The Effects of Plant Growth Regulators on Cell Growth, Protein, Carotenoid, PUFAs and Lipid Production of Chlorella pyrenoidosa ZF Strain
Abstract
:1. Introduction
2. Results
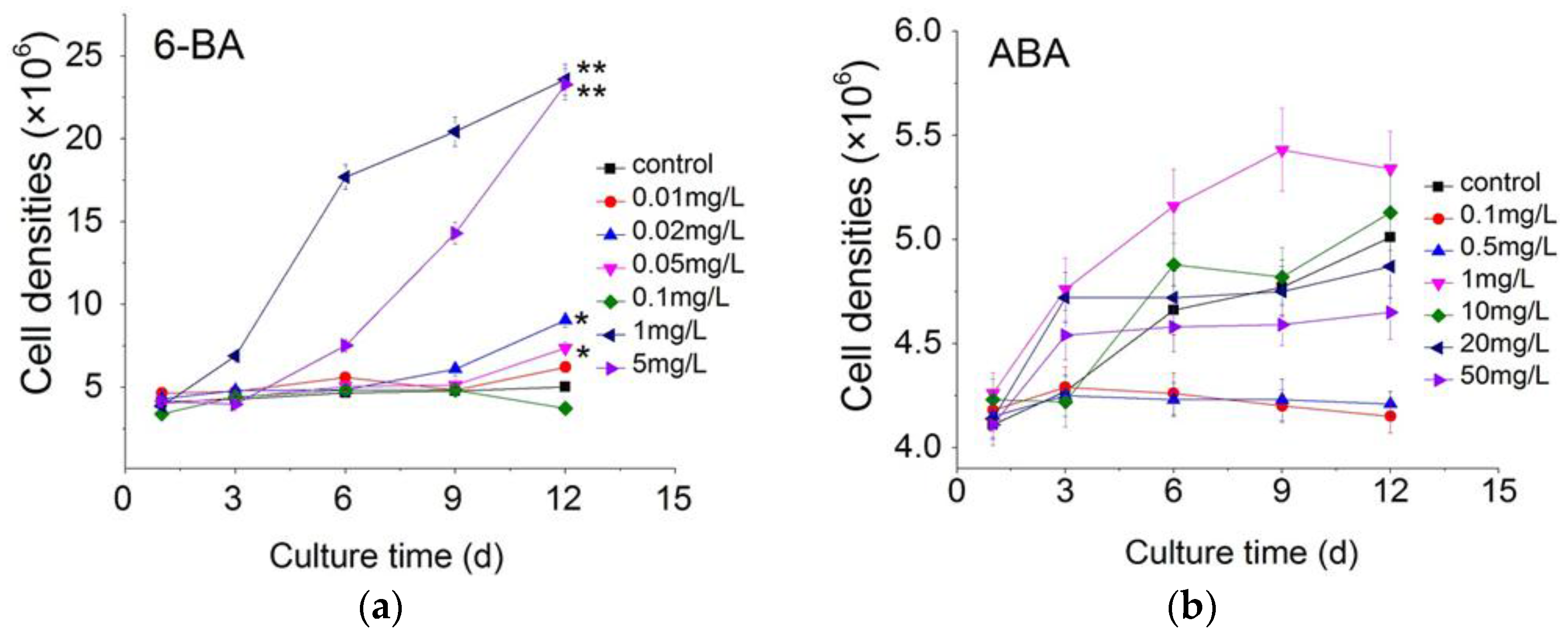
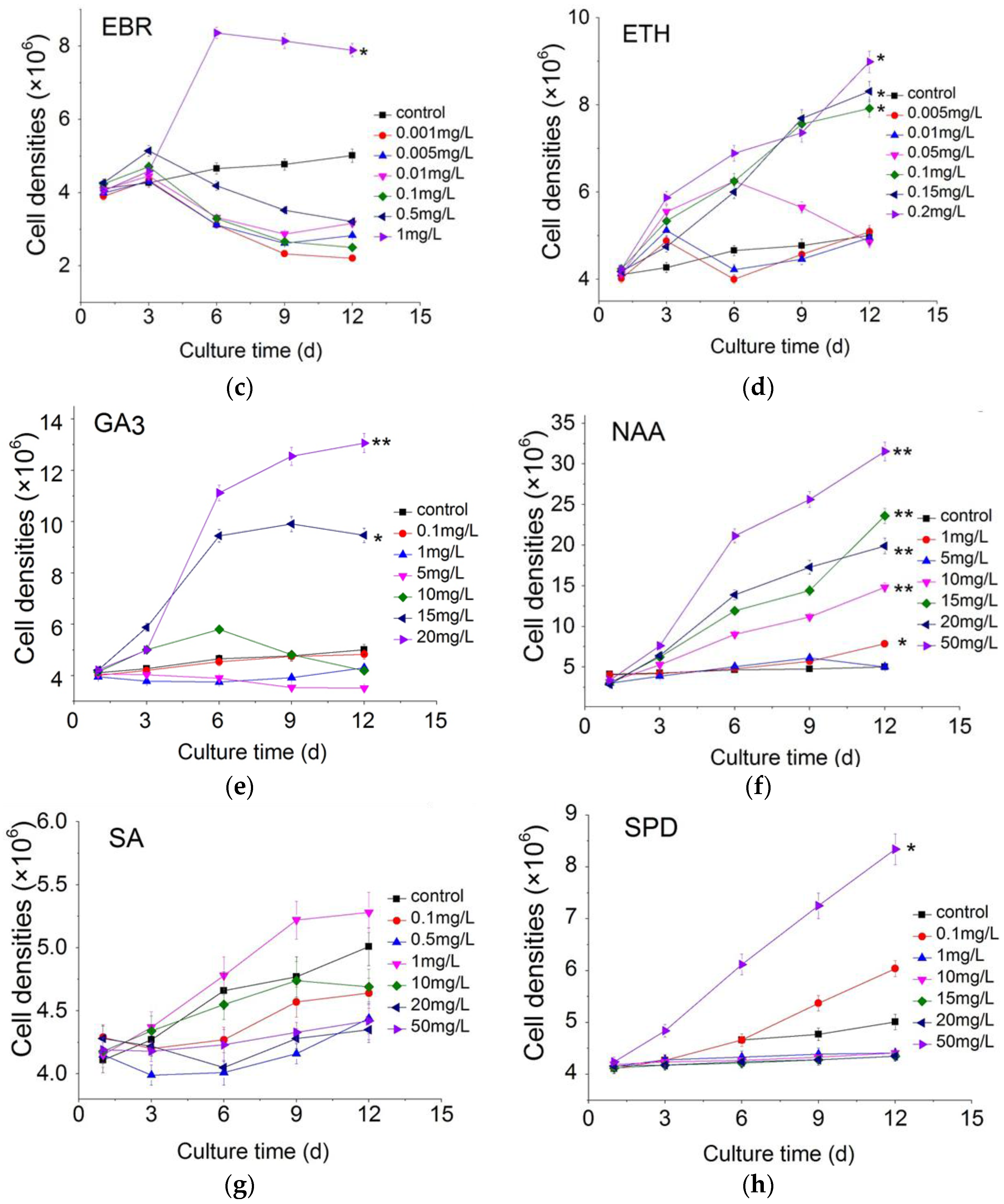
2.1. Effect of Plant Growth Regulators on Microalgal Growth
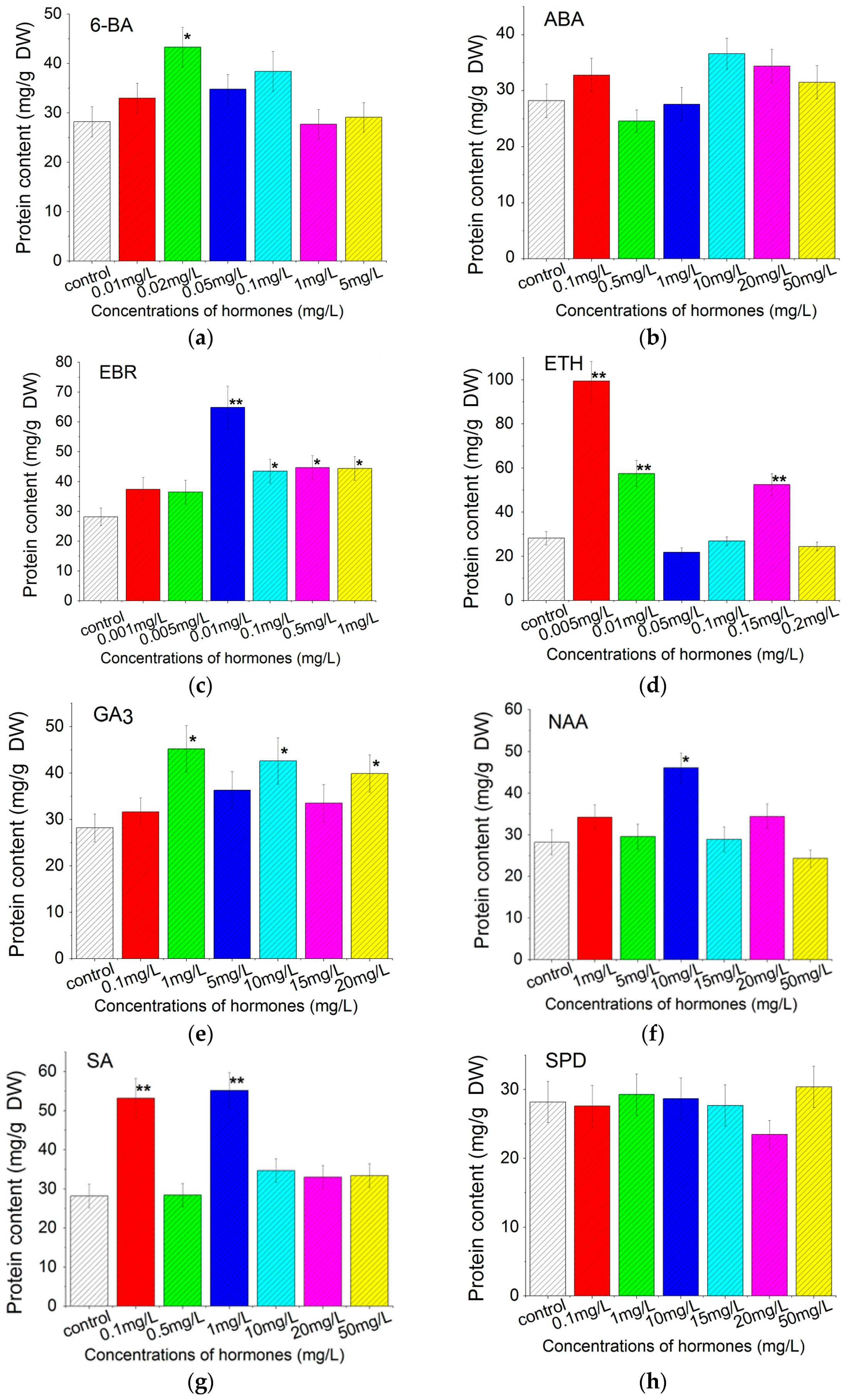
2.2. Effect on Crude Protein Contents
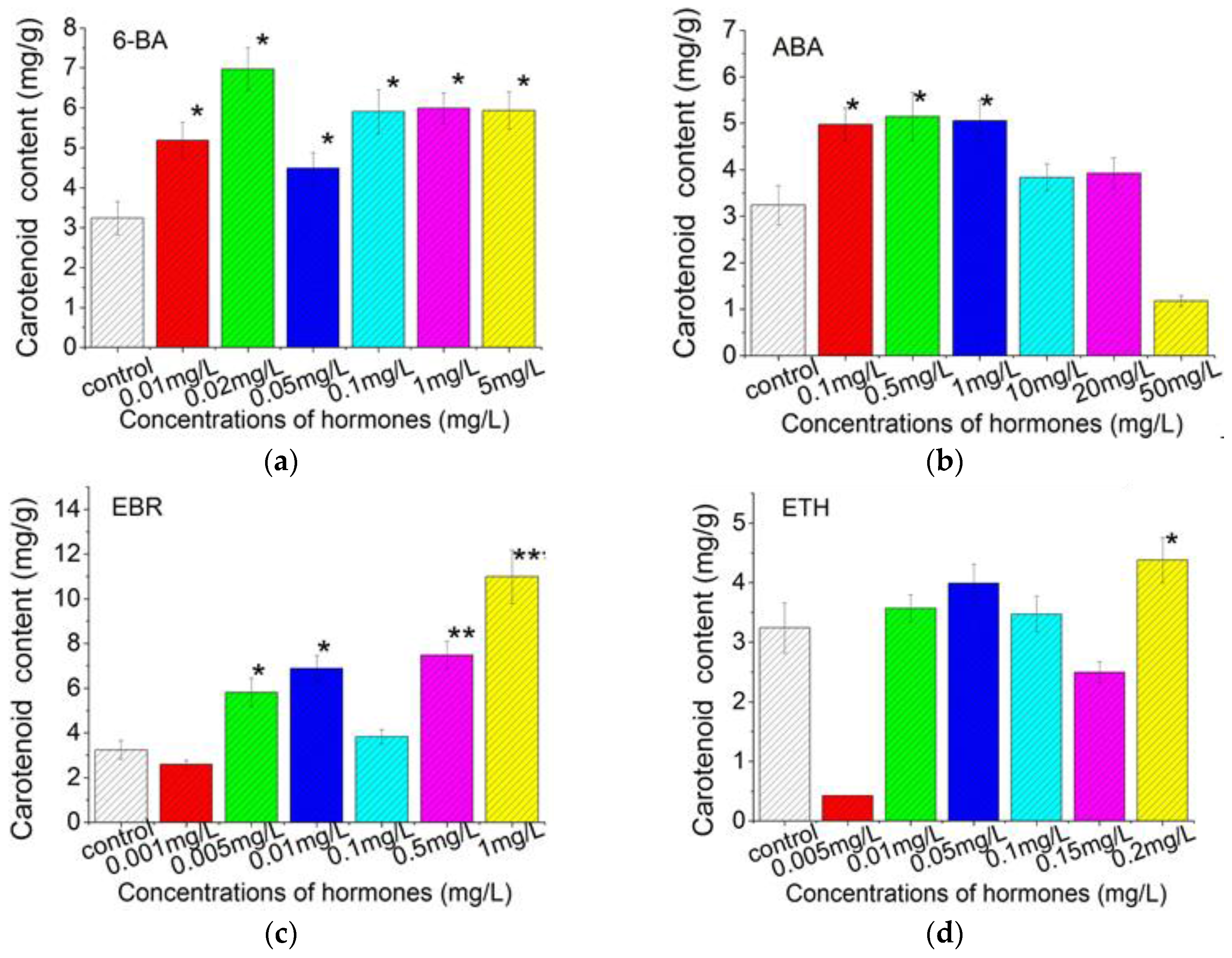
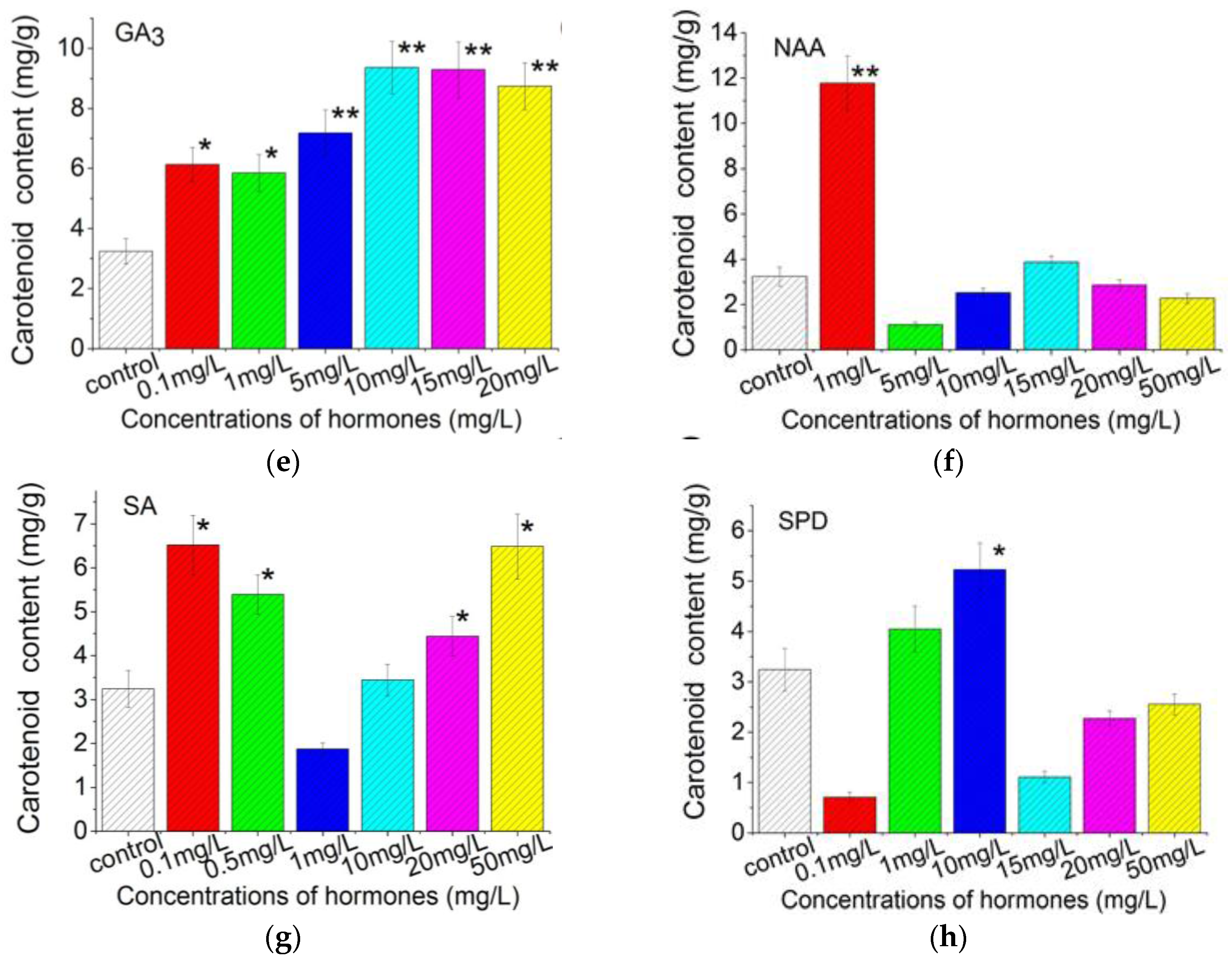
2.3. Plant Growth Regulators Induced Carotenoids Production in C. Pyrenoidosa ZF Cells
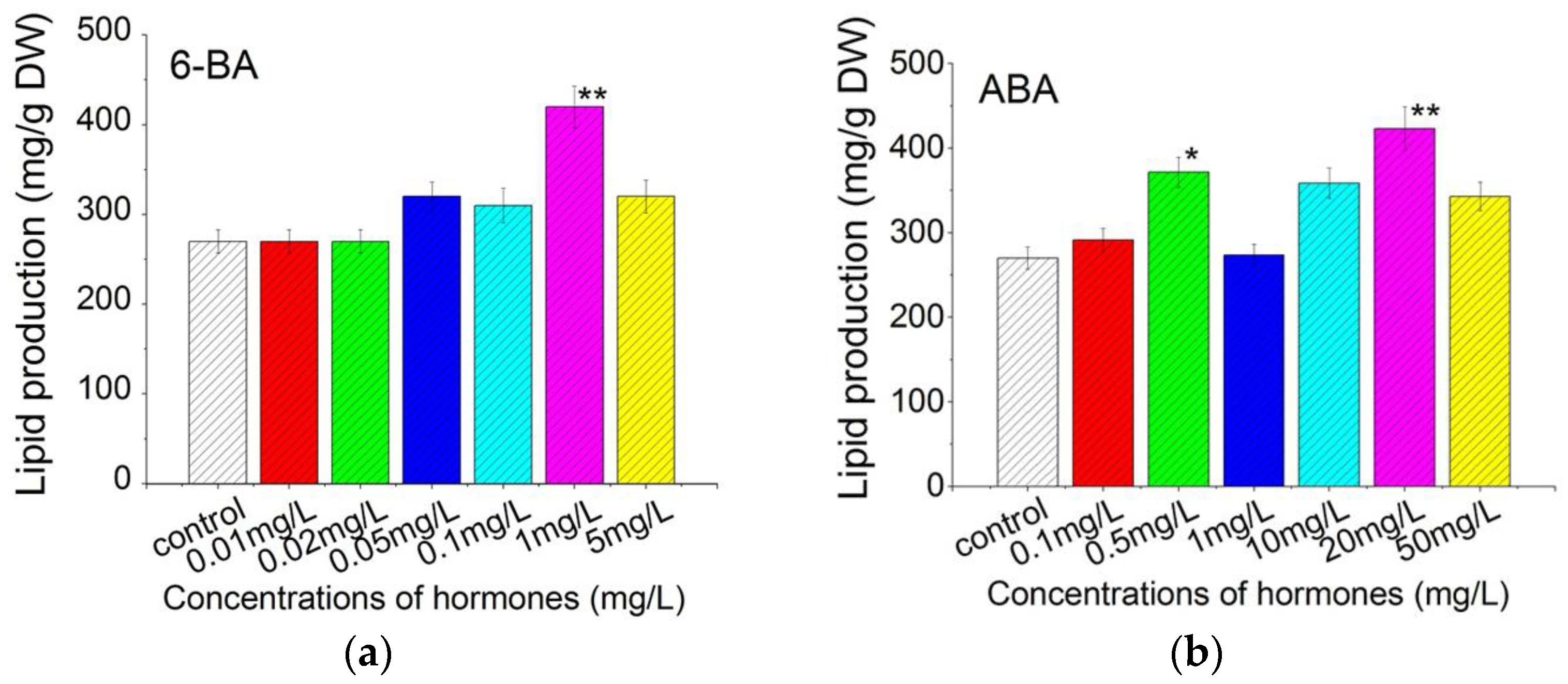
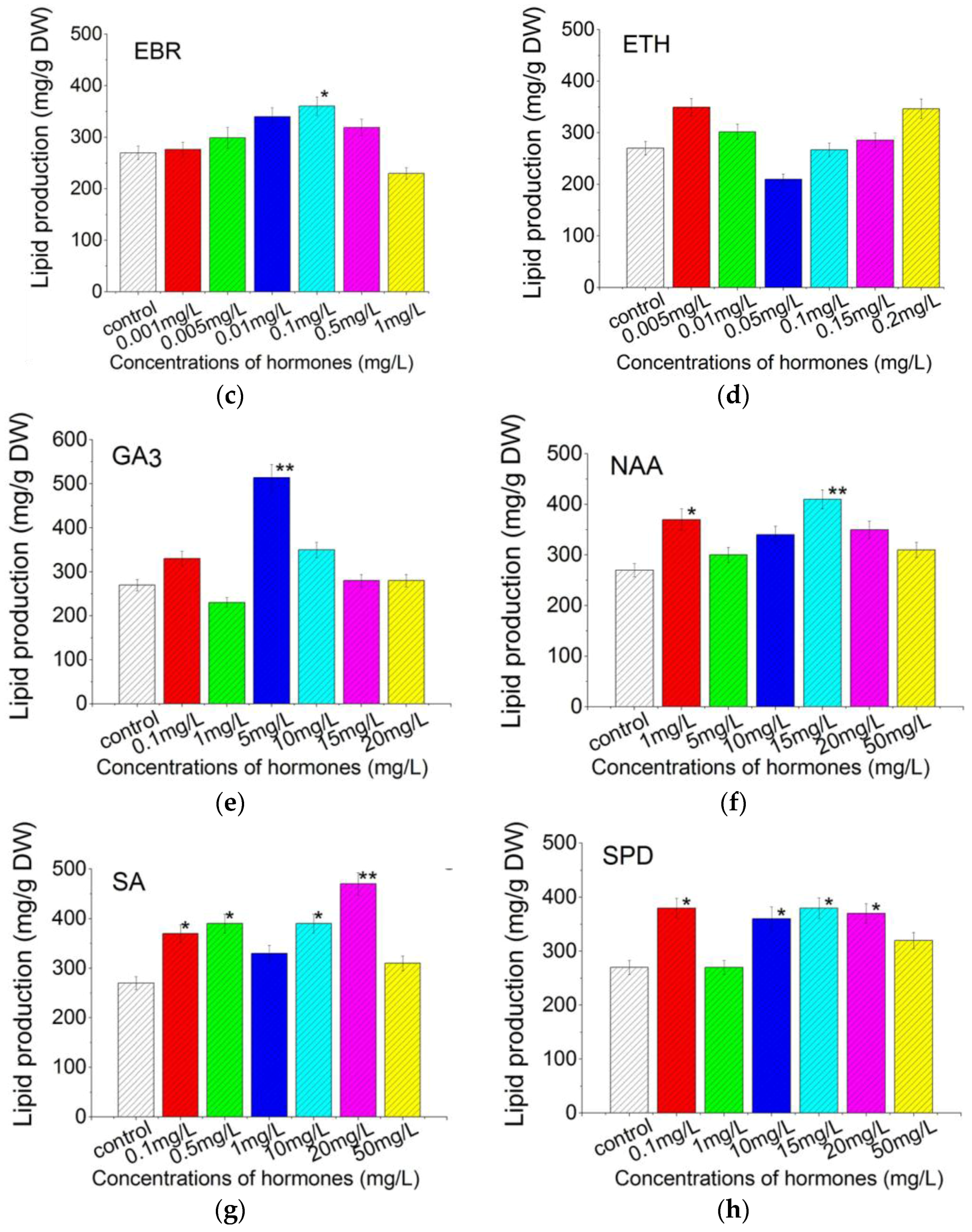
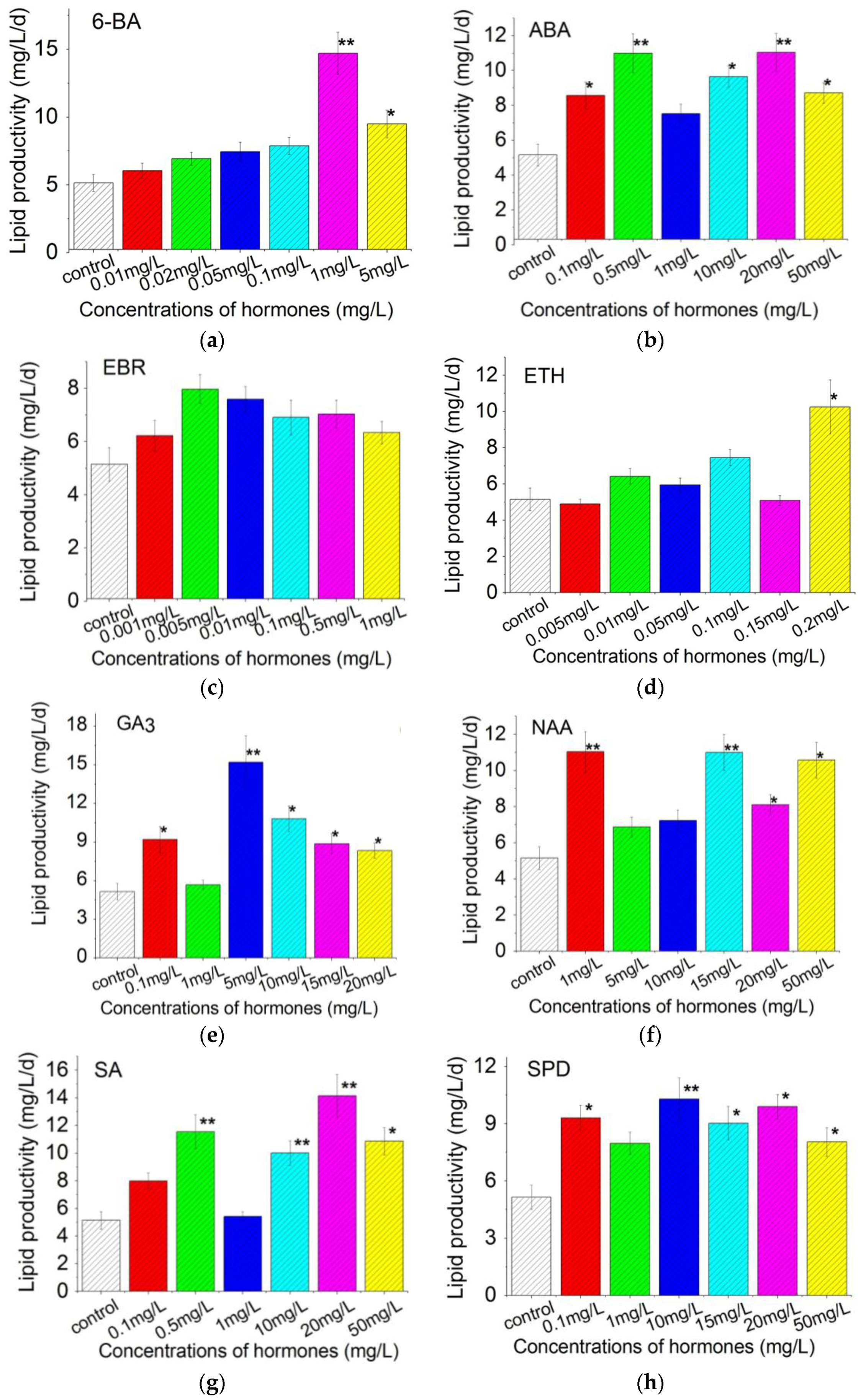
2.4. Plant Growth Regulators Induced Lipid Production in C. pyrenoidosa ZF Strain Cells
2.5. Effect on Fatty Acid Compositions
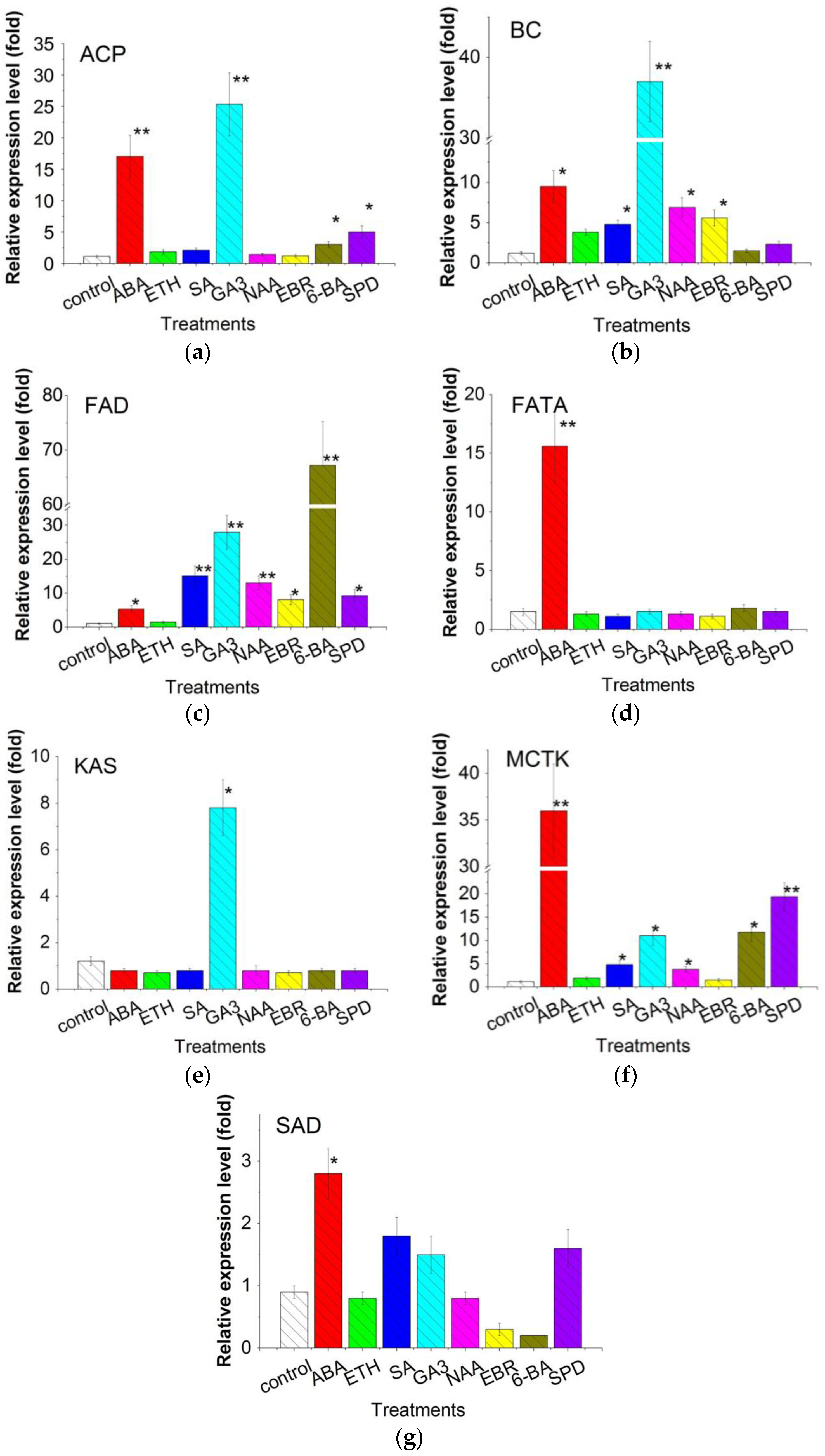
2.6. Expression of Fatty Acid Synthesis Related Genes
3. Discussion
3.1. The Cell Growth Analysis in C. pyrenoidosa ZF Strain
3.2. Total Soluble Protein and Carotenoids Production
3.3. The Lipids and High Value PUFAs Analysis
3.4. Transcriptional Expression of 7 Genes Related Fatty Acid Synthesis
4. Materials and Methods
4.1. Culture of C. pyrenoidosa ZF Strain
4.2. Treatment of C. pyrenoidosa ZF Strain Cultures
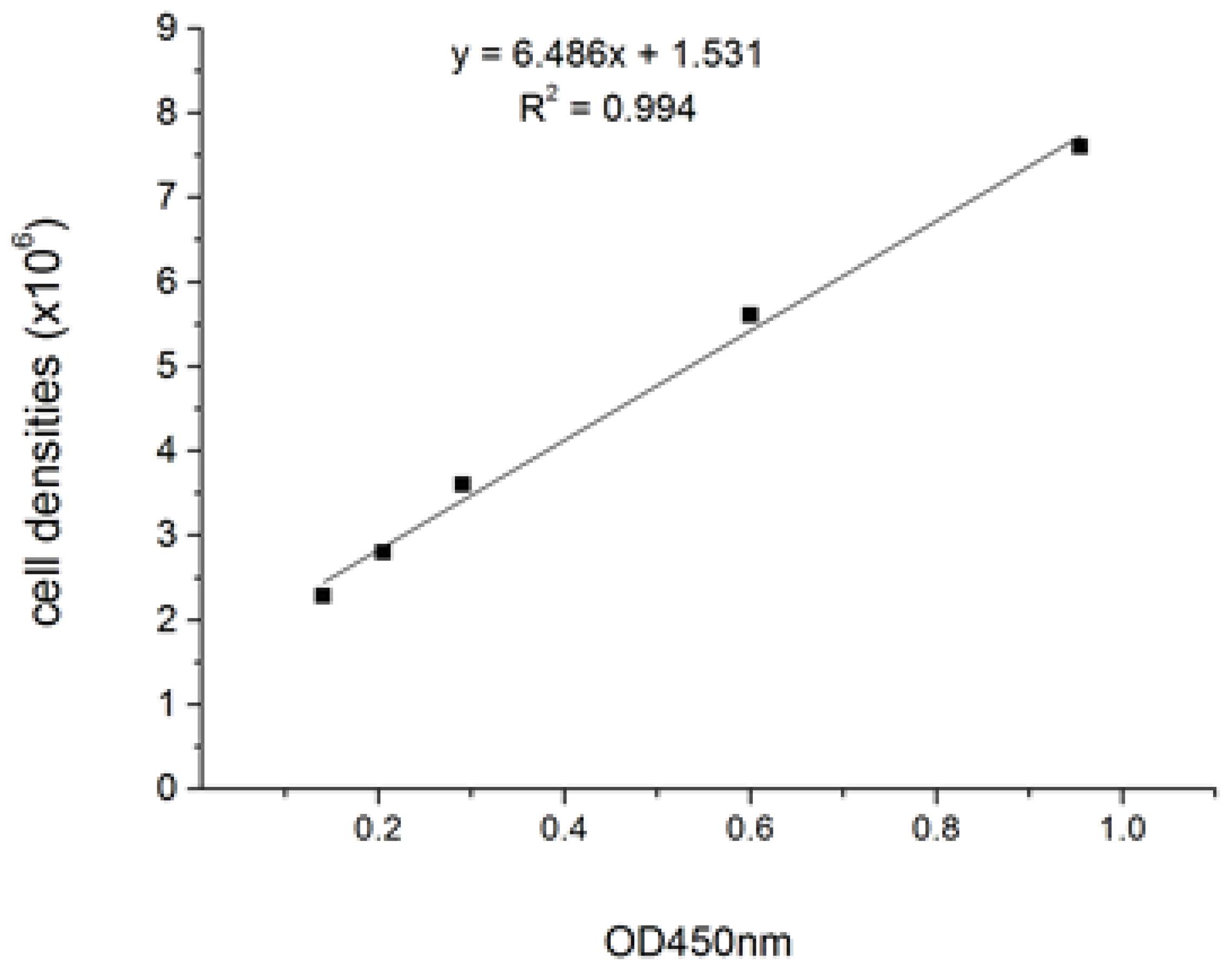
4.3. Determination of Growth of C. pyrenoidosa ZF Cultures
4.4. Determination of Total Soluble Protein Contents
4.5. Measurement of Total Carotenoid Contents
4.6. Measurement of Total Lipid Contents
4.7. Analysis of Fatty Acid Methyl Esters (FAMEs)
4.8. Expression Profiling of Fatty Acid Biosynthesis Genes
4.9. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fan, J.H.; Cui, Y.B.; Wan, M.X.; Wang, W.L.; Li, Y.G. Lipid accumulation and biosynthesis genes response of the oleaginous Chlorella pyrenoidosa under three nutrition stressors. Biotechnol. Biofuel 2014, 7, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Wan, M.X.; Liu, P.; Xia, J.L.; Rosenberg, J.L.; Oyler, G.; Betenbaugh, M.; Nie, Z.Y.; Qiu, G.Z. The effect of mixotrophy on microalgal growth, lipid content, and expression levels of three pathway genes in Chlorella sorokiniana. Appl. Microbiol. Biotechnol. 2011, 91, 835–844. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, J.N.; Oyler, G.; Wilkinson, L.; Betenbaugh, M.J. A green light for engineered algae: Redirecting metabolism to fuel a biotechnology revolution. Curr. Opin. Biotechnol. 2008, 19, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Kumari, S.; Guldhe, A.; Misra, R.; Rawat, I.; Bux, F. Trends and novel strategies for enhancing lipid accumulation and quality in microalgae. Renew. Sustain. Energy Rev. 2016, 55, 1–16. [Google Scholar] [CrossRef]
- Ho, S.H.; Chen, C.Y.; Chang, J.S. Effect of light intensity and nitrogen starvation on CO2 fixation and lipid/carbohydrate production of an indigenous microalga Scenedesmus obliquus CNW-N. Bioresour. Technol. 2012, 113, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Hockin, N.L.; Mock, T.; Mulholland, F.; Kopriva, S.; Malin, G. The response of diatom central carbon metabolism to nitrogen starvation is different from that of green algae and higher plants. Plant Physiol. 2012, 158, 299–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, X.H.; Chen, L.; Zhan, W.W. Chemicals to enhance microalgal growth and accumulation of high-value bioproducts. Front. Microbiol. 2015, 6, 56. [Google Scholar] [CrossRef] [PubMed]
- Evans, L.V.; Trewavas, A.J. Is algal development controlled by plant growth substances? J. Phycol. 1991, 27, 322–326. [Google Scholar] [CrossRef]
- Bradley, P.M. Plant hormones do have a role in controlling growth and development of algae. J. Phycol. 1991, 27, 317–321. [Google Scholar] [CrossRef]
- Tarakhovskaya, E.R.; Maslov, Y.I.; Shishova, M.F. Phytohormones in algae. Russ. J. Plant Physiol. 2007, 54, 186–194. [Google Scholar] [CrossRef]
- Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.T.; Liu, F.; Wang, C.; Wang, Z.Y.; Li, Y.Q. The boosted biomass and lipid accumulation in Chlorella vulgaris by supplementation of synthetic phytohormone analogs. Bioresour. Technol. 2017, 232, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.D.; Xu, J. Phytohormones in microalgae: A new opportunity for microalgal biotechnology? Trends Plant Sci. 2015, 20, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Bajguz, A.; Tretyn, A. The chemical characteristics and distribution of brassinosteroids in plants. Phytochemistry 2003, 62, 1027–1046. [Google Scholar] [CrossRef]
- Howell, W.M.; Keller, G.E., III; Kirkpatrick, J.D.; Jenkins, R.L.; Hunsinger, R.N.; Mclaughlin, E.W. Effects of the plant steroidal hormone, 2,4-epibrassinolide, on the mitotic index and growth of onion (Allium cepa) root tips. Genet. Mol. Res. 2007, 6, 50–58. [Google Scholar] [PubMed]
- Brock, A.K.; Willmann, R.; Kolb, D.; Grefen, L.; Lajunen, H.M.; Bethke, G.; Lee, J.; Nürnberger, T.; Gust, A.A. The Arabidopsis mitogen-activated protein kinase phosphatase PP2C5 affects seed germination, stomatal aperture, and abscisic acid-inducible gene expression. Plant Physiol. 2010, 153, 1098–1111. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yang, Y.N.; Xue, L.J.; Zou, M.J.; Liu, J.Y.; Chen, F.; Xue, H.W. Rice ABI5-Like1 regulates abscisic acid and auxin responses by affecting the expression of ABRE-containing genes. Plant Physiol. 2011, 156, 1397–1409. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.Q.; Miao, X.X.; Zhang, X.W.; Wu, G.X.; Guo, Y.Y.; Wang, M.M.; Li, B.; Li, X.B.; Gao, Y.H.; Hu, S.; et al. Comparative fatty acid transcriptomic test and iTRAQ-based proteomic analysis in Haematococcus pluvialis, upon salicylic acid (SA) and jasmonic acid (JA) inductions. Algal Res. 2016, 17, 277–284. [Google Scholar] [CrossRef]
- Czerpak, R.; Bajguz, A.; Gromek, M.; Kozłowska, G.; Nowak, I. Activity of salicylic acid on the growth and biochemism of Chlorella vulgaris Beijerinck. Acta Physiol. Plant. 2002, 24, 45–52. [Google Scholar] [CrossRef]
- Falkowska, M.; Pietryczuk, A.; Piotrowska, A.; Bajguz, A.; Grygoruk, A.; Czerpak, R. The effect of gibberellic acid ga(3) on growth, metal biosorption and metabolism of the green algae Chlorella vulgaris (Chlorophyceae) Beijerinck exposed to cadmium and lead stress. Pol. J. Environ. Stud. 2011, 20, 53–59. [Google Scholar]
- Raman, V.; Ravi, S. Effect of salicylic acid and methyl jasmonate on antioxidant systems of Haematococcus pluvialis. Acta Physiol. Plant. 2011, 33, 1043–1049. [Google Scholar] [CrossRef]
- Gao, Z.Q.; Meng, C.X.; Gao, H.Z.; Ye, N.H.; Zhang, X.W.; Su, Y.F.; Zhao, Y.R.; Wang, Y.Y. Effect of exogenous ethylene (ET) on regulating carotenogenesis expression and astaxanthin accumulation in Haematococcus pluvialis. J. Pure Appl. Microbiol. 2013, 7, 2503–2511. [Google Scholar]
- Gao, Z.Q.; Gao, H.Z.; Meng, C.X. Effects of Abscisic acid (ABA) carotenogenesis expression and astaxanthin accumulation in Haematococcus pluvialis. Res. J. Biotechol. 2013, 8, 9–15. [Google Scholar]
- Gao, Z.Q.; Meng, C.X.; Gao, H.Z.; Li, Y.; Zhang, X.W.; Xu, D.; Zhou, S.T.; Liu, B.H.; Su, Y.F.; Ye, N.H. Carotenoid genes transcriptional regulation for astaxanthin accumulation in fresh water unicellular alga Haematococcus pluvialis by gibberellin A3 (GA3). Indian J. Biochem. Biophys. 2013, 50, 548–553. [Google Scholar] [PubMed]
- Gao, Z.Q.; Meng, C.X.; Gao, H.Z.; Zhang, X.W.; Xu, D.; Su, Y.F.; Wang, Y.Y.; Zhao, Y.R.; Ye, N.H. Analysis of mRNA expression profiles of carotenogenesis and astaxanthin production of Haematococcus pluvialis under exogenous 2,4-epibrassinolide (EBR). Biol. Res. 2013, 46, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.Y.; Qiu, W.; Song, Y.M. Stimulatory effect of auxins on the growth and lipid productivity of Chlorella pyrenoidosa, and Scenedesmus quadricauda. Algal Res. 2016, 18, 273–280. [Google Scholar] [CrossRef]
- Park, W.K.; Yoo, G.; Moon, M.; Kim, C.W.; Choi, Y.E.; Yang, J.W. Phytohormone supplementation significantly increases growth of Chlamydomonas reinhardtii cultivated for biodiesel production. Appl. Biochem. Biotechnol. 2013, 171, 1128–1142. [Google Scholar] [CrossRef] [PubMed]
- Cowan, A.K.; Rose, P.D. Abscisic acid metabolism in salt-stressed cells of Dunaliella salina. Plant Physiol. 1991, 97, 798–803. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Todoroki, Y.; Hirai, N.; Kurimura, Y.; Ohigashi, H.; Tsuji, Y. Biological activities of abscisic acid analogs in the morphological change of the green alga Haematococcus pluvialis. J. Ferment. Bioeng. 1998, 85, 529–531. [Google Scholar] [CrossRef]
- Anuradha, S.; Rao, S.S.R. Effect of 2,4-epibrassinolide on the photosynthetic activity of radish plants under cadmium stress. Photosynthetica 2009, 47, 317–320. [Google Scholar] [CrossRef]
- Ding, J.; Shi, K.; Zhou, Y.H. Effects of root and foliar applications of 24-epibrassinolide on fusarium wilt and antioxidant metabolism in cucumber roots. Hortscience 2009, 44, 1340–1345. [Google Scholar]
- Swain, S.M.; Olszewski, N.E. Genetic analysis of gibberellin signal transduction. Plant Physiol. 1996, 112, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Navarro, L.; Bari, R.; Achard, P.; Lison, P.; Nemri, A.; Harberd, N.; Jones, J.D. Dellas control plant immune responses by modulating the balance of jasmonic acid and salicylic acid signaling. Curr. Biol. 2008, 18, 650–655. [Google Scholar] [CrossRef] [PubMed]
- Anerson, J.P.; Badruzsaufari, E.; Schenk, P.M.; Manners, J.M.; Desmond, O.J.; Ehlert, C.; Maclean, D.J.; Ebert, P.R.; Kazan, K. Antagonistic interaction between abscisic acid and jasmonate-ethylene signaling pathways modulates defense gene expression and disease resistance in Arabidopsis. Plant Cell 2004, 16, 3460–3479. [Google Scholar] [CrossRef] [PubMed]
- Jusoh, M.; Loh, S.H.; Chuah, T.S.; Azizc, A.; Cha, T.S. Elucidating the role of jasmonic acid in oil accumulation, fatty acid composition and gene expression in Chlorella vulgaris, (Trebouxiophyceae) during early stationary growth phase. Algal Res. 2015, 9, 14–20. [Google Scholar] [CrossRef]
- Illman, A.M.; Scragg, A.H.; Shales, S.W. Increase in Chlorella strains calorific values when grown in low nitrogen medium. Enzym. Microb. Technol. 2000, 27, 631–635. [Google Scholar] [CrossRef]
- Xu, H.; Miao, X.L.; Wu, Q.Y. High quality biodiesel production from a microalga Chlorella protothecoides by heterotrophic growth in fermenters. J. Biotechnol. 2006, 126, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Yeh, K.L.; Chang, J.S. Nitrogen starvation strategies and photobioreactor design for enhancing lipid content and lipid production of a newly isolated microalga Chlorella vulgaris ESP-31: Implications for biofuels. Biotechnol. J. 2011, 6, 1358–1366. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.H.; Huang, J.K.; Li, Y.G.; Han, F.F.; Wang, J.; Li, X.W.; Wang, W.L.; Li, S.L. Sequential heterotrophy-dilution-photoinduction cultivation for efficient microalgal biomass and lipid production. Bioresour. Technol. 2012, 112, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Han, F.F.; Huang, J.K.; Li, Y.G.; Wang, W.L.; Wang, J.; Fan, J.H.; Shen, G.M. Enhancement of microalgal biomass and lipid productivities by a model of photoautotrophic culture with heterotrophic cells as seed. Bioresour. Technol. 2012, 118, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Hunt, R.W.; Chinnasamy, S.; Bhatnagar, A.; Das, K.C. Effect of biochemical stimulants on biomass productivity and metabolite content of the microalga, Chlorella sorokiniana. Appl. Biochem. Biotechnol. 2010, 162, 2400–2414. [Google Scholar] [CrossRef] [PubMed]
- Stirk, W.A.; Bálint, P.; Tarkowská, D.; Novák, O.; Maróti, G.; Ljung, K.; Turečková, V.; Strnad, M.; Ördög, V.; Staden, J. Effect of light on growth and endogenous hormones in Chlorella minutissima, (Trebouxiophyceae). Plant Physiol. Biochem. 2014, 79, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Young, A.J. The photoprotective role of carotenoids in higher plants. Physiol. Plant. 1991, 83, 702–708. [Google Scholar] [CrossRef]
- Rodolfi, L.; Zittelli, G.C.; Bassi, N.; Padovani, G.; Biondi, N.; Bonini, G.; Tredici, M.R. Microalgae for oil: Strain selection, induction of lipid synthesis and outdoor mass cultivation in a low-cost photobioreactor. Biotechnol. Bioeng. 2009, 102, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Damiani, M.C.; Popovich, C.A.; Constenla, D.; Leonardi, P.I. Lipid analysis in Haematococcus pluvialis to assess its potential use as a biodiesel feedstock. Bioresour. Technol. 2010, 101, 3801–3807. [Google Scholar] [CrossRef] [PubMed]
- Roessler, P.G. Environmental control of glycerol lipid metabolism in microalgae: Commercial implications and future research directions. J. Phycol. 1990, 26, 393–399. [Google Scholar] [CrossRef]
- Zhukova, N.V.; Titlyanov, E.A. Effect of light intensity on the fatty acid composition of dinoflagellates symbiotic with hermatypic corals. Bot. Mar. 2006, 49, 339–346. [Google Scholar] [CrossRef]
- Norman, H.A.; Thompson, G.A. Effects of low-temperature stress on the metabolism of phosphatidylglycerol molecular species in Dunaliella salina. Arch. Biochem. Biophys. 1985, 242, 168–175. [Google Scholar] [CrossRef]
- Zažímalová, E.; Petrášek, J.; Benková, E. Auxin and Its Role in Plant Development; Springer: Vienna, Australia, 2014. [Google Scholar]
- Cho, K.; Kim, K.N.; Lim, N.L.; Kim, M.S.; Ha, J.C.; Shin, H.H.; Kim, M.K.; Roh, S.W.; Kim, D.; Oda, T. Enhanced biomass and lipid production by supplement of myo-inositol with oceanic microalga Dunaliella salina. Biomass Bioenergy 2015, 72, 1–7. [Google Scholar] [CrossRef]
- Jusoh, M.; Loh, S.H.; Chuah, T.S.; Aziz, A.; Cha, T.S. Indole-3-acetic acid (IAA) induced changes in oil content, fatty acid profiles and expression of four fatty acid biosynthetic genes in Chlorella vulgaris at early stationary growth phase. Phytochemistry 2015, 111, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Sommerfeld, M.; Jarvis, E.; Ghirardi, M.; Posewitz, M.; Seibert, M.; Darzins, A. Microalgal triacylglycerols as feedstocks for biofuel production: Perspectives and advances. Plant J. 2008, 54, 621–639. [Google Scholar] [CrossRef] [PubMed]
- Maršálek, B.; Zahradníčková, H.; Hronková, M. Extracellular Abscisic acid produced by cyanobacteria under salt stress. J. Plant Physiol. 1992, 139, 506–508. [Google Scholar] [CrossRef]
- Tominaga, N.; Takahata, M.; Tominaga, H. Effects of NaCl and KNO3, concentrations on the abscisic acid content of Dunaliella sp. (Chlorophyta). Hydrobiologia 1993, 267, 163–168. [Google Scholar] [CrossRef]
- Kobayashi, M.; Hirai, N.; Kurimura, Y.; Ohigashi, H.; Tsuji, Y. Abscisic acid-dependent algal morphogenesis in the unicellular green alga Haematococcus pluvialis. Plant Growth Regul. 1997, 22, 79–85. [Google Scholar] [CrossRef]
- Yoshida, K.; Igarashi, E.; Mukai, M.; Hirata, K.; Miyamoto, K. Induction of tolerance to oxidative stress in the green alga, Chlamydomonas reinhardtii, by abscisic acid. Plant Cell Environ. 2003, 26, 451–457. [Google Scholar] [CrossRef]
- Yoshida, K.; Igarashi, E.; Wakatsuki, E.; Miyamoto, K.; Hirata, K. Mitigation of osmotic and salt stresses by abscisic acid through reduction of stress-derived oxidative damage in Chlamydomonas reinhardtii. Plant Sci. 2004, 167, 1335–1341. [Google Scholar] [CrossRef]
- Lu, Y.D.; Arkowska, D.; Tureckova, V.; Luo, T.W.; Xin, Y.; Li, J.; Wang, Q.T.; Jiao, N.Z.; Strnad, M.; Xu, J. Antagonistic roles of abscisic acid and cytokinin during response to nitrogen depletion in oleaginous microalga Nannochloropsis oceanica expand the evolutionary breadth of phytohormone function. Plant J. 2014, 80, 52–68. [Google Scholar] [CrossRef] [PubMed]
- Salama, E.S.; Kabra, A.N.; Ji, M.K.; Kim, J.R.; Min, B.; Jeon, B.H. Enhancement of microalgae growth and fatty acid content under the influence of phytohormones. Bioresour. Technol. 2014, 172, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Mu, J.X.; Li, S.T.; Chen, D.; Xu, H.; Han, F.X.; Feng, B.; Li, Y.Q. Enhanced biomassand oil production from sugarcane bagasse hydrolysate (SBH) by heterotrophic oleaginous microalga Chlorella protothecoides. Bioresour. Technol. 2015, 185, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Khozingoldberg, I.; Cohen, Z. Unraveling algal lipid metabolism: Recent advances in gene identification. Biochimie 2011, 93, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Cheesbrough, T.M. Changes in enzymes for fatty acid synthesis and desaturation during acclimation of developing soybean seeds to altered growth temperature. Plant Physiol. 1989, 90, 760–764. [Google Scholar] [CrossRef] [PubMed]
- Iba, K. Acclimative response to temperature stress in higher plants: Approaches of gene engineering for temperature tolerance. Annu. Rev. Plant Biol. 2002, 53, 225–245. [Google Scholar] [CrossRef] [PubMed]
- Vega, S.E.; Rio, A.H.D.; Bamberg, J.B.; Palta, J.P. Evidence for the up-regulationof stearoyl-ACP (D9) desaturase gene expression during cold acclimation. Am. J. Potato Res. 2004, 81, 125–135. [Google Scholar] [CrossRef]
- Upchurch, R.G. Fatty acid unsaturation, mobilization, and regulation in the response of plants to stress. Biotechnol. Lett. 2008, 30, 967–977. [Google Scholar] [CrossRef] [PubMed]
- Thompson, G.A. Lipids and membrane function in green algae. Biochim. Biophys. Acta 1996, 13, 17–45. [Google Scholar] [CrossRef]
- Griffiths, M.J.; van Hille, R.P.; Harrison, S.T. Selection of direct transesterification as the preferred method for assay of fatty acid content of microalgae. Lipids 2010, 45, 1053–1060. [Google Scholar] [CrossRef] [PubMed]
- Lei, A.P.; Chen, H.; Shen, G.M.; Hu, Z.L.; Chen, L.; Wang, J.X. Expression of fatty acid synthesis genes and fatty acid accumulation in Haematococcus pluvialis under different stressors. Biotechnol. Biofuels 2012, 5, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Harwood, J.L.; Guschina, I.A. The versatility of algae and their lipid metabolism. Biochimie 2009, 91, 679–684. [Google Scholar] [CrossRef] [PubMed]
- Riekhof, W.R.; Sears, B.B.; Benning, C. Annotation of genes involved in glycerol lipid biosynthesis in Chlamydomonas reinhardtii: Discovery of the betaine lipid synthase BTA1Cr. Eukaryot. Cell 2005, 4, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Battah, M.; El-Ayoty, Y.; Abomohra, A.E.F.; El-Ghany, S.A.; Esmael, A. Effect of Mn2+, Co2+ and H2O2 on biomass and lipids of the green microalga Chlorella vulgaris as a potential candidate for biodiesel production. Ann. Microbiol. 2015, 65, 155–162. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Folch, J.M.L.; Lees, M.P.; Stanley, G.H.A.S. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [PubMed]
- Cha, T.S.; Chen, J.W.; Goh, E.G.; Aziz, A.; Loh, S.H. Differential regulation of fatty acid biosynthesis in two Chlorella species in response to nitrate treatments and the potential of binary blending microalgae oils for biodiesel application. Bioresour. Technol. 2011, 102, 10633–10640. [Google Scholar] [CrossRef] [PubMed]
| Components (%) | C12:0 | C15:1 | C16:1 | C16:0 | C17:1 | C17:0 | C18:1n9t | C18:2n6 | C18:3n3 | C20:4n6 | C22:6n3 | Others |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | 4.59 ± 0.57 | 8.02 ± 1.10 | 3.82 ± 0.48 | 6.28 ± 0.52 | 26.35 ± 3.27 | 6.57 ± 0.62 | - | 1.31 ± 0.22 | - | 0.71 ± 0.08 | - | 42.35 ± 3.86 |
| 0.5 mg·L−1 ABA | 3.67 ± 0.28 | 1.32 ± 0.10 | - | 9.54 ± 1.02 * | 51.30 ± 5.34 ** | 9.28 ± 1.02 * | - | - | - | - | - | 24.89 ± 2.73 |
| 10 mg·L−1 ABA | 3.15 ± 0.33 | 0.85 ± 0.04 | - | 8.89 ± 0.54 * | 43.11 ± 3.89 ** | 11.40 ± 0.86 ** | - | - | - | - | - | 32.60 ± 4.05 |
| 20 mg·L−1 ABA | 2.88 ± 0.30 | 1.26 ± 0.10 | - | 7.49 ± 0.64 | 32.59 ± 4.02 | 15.28 ± 1.32 ** | 3.54 ± 0.22 | - | - | - | - | 36.96 ± 4.52 |
| 50 mg·L−1 ABA | 1.74 ± 0.23 | 11.83 ± 1.02 * | - | 13.04 ± 1.02 ** | 51.45 ± 4.85 ** | 9.35 ± 1.00 * | - | - | - | - | - | 12.59 ± 1.23 |
| 0.05 mg·L−1 6-BA | 2.96 ± 0.31 | 7.75 ± 0.52 | - | 7.62 ± 0.55 | 43.23 ± 3.99 ** | 6.20 ± 0.38 | - | - | - | - | - | 32.24 ± 2.88 |
| 0.1 mg·L−1 6-BA | 2.55 ± 0.25 | - | - | 6.45 ± 0.63 | 33.48 ± 3.48 | 8.47 ± 0.58 * | - | - | - | - | - | 49.05 ± 4.36 |
| 1 mg·L−1 6-BA | 4.26 ± 0.18 | 0.82 ± 0.13 | - | 12.08 ± 0.87 ** | 35.23 ± 2.36 * | 10.28 ± 0.72 ** | 3.06 ± 0.11 | - | - | - | - | 34.27 ± 2.96 |
| 5 mg·L−1 6-BA | 3.07 ± 0.15 | 2.23 ± 0.23 | - | 6.77 ± 0.74 | 44.56 ± 3.89 ** | 3.45 ± 0.31 | - | - | - | - | - | 39.92 ± 4.03 |
| 0.005 mg·L−1 EBR | 2.48 ± 0.22 | - | - | 13.55 ± 1.24 ** | 38.13 ± 3.56 * | 5.24 ± 0.35 | 4.16 ± 0.21 | - | - | 0.77 ± 0.05 | - | 35.67 ± 2.86 |
| 0.01 mg·L−1 EBR | 2.47 ± 0.19 | - | - | 7.83 ± 0.56 | 42.87 ± 0.23 ** | - | - | - | - | - | - | 46.83 ± 4.21 |
| 0.1 mg·L−1 EBR | 4.78 ± 0.55 | 0.87 ± 0.05 | - | 5.52 ± 0.36 | 47.37 ± 2.53 ** | 8.07 ± 0.50 | - | - | - | - | - | 33.39 ± 2.89 |
| 0.5 mg·L−1 EBR | 1.79 ± 0.22 | 1.61 ± 0.11 | - | 11.38 ± 1.22 ** | 58.45 ± 4.82 ** | 2.15 ± 0.15 | - | - | - | - | - | 24.62 ± 3.08 |
| 0.005 mg·L−1 ETH | 15.49 ± 1.02 ** | 16.67 ± 2.10 ** | 3.78 ± 0.44 | 4.49 ± 0.42 | 16.83 ± 1.83 | 33.26 ± 3.78 ** | - | 1.24 ± 0.12 | - | - | - | 8.24 ± 0.58 |
| 0.01 mg·L−1 ETH | 10.27 ± 0.89 ** | 7.11 ± 0.36 | 3.05 ± 0.25 | 3.12 ± 0.21 | 53.74 ± 5.62 ** | 14.74 ± 1.23 ** | - | 0.76 ± 0.03 | - | - | - | 7.21 ± 0.32 |
| 0.1 mg·L−1 ETH | 9.16 ± 0.92 | 8.56 ± 1.00 | 4.02 ± 0.45 | 3.03 ± 0.18 | 54.76 ± 7.23 ** | 0.46 ± 0.05 | - | 0.62 ± 0.07 | - | - | - | 19.39 ± 175 |
| 0.15 mg·L−1 ETH | 11.61 ± 1.22 ** | 13.02 ± 1.52 * | 3.18 ± 0.30 | 5.10 ± 0.45 | 52.75 ± 4.78 ** | 1.30 ± 0.10 | - | 1.46 ± 0.13 | - | - | - | 11.58 ± 1.05 |
| 0.2 mg·L−1 ETH | 7.38 ± 0.98 * | 8.30 ± 0.89 | 3.19 ± 0.26 | 4.56 ± 0.45 | 45.75 ± 4.55 ** | 18.59 ± 2.92 ** | - | 1.82 ± 0.23 | - | - | - | 10.41 ± 1.02 |
| 0.1 mg·L−1 GA3 | 4.73 ± 0.38 | 8.93 ± 1.08 | 10.62 ± 1.32 ** | 8.11 ± 0.75 | 38.57 ± 4.31 * | 15.57 ± 2.57 ** | - | 1.74 ± 0.21 | - | - | - | 11.73 ± 1.51 |
| 5 mg·L−1 GA3 | 2.37 ± 0.11 | 6.47 ± 0.32 | 12.83 ± 0.98 ** | 6.81 ± 0.45 | 32.25 ± 1.88 | 12.28 ± 0.86 ** | 0.93 ± 0.02 | 0.87 ± 0.12 | 0.57 ± 0.03 | 2.53 ± 0.15 * | 0.44 ± 0.05 | 21.65 ± 1.42 |
| 10 mg·L−1 GA3 | 6.78 ± 0.30 | 10.23 ± 1.02 | - | 9.18 ± 1.02 * | 39.27 ± 2.88 * | 10.35 ± 1.02 ** | - | 1.25 ± 0.18 | - | - | - | 22.94 ± 1.98 |
| 15 mg·L−1 GA3 | 4.64 ± 0.35 | 6.51 ± 0.39 | - | 2.79 ± 0.23 | 56.43 ± 6.15 ** | 18.62 ± 1.53 ** | - | 0.53 ± 0.05 | - | - | - | 10.48 ± 1.12 |
| 20 mg·L−1 GA3 | 4.85 ± 0.41 | 9.81 ± 0.83 | - | 1.51 ± 0.21 | 55.32 ± 4.86 ** | 8.77 ± 1.02 * | - | 0.39 ± 0.02 | - | - | - | 19.35 ± 2.14 |
| 1 mg·L−1 NAA | 3.17 ± 0.38 | 8.25 ± 1.02 | - | 3.80 ± 0.41 | 4 .35 ± 3.02 ** | 7.47 ± 0.65 | 1.21 ± 0.07 | 0.57 ± 0.03 | 0.65 ± 0.01 | 1.64 ± 0.12* | - | 27.89 ± 2.76 |
| 10 mg·L−1 NAA | 1.59 ± 0.15 | 12.77 ± 1.21 * | - | 8.05 ± 0.26 | 36.71 ± 2.23 * | 5.28 ± 0.32 | - | - | - | - | - | 35.60 ± 3.76 |
| 15 mg·L−1 NAA | 2.35 ± 0.22 | 9.26 ± 0.41 | - | 11.84 ± 1.08 ** | 26.61 ± 3.03 | 4.67 ± 0.03 | - | - | - | - | - | 45.27 ± 4.16 |
| 20 mg·L−1 NAA | 2.02 ± 0.18 | 11.19 ± 0.28 * | - | 8.31 ± 0.38 * | 45.87 ± 2.30 ** | 1.81 ± 0.26 | - | - | - | - | - | 30.80 ± 2.57 |
| 0.1 mg·L−1 SA | 8.42 ± 0.76 * | 11.36 ± 1.32 * | 2.54 ± 0.25 | 3.12 ± 0.25 | 40.09 ± 4.87 * | 27.26 ± 2.85 ** | - | 1.30 ± 0.09 | - | - | - | 5.91 ± 0.43 |
| 0.5 mg·L−1 SA | 2.25 ± 0.18 | 2.73 ± 0.23 | - | 8.23 ± 0.48 * | 25.00 ± 2.87 | 19.87 ± 1.48 ** | - | - | - | - | - | 41.92 ± 3.94 |
| 1 mg·L−1 SA | 12.62 ± 2.35 ** | 7.47 ± 0.92 | 2.46 ± 0.44 | 3.33 ± 0.26 | 45.55 ± 6.95 ** | 16.17 ± 1.98 ** | - | 0.89 ± 0.04 | - | - | - | 11.51 ± 1.22 |
| 10 mg·L−1 SA | 13.73 ± 1.12 ** | 8.73 ± 1.22 | 3.19 ± 0.15 | 5.53 ± 0.75 | 41.10 ± 6.01 * | 15.25 ± 3.59 ** | - | 1.07 ± 0.11 | - | - | - | 11.40 ± 1.24 |
| 20 mg·L−1 SA | 15.94 ± 1.05 ** | 9.78 ± 1.02 | 3.09 ± 0.30 | 3.08 ± 0.26 | 42.07 ± 4.98 ** | 18.22 ± 3.02 ** | - | 0.96 ± 0.5 | - | - | - | 6.86 ± 0.84 |
| 50 mg·L−1 SA | 7.56 ± 0.53 * | 2.26 ± 0.12 | - | 5.82 ± 0.36 | 30.36 ± 2.36 | 15.24 ± 1..63 ** | - | - | - | - | - | 38.76 ± 4.15 |
| 0.1 mg·L−1 SPD | - | 2.67 ± 0.21 | - | 3.82 ± 0.22 | 34.97 ± 3.48 * | 12.29 ± 1.12 ** | - | - | - | - | - | 46.25 ± 5.82 |
| 10 mg·L−1 SPD | 1.30 ± 0.18 | 8.13 ± 0.56 | 2.77 ± 0.27 | 11.89 ± 0.98 ** | 54.56 ± 4.99 ** | 2.44 ± 0.15 | 2.76 ± 0.22 | 0.46 ± 0.02 | - | - | - | 15.69 ± 1.28 |
| 20 mg·L−1 SPD | 2.93 ± 0.15 | 5.23 ± 0.22 | - | 10.21 ± 0.86 * | 38.28 ± 3.26 * | 1.95 ± 0.23 | - | 0.37 ± 0.05 | - | - | - | 41.03 ± 4.53 |
| 50 mg·L−1 SPD | 5.34 ± 0.37. | 5.39 ± 0.66 | - | 8.98 ± 1.03 * | 38.55 ± 4.02 * | 2.40 ± 0.23 | - | - | - | - | - | 39.34 ± 3.64 |
| PGR Kinds | Concentrations of Plant Growth Regulators (mg·L−1) | |||||
|---|---|---|---|---|---|---|
| EBR | 0.001 | 0.005 | 0.010 | 0.100 | 0.500 | 1.000 |
| GA3 | 0.100 | 1.000 | 5.000 | 10.000 | 15.000 | 20.000 |
| ETH | 0.005 | 0.010 | 0.050 | 0.100 | 0.150 | 0.200 |
| SPD | 0.100 | 1.000 | 10.000 | 15.000 | 20.000 | 50.000 |
| 6-BA | 0.010 | 0.020 | 0.050 | 0.100 | 1.000 | 5.000 |
| NAA | 1.000 | 5.000 | 10.000 | 15.000 | 20.000 | 50.000 |
| SA | 0.100 | 0.500 | 1.000 | 10.000 | 20.000 | 50.000 |
| ABA | 0.100 | 0.500 | 1.000 | 10.000 | 20.000 | 50.000 |
| Name | Primer Sequence (5′-3′) |
|---|---|
| BC | F: CAAGAAGGTGATGATCGCCA |
| R: GACGTGCAGCGAGTTCTTGTC | |
| FATA | F: AGACTCGTTCAGCGAGGAGC |
| R: CATGCCCACAGCATGGTTC | |
| ACP | F: CAGCTCGGCACTGACCTTG |
| R: CAAGGGTCAGCTCGAACTTCTC | |
| SAD | F: CCGAGCCCAAGCTTCTAGTG |
| R: TTTGCCTCCATGTAATCCCC | |
| MCTK | F: GGTGAGGACAAGGCGGTG |
| R: TCATCCTGGCCTTGAAGCTC | |
| FAD | F: GTAGGTCACCACGTCCAGCC |
| R: CTTGATAGGCATGCTGGGTGT | |
| KAS | F: CACCCCACTCTGAACCAGGA |
| R: GACCTCCAAACCCGAAGGAG |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, H.; Ahmed, F.; Lin, B.; Li, Z.; Huang, Y.; Sun, G.; Ding, H.; Wang, C.; Meng, C.; Gao, Z. The Effects of Plant Growth Regulators on Cell Growth, Protein, Carotenoid, PUFAs and Lipid Production of Chlorella pyrenoidosa ZF Strain. Energies 2017, 10, 1696. https://doi.org/10.3390/en10111696
Du H, Ahmed F, Lin B, Li Z, Huang Y, Sun G, Ding H, Wang C, Meng C, Gao Z. The Effects of Plant Growth Regulators on Cell Growth, Protein, Carotenoid, PUFAs and Lipid Production of Chlorella pyrenoidosa ZF Strain. Energies. 2017; 10(11):1696. https://doi.org/10.3390/en10111696
Chicago/Turabian StyleDu, Huanmin, Faruq Ahmed, Bin Lin, Zhe Li, Yuhan Huang, Guang Sun, Huan Ding, Chang Wang, Chunxiao Meng, and Zhengquan Gao. 2017. "The Effects of Plant Growth Regulators on Cell Growth, Protein, Carotenoid, PUFAs and Lipid Production of Chlorella pyrenoidosa ZF Strain" Energies 10, no. 11: 1696. https://doi.org/10.3390/en10111696




