A Two-Dimensional Multiphysics Coupling Model of a Middle and Low Temperature Solar Receiver/Reactor for Methanol Decomposition
Abstract
:1. Introduction
- (1)
- To reveal the fluid dynamic, thermal and chemical reaction characteristics of the MLTSRR, a mathematical model for analyzing the properties of the MLTSRR is implemented by the finite element method (FEM), which couples momentum equation in porous catalyst bed, the governing mass conservation with chemical reaction, and energy conservation incorporating conduction/convection/radiation heat transfer.
- (2)
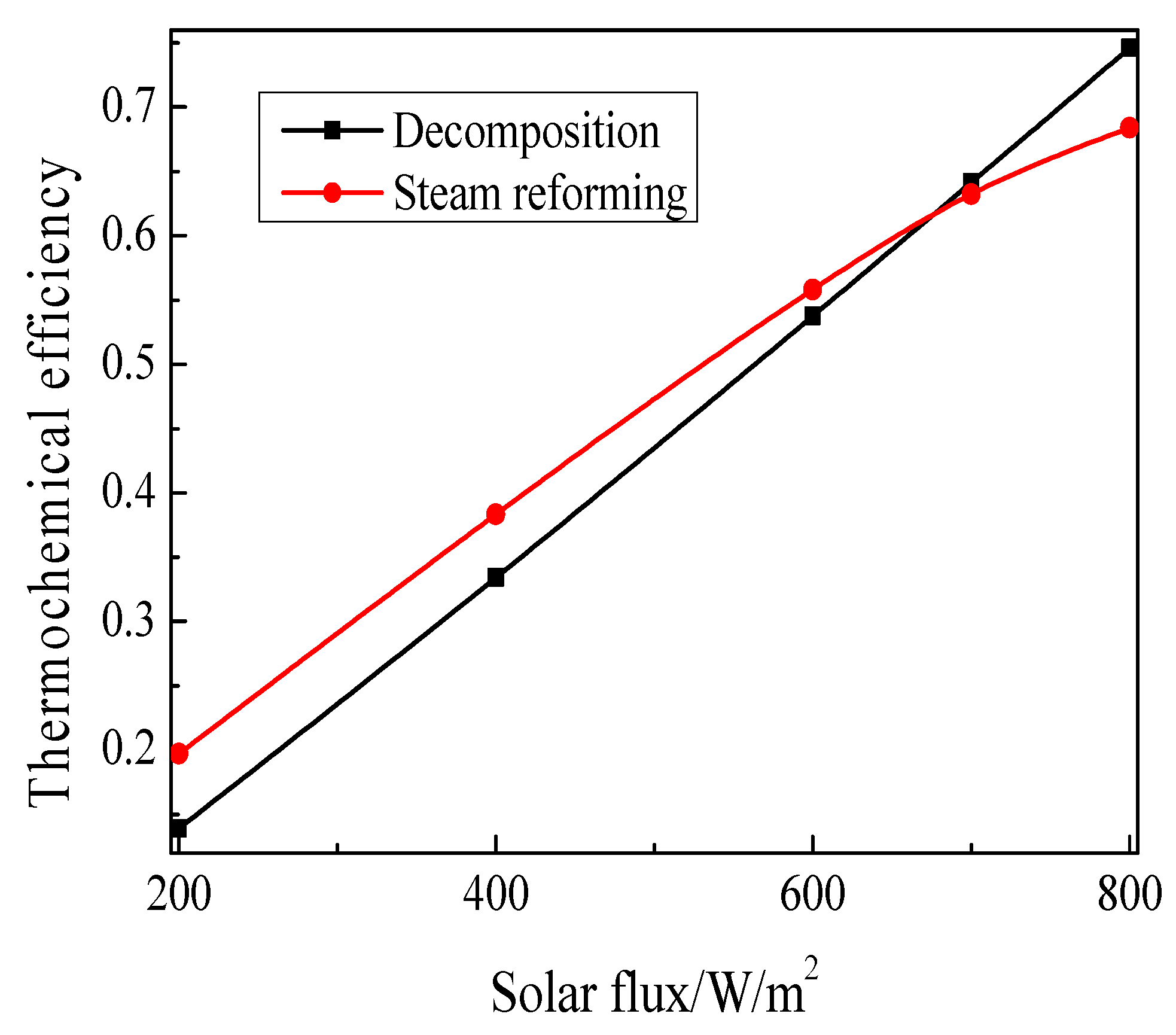
- Two approaches of methanol for solar energy utilization: methanol decomposition and methanol steam reforming with direct normal irradiation (DNIs) were compared.
- (3)
- The effects of the annulus vacuum space and the glass tube on the properties of the middle and low temperature solar receiver/reactor (MLTSRR) were disclosed.
2. Physical Model of the MLTSRR
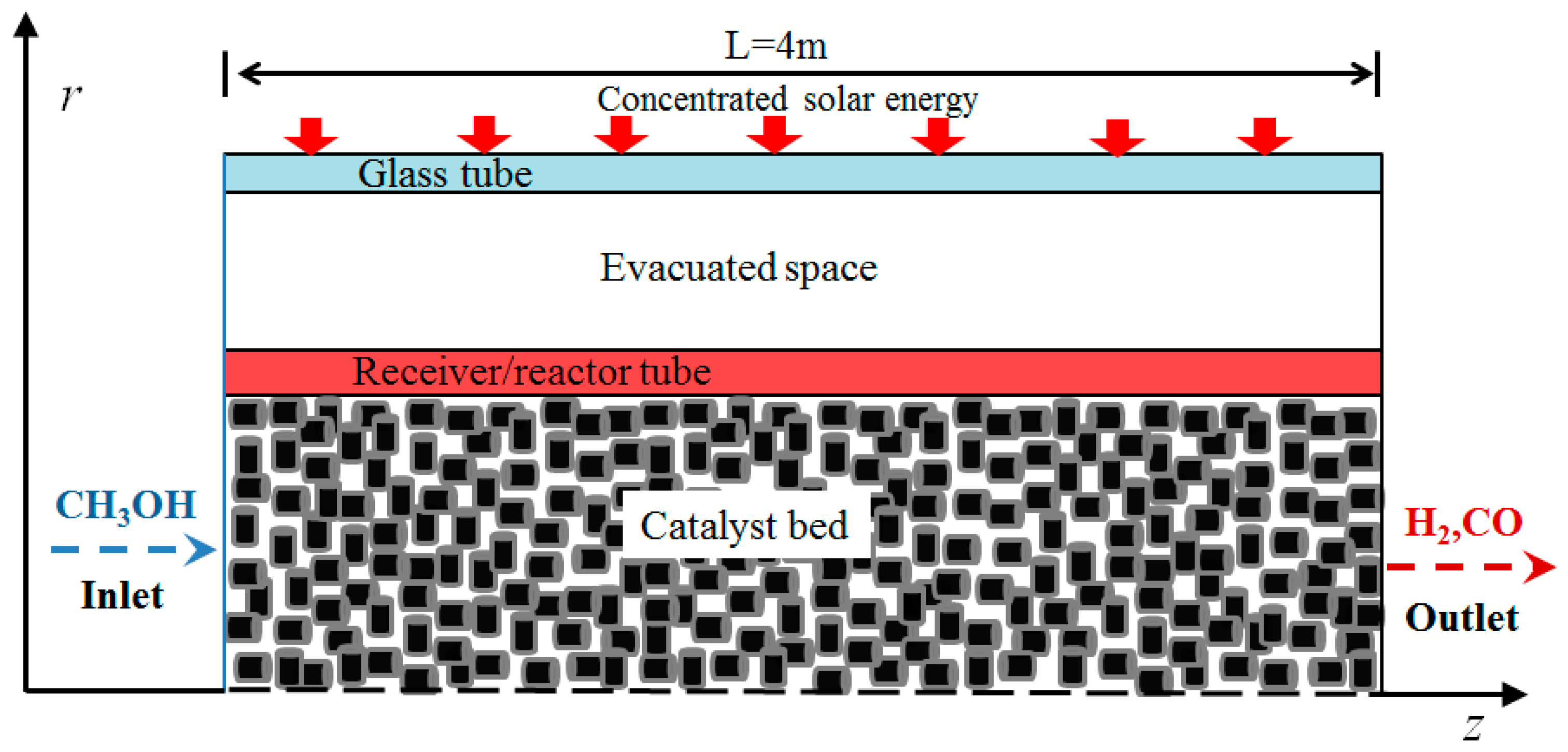
2.1. Configuration of the MLTSRR
2.2. Multiphysics Coupling Model of the MLTSRR
2.3. Numerical Methods
3. Results and Discussion
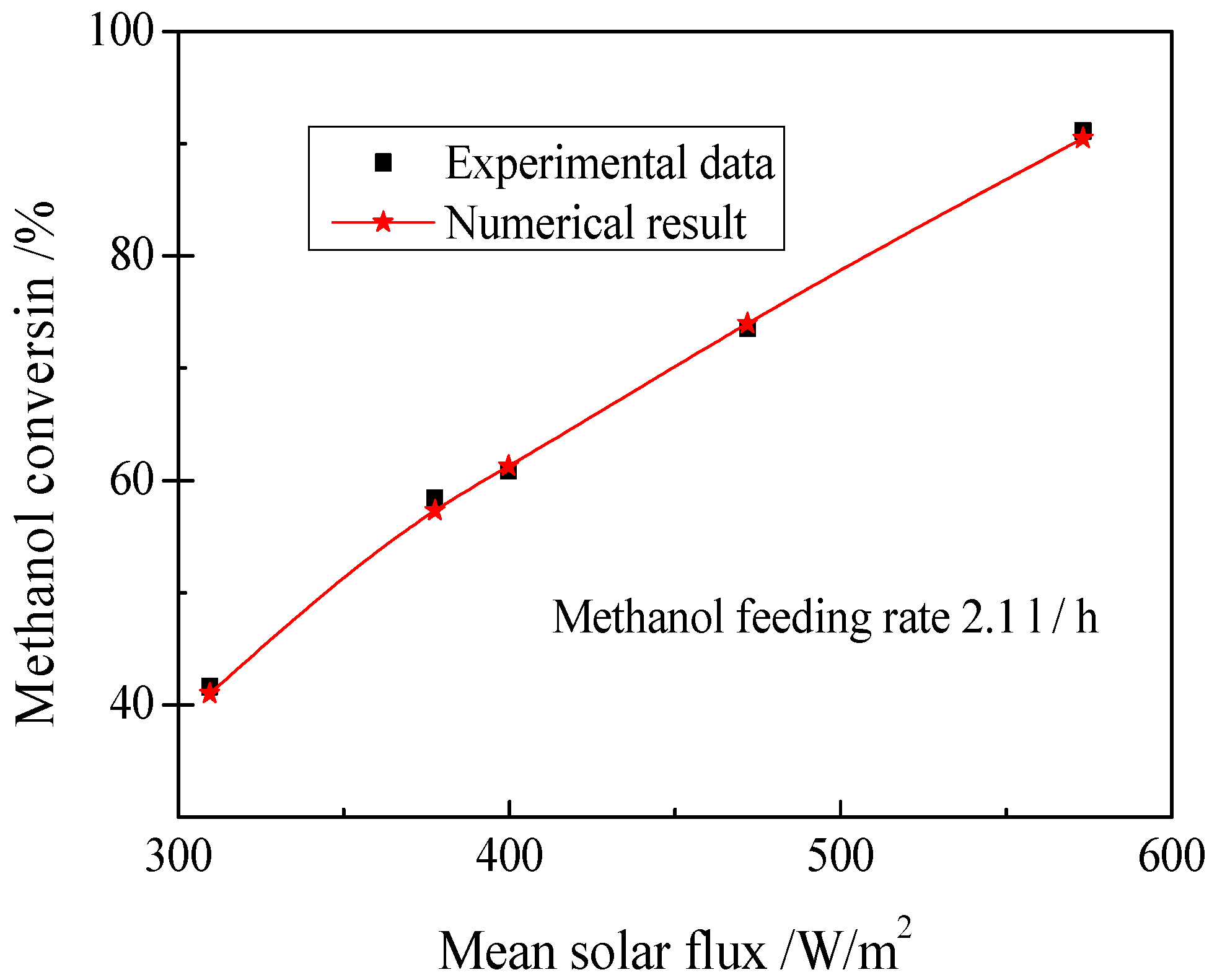
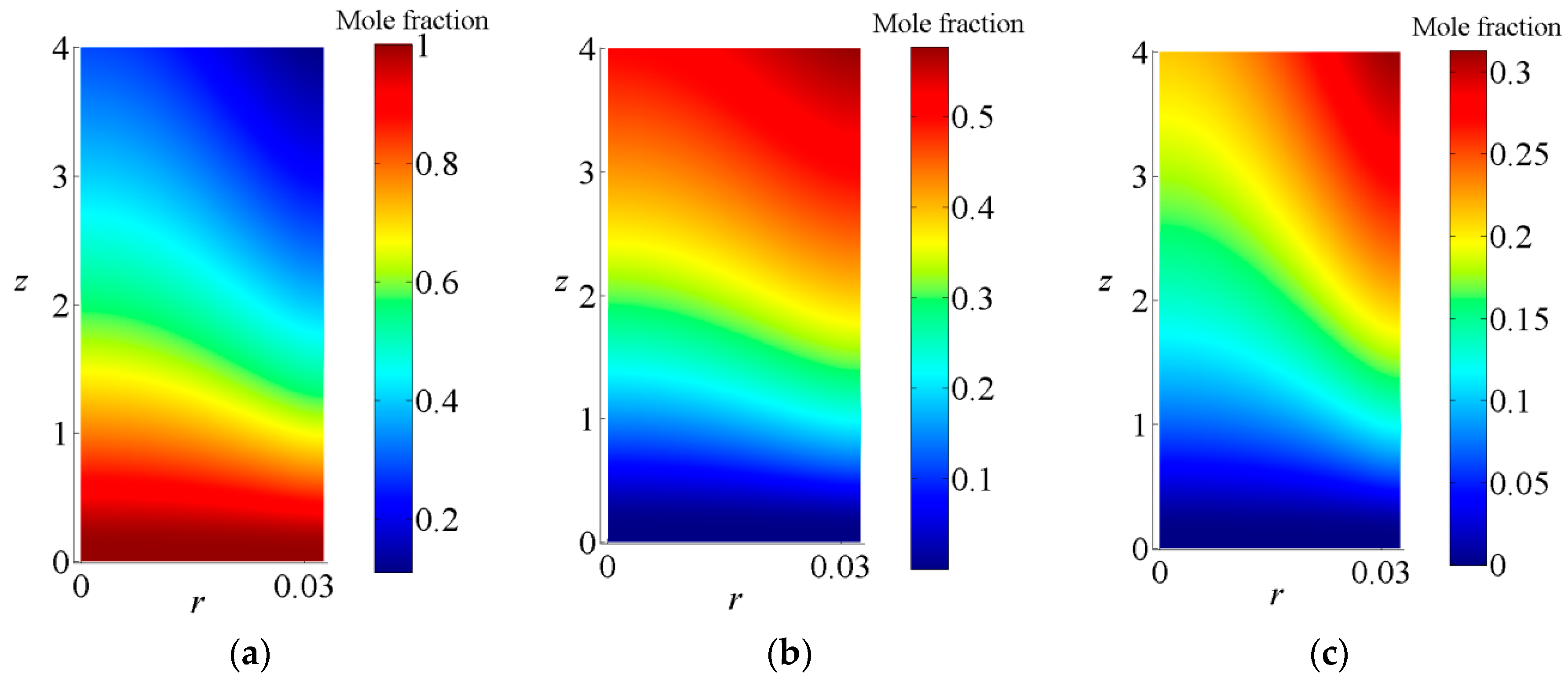
3.1. Thermochemical Properties of the MLTSRR
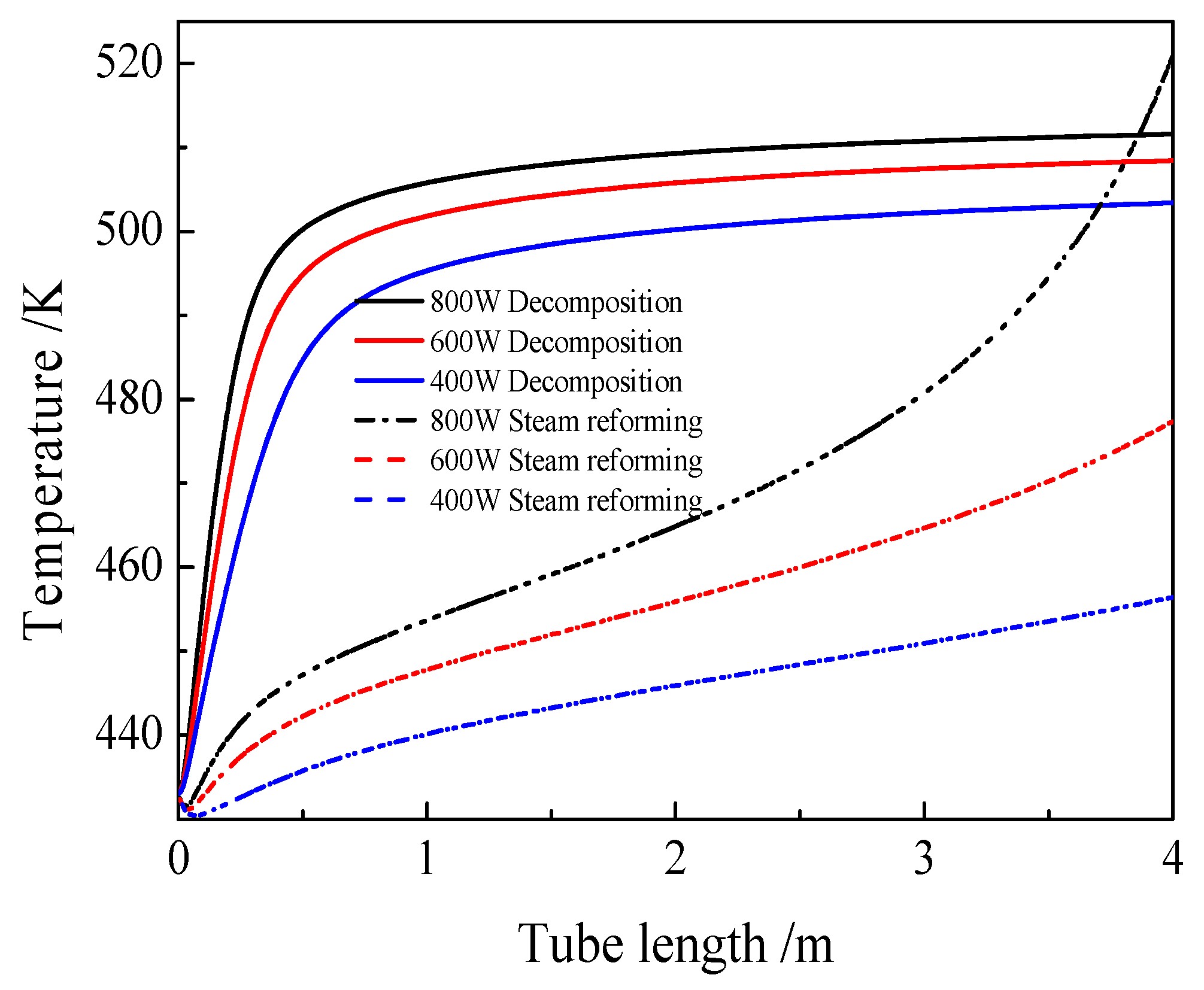
3.2. Comparison of the Thermochemical Performance between Methanol Decomposition and Methanol Steam Reforming
3.3. Characteristics of Different Operation Conditions
4. Conclusions
- (1)
- The characteristics of chemical reaction, coupled heat transfer, and the whole components distributions and temperature fields in the receiver/reactor were obtained, when the reactants feeding rate of methanol is 11.3 kg/h, the DNI is 800 W/m2, the efficiency of the solar energy transformed into the chemical energy can reach 0.74.
- (2)
- Two ways of methanol for solar energy utilization: methanol decomposition and methanol steam reforming were compared. The thermochemical efficiency of the methanol steam reforming can be 7.2%, which is higher than that of methanol decomposition when the solar flux is 200 W/m2, and the thermochemical efficiency of the methanol decomposition method can be 6.2% higher than that of methanol steam reforming when the solar flux is 800 W/m2.
- (3)
- The effects of the annulus vacuum space and the glass tube on the property of the MLTSRR were investigated. When the reactants feeding rate of methanol is 13 kg/h, the solar flux increases from 500 W/m2 to 1000 W/m2, the solar flux increases from 500 W/m2 to 1000 W/m2, the methanol conversion can fall by 6.8–8.9% with air in the annulus, and methanol conversion can decrease by 21.8–28.9% when the glass is removed from the receiver/reactor.
Acknowledgments
Author Contributions
Conflicts of Interest
Nomenclature
| ηso-th | Efficiency of solar thermal energy converted into chemical energy |
| nun,CH3OH | Mole flow rate of methanol which is not reacted |
| nCH3OH | Mole flow rate of methanol |
| ε | Porosity of the catalyst bed |
| SA | Effect area of the parabolic trough concentrators |
| κ | Hydraulic permeability κ [m2] |
| XCH3OH | Methanol conversion rate |
| η | Dynamic viscosity [kg/(m·s)] |
| ΔrH | Reaction enthalpy of methanol [J/mol] |
References
- Steinfeld, A.; Palumbo, R. Solar thermochemical process technology. Encycl. Phys. Sci. Technol. 2001, 15, 237–256. [Google Scholar]
- Fletcher, E.A. Solarthermal processing: A review. J. Sol. Energy Eng. 2001, 123, 63–74. [Google Scholar] [CrossRef]
- Chueh, W.C.; Falter, C.; Abbott, M.; Scipio, D.; Furler, P.; Haile, S.M.; Steinfeld, A. High-flux solar-driven thermochemical dissociation of CO2 and H2O using nonstoichiometric ceria. Science 2010, 330, 1797–1801. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, D.; Steinfeld, A. Solar hydrogen production by thermal decomposition of natural gas using a vortex-flow reactor. Int. J. Hydrogen Energy 2004, 29, 47–55. [Google Scholar] [CrossRef]
- Wang, J.J.; Lu, Y.C.; Yang, Y.; Mao, T.Z. Thermodynamic performance analysis and optimization of a solar-assisted combined cooling, heating and power system. Energy 2016, 115, 49–59. [Google Scholar] [CrossRef]
- Hong, H.; Liu, Q.B.; Jin, H.G. Solar hydrogen production integrating low-grade solar thermal energy and methanol steam reforming. J. Energy Resour. Technol. 2009, 131, 012601. [Google Scholar] [CrossRef]
- Sui, J.; Liu, Q.B.; Dang, J.G.; Guo, D.; Jin, H.G.; Ji, J. Experimental investigation of methanol decomposition with mid- and low-temperature solar thermal energy. Energy Res. 2011, 35, 61–67. [Google Scholar] [CrossRef]
- Wang, J.J.; Yang, Y. Energy, exergy and environmental analysis of a hybrid combined cooling heating and power system utilizing biomass and solar energy. Energy Convers. Manag. 2016, 124, 566–577. [Google Scholar] [CrossRef]
- Jin, H.; Sui, J.; Hong, H.; Wang, Z.; Zheng, D.; Hou, Z. Prototype of solar receiver/reactor with parabolic troughs. J. Sol. Energy Eng. 2007, 129, 378–381. [Google Scholar] [CrossRef]
- Fogler, H.S. Elements of Chemical Reactor Engineering; Prentice-Hall: Englewood Cliffs, NJ, USA, 1986. [Google Scholar]
- Kanouff, M.P.; Gharagozloo, P.E.; Salloum, M.; Shugard, A.D. A multiphysics numerical model of oxidation and decomposition in a uranium hydride bed. Chem. Eng. Sci. 2013, 93, 212–225. [Google Scholar] [CrossRef]
- Jiang, C.J.; Trimm, D.L.; Wainwright, M.S.; Cant, N.W. Kinetic mechanism for the reaction between methanol and water over a Cu-ZnO-Al2O3 catalyst. Appl. Catal. A Gen. 1993, 97, 145–158. [Google Scholar] [CrossRef]
- Wesselingh, J.A.; Krishna, R. Mass Transfer in Multicomponent Mixtures; Delft University Press: Delft, The Netherlands, 2000. [Google Scholar]
- Poling, B.E.; Prausnitz, J.M.; O’connell, J.P. The Properties of Gases and Liquids; McGraw-Hill: New York, NY, USA, 2001. [Google Scholar]
- Price, H.; Lüpfert, E.; Kearney, D. Advances in parabolic trough solar power technology. J. Sol. Energy Eng. 2002, 124, 109–125. [Google Scholar] [CrossRef]



| Items | Parameter |
|---|---|
| Mirror reflectivity | 0.93 |
| Aperture Length [m] | 4 |
| Aperture Width [m] | 2.5 |
| Glass tue’s outer diameter [m] | 0.115 |
| glass tube’s inner diameter [m] | 0.109 |
| Reactor’s outer diameter [m] | 0.07 |
| Reactor’s inner diameter [m] | 0.065 |
| Coating absorbance | 0.95 |
| Glass tube’s transmittance | 0.95 |
| Coating emissivity [°C] | 0.04795 + 0.0002331T |
| Rate Constant or Equilibrium Constant | or | or |
|---|---|---|
| 3.8 × 1020 | 170 | |
| 30.0 | −20.0 | |
| 30.0 | −20.0 | |
| −46.2 | −50.0 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Liu, Q.; Lei, J.; Li, J.; Chen, C. A Two-Dimensional Multiphysics Coupling Model of a Middle and Low Temperature Solar Receiver/Reactor for Methanol Decomposition. Energies 2017, 10, 1705. https://doi.org/10.3390/en10111705
Wang Y, Liu Q, Lei J, Li J, Chen C. A Two-Dimensional Multiphysics Coupling Model of a Middle and Low Temperature Solar Receiver/Reactor for Methanol Decomposition. Energies. 2017; 10(11):1705. https://doi.org/10.3390/en10111705
Chicago/Turabian StyleWang, Yanjuan, Qibin Liu, Jing Lei, Jiwei Li, and Can Chen. 2017. "A Two-Dimensional Multiphysics Coupling Model of a Middle and Low Temperature Solar Receiver/Reactor for Methanol Decomposition" Energies 10, no. 11: 1705. https://doi.org/10.3390/en10111705




