scholar]
- Guillard, R.R.; Ryther, J.H. Studies of marine planktonic diatoms: I. Cyclotella nana Hustedt, and Detonula confervacea (CLEVE) Gran. Can. J. Microbiol. 1962, 8, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Lau, K.Y.; Pleissner, D.; Lin, C.S.K. Recycling of food waste as nutrients in Chlorella vulgaris cultivation. Bioresour. Technol. 2014, 170, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Arrese-Igor, C.; Garcia-Plazaola, J.I.; Diaz, A.; Apaparico-Tejo, P.M. Distribution of nitrate reductase activity in nodulated lucerne plants. Plant Soil 1991, 131, 107–113. [Google Scholar] [CrossRef]
- Jauregui, L.; Aparicio-Tejo, P.M.; Avila, C.; Cañas, R.; Sakalauskiené, S.; Aranjuelo, I. Root-shoot interactions explain the reduction of leaf mineral content in Arabidopsis plants grown under elevated [CO2] conditions. Physiol. Plant. 2016, 158, 65–79. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.K.Y.; Garg, S.; Timmins, M.; Zhang, E.S.B.; Thomas-Hall, S.R.; Schuhmann, H. Isolation and evaluation of oil-producing microalgae from subtropical coastal and brackish waters. PLoS ONE 2012, 7, e40751. [Google Scholar] [CrossRef] [PubMed]
- Bligh, E.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]







| Treatments of Nd: YAG Laser | Biomass g L−1 | Total Lipid g L−1 | Lipid Productivity mg L−1 d−1 |
|---|---|---|---|
| Control | 0.258 ± 0.017 | 0.033 ± 0.003 | 8.254 ± 1.662 |
| 1 min | 0.322 ± 0.025 * | 0.064 ± 0.006 ** | 16.004 ± 2.313 ** |
| 2 min | 0.251 ± 0.019 | 0.088 ± 0.008 ** | 22.001 ± 3.278 ** |
| 4 min | 0.417 ± 0.028 ** | 0.061 ± 0.004 ** | 15.246 ± 2.557 ** |
| 8 min | 0.303 ± 0.023 | 0.048 ± 0.004 * | 11.995 ± 1.382 ** |
| 12 min | 0.289 ± 0.021 | 0.042 ± 0.003 * | 10.497 ± 1.104 |
| 16 min | 0.329 ± 0.021 * | 0.055 ± 0.051 * | 13.753 ± 1.652 ** |
| Treatments of He-Ne Laser | Biomass g L−1 | Total Lipid g L−1 | Lipid Productivity mgL−1 d−1 |
|---|---|---|---|
| Control | 0.258 ± 0.017 | 0.033 ± 0.003 | 8.254 ± 1.662 |
| 1 min | 0.341 ± 0.028 * | 0.056 ± 0.003 * | 14.002 ± 1.274 * |
| 2 min | 0.214 ± 0.021 * | 0.046 ± 0.0065 ** | 11.493 ± 1.531 ** |
| 4 min | 0.449 ± 0.020 ** | 0.065 ± 0.007 ** | 16.254 ± 2.452 ** |
| 8 min | 0.317 ± 0.035 | 0.035 ± 0.003 | 8.746 ± 1.512 |
| 12 min | 0.242 ± 0.031 * | 0.045 ± 0.006 * | 11.249 ± 1. 668 * |
| 16 min | 0.393 ± 0.021 ** | 0.032 ± 0.003 | 8.004 ± 1.032 |
| Treatments of Semiconductor Laser | Biomass g L−1 | Total Lipid g L−1 | Lipid Productivity mg L−1 d−1 |
|---|---|---|---|
| Control | 0.258 ± 0.017 | 0.033 ± 0.003 | 8.254 ± 1.662 |
| 1 min | 0.324 ± 0.022 * | 0.026 ± 0.003 | 6.495 ± 0.344 |
| 2 min | 0.435 ± 0.028 ** | 0.364 ± 0.003 | 9.004 ± 0. 886 |
| 4 min | 0.354 ± 0.031 * | 0.077 ± 0.054 ** | 19.247 ± 1.781 ** |
| 8 min | 0.542 ± 0.019 ** | 0.059 ± 0.005 ** | 14.749 ± 2.109 * |
| 12 min | 0.423 ± 0.029 ** | 0.043 ± 0.005 * | 10.753 ± 1.607 |
| 16 min | 0.449 ± 0.002 ** | 0.028 ± 0.003 | 6.997 ± 0.914 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Gao, Z.; Li, Z.; Du, H.; Lin, B.; Cui, M.; Yin, Y.; Lei, F.; Yu, C.; Meng, C. Laser Radiation Induces Growth and Lipid Accumulation in the Seawater Microalga Chlorella pacifica. Energies 2017, 10, 1671. https://doi.org/10.3390/en10101671
Zhang H, Gao Z, Li Z, Du H, Lin B, Cui M, Yin Y, Lei F, Yu C, Meng C. Laser Radiation Induces Growth and Lipid Accumulation in the Seawater Microalga Chlorella pacifica. Energies. 2017; 10(10):1671. https://doi.org/10.3390/en10101671
Chicago/Turabian StyleZhang, Haonan, Zhengquan Gao, Zhe Li, Huanmin Du, Bin Lin, Meng Cui, Yonghao Yin, Fengming Lei, Chunyu Yu, and Chunxiao Meng. 2017. "Laser Radiation Induces Growth and Lipid Accumulation in the Seawater Microalga Chlorella pacifica" Energies 10, no. 10: 1671. https://doi.org/10.3390/en10101671





