Supercritical Water Gasification of Biomass in a Ceramic Reactor: Long-Time Batch Experiments
Abstract
:1. Introduction
2. Results and Discussion
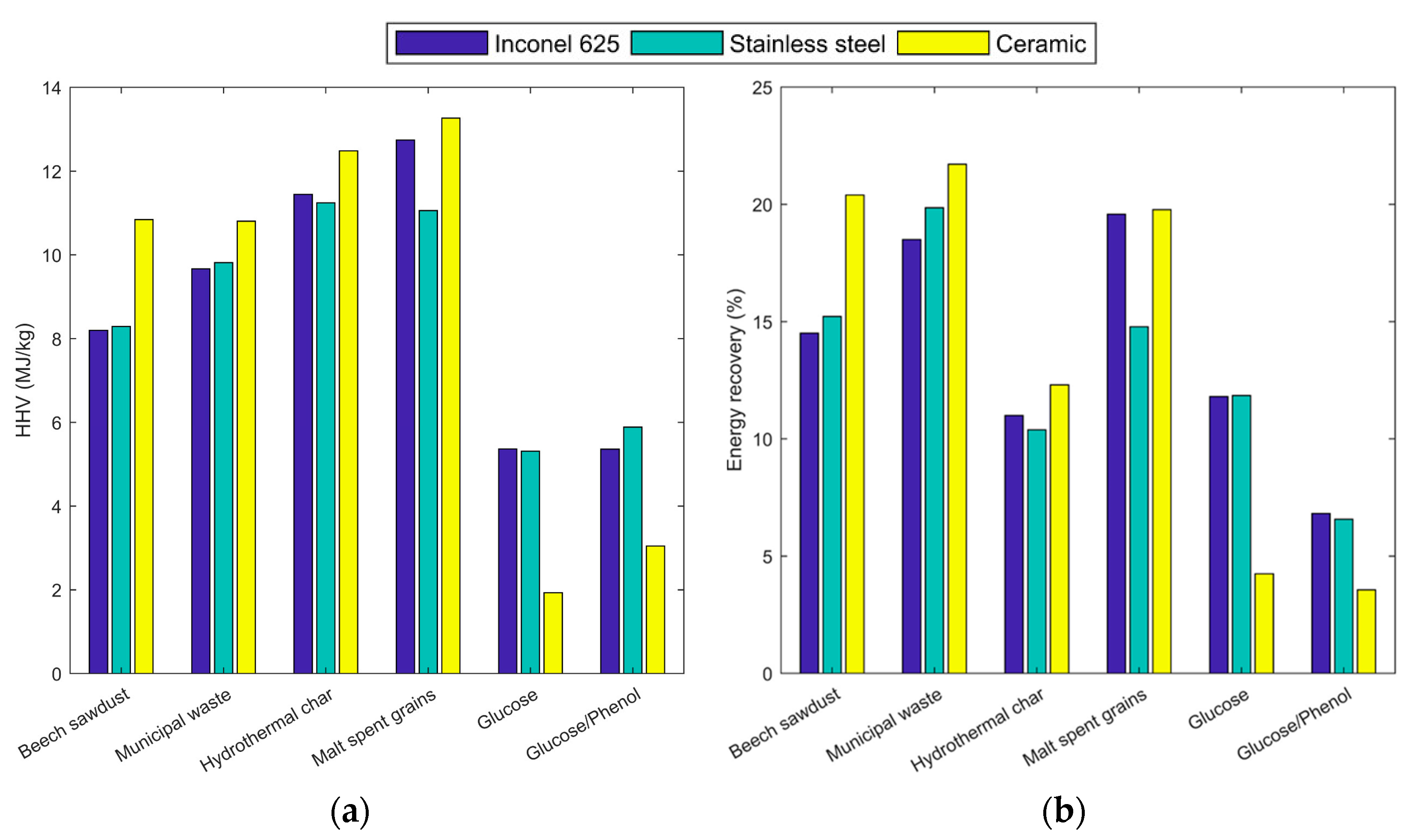
2.1. Elemental Analysis and Higher Heating Value of the Feedstock
2.2. Gas, Liquid and Solid Yields
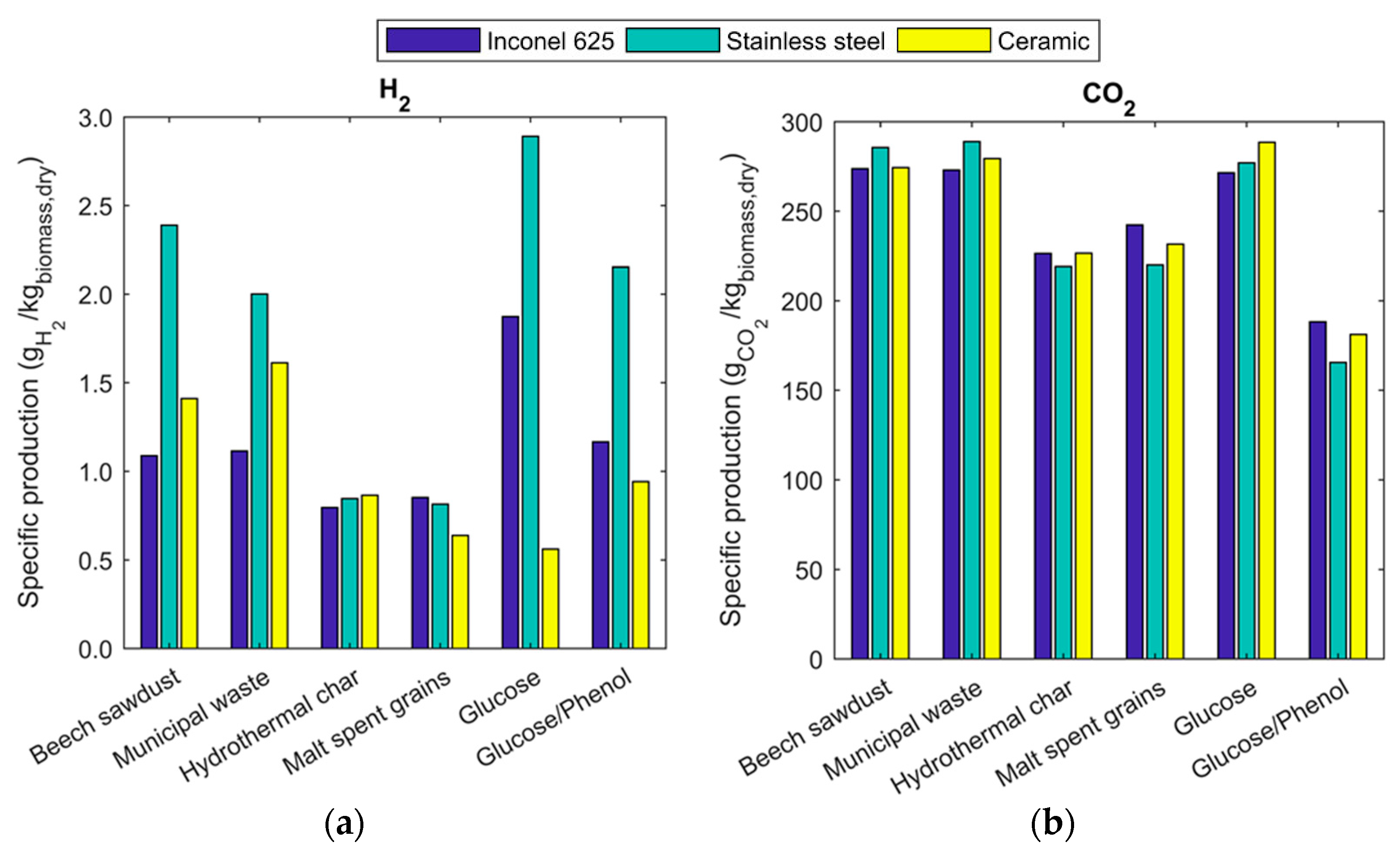
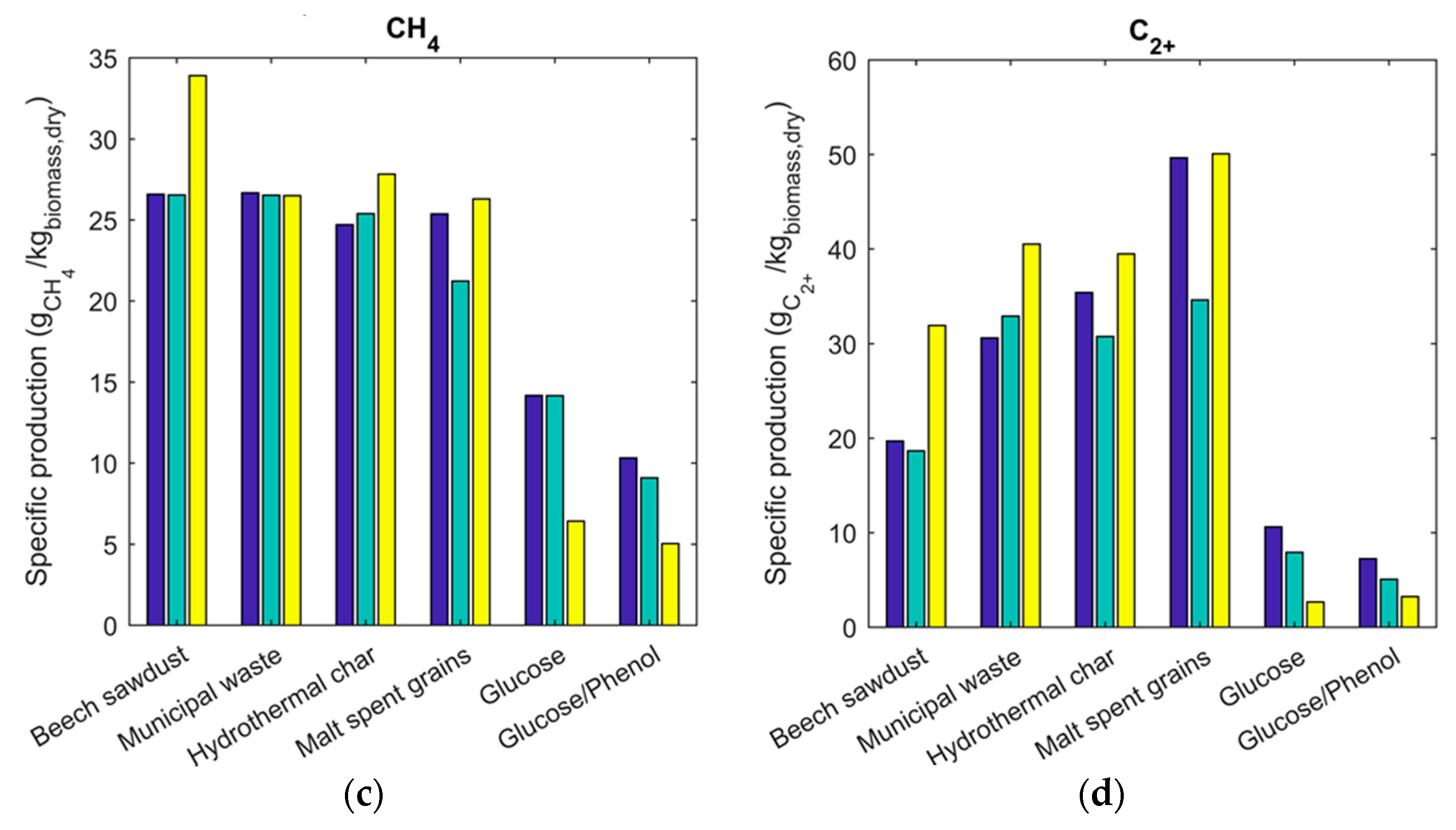
2.3. Gas Composition and Equilibrium Yields
2.4. Energy Recovery
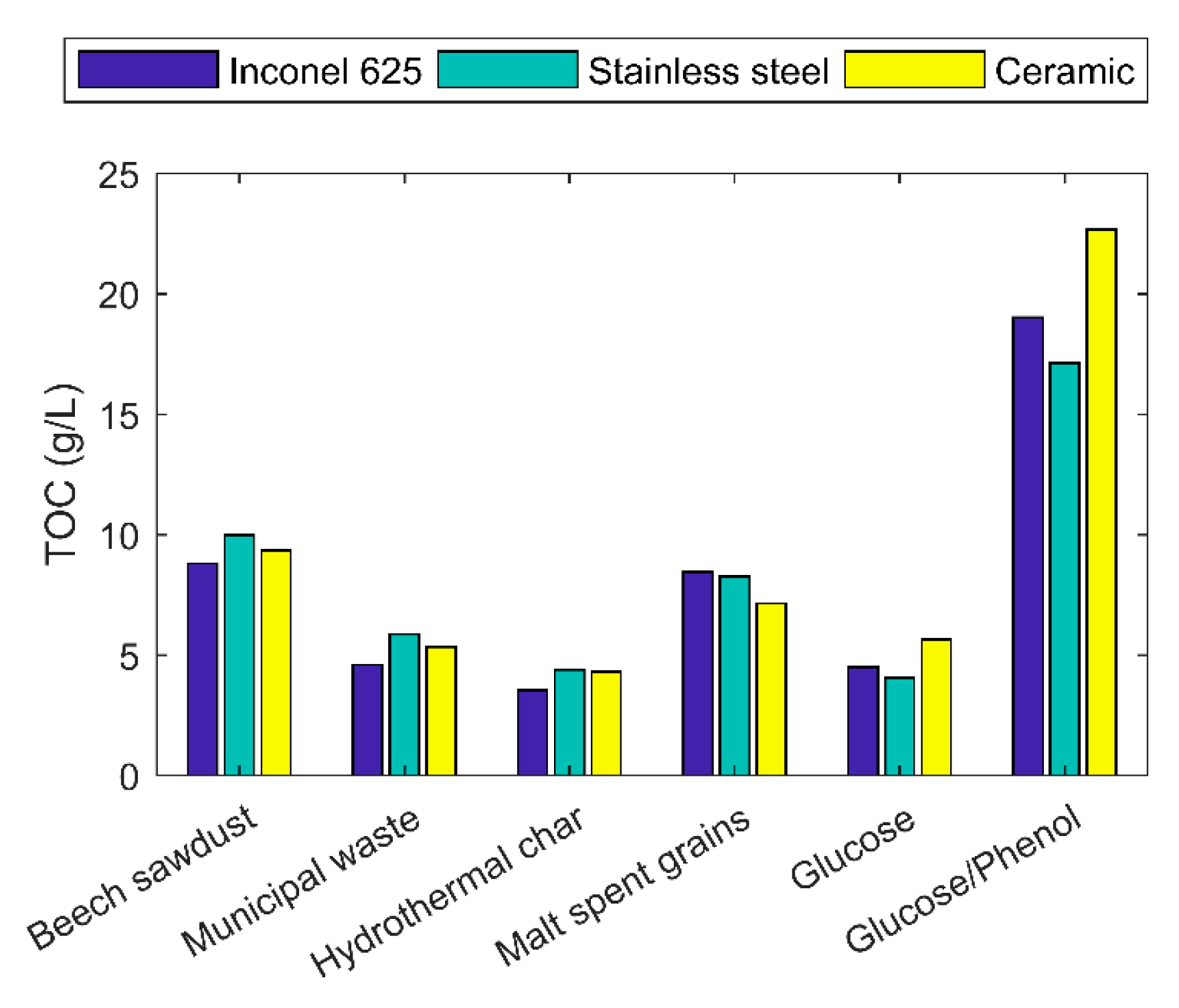
2.5. Liquid Products
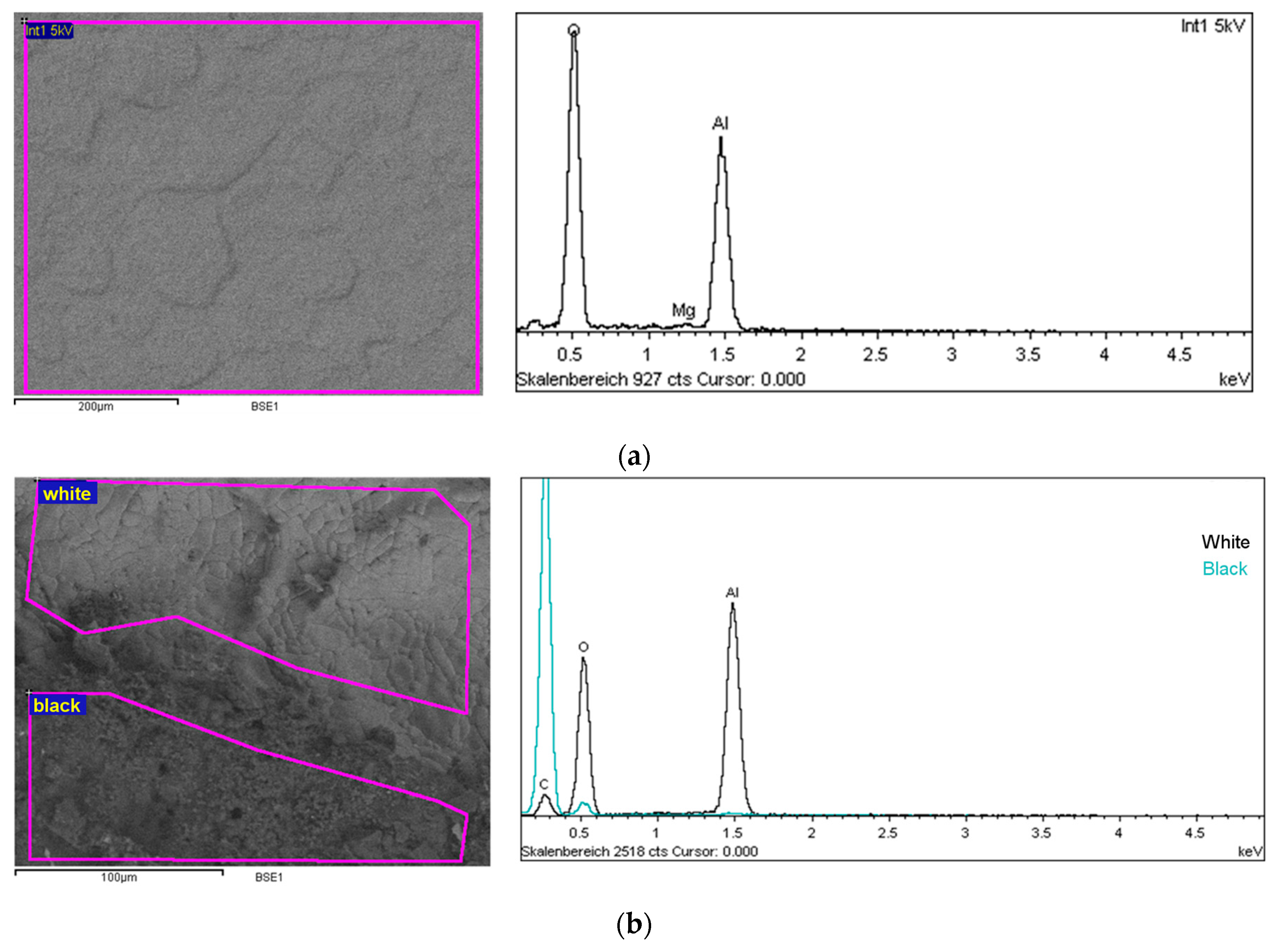
2.6. Observation of the Alumina Surface
3. Materials and Methods
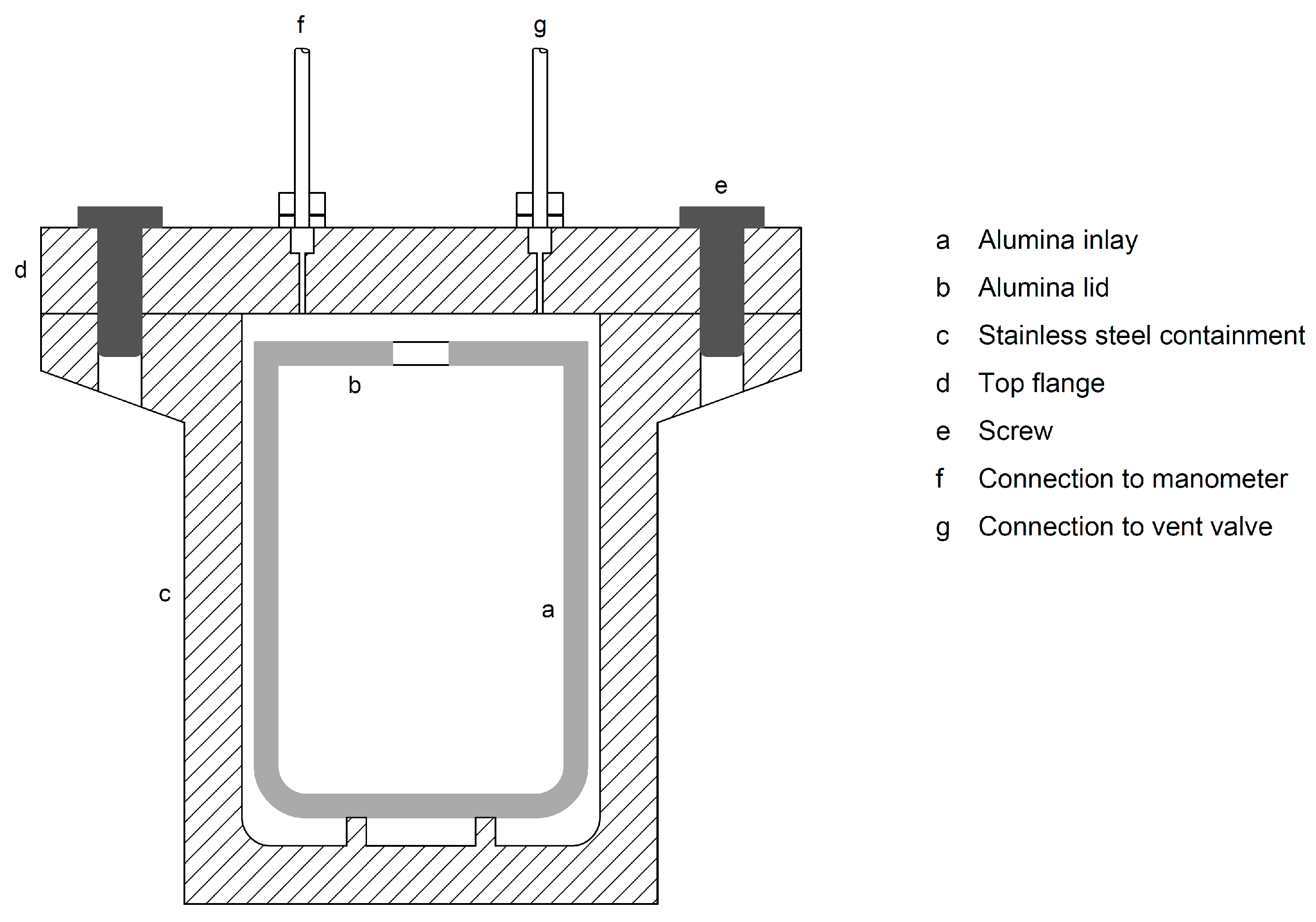
3.1. Reactors and Feedstock
3.2. Experimental Procedure and Analytics
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Molino, A.; Chianese, S.; Musmarra, D. Biomass gasification technology: The state of the art overview. J. Energy Chem. 2016, 25, 10–25. [Google Scholar] [CrossRef]
- Matsumura, Y.; Minowa, T.; Potic, B.; Kersten, S.; Prins, W.; van Swaaij, W.; van de Beld, B.; Elliott, D.; Neuenschwander, G.; Kruse, A.; et al. Biomass gasification in near- and super-critical water: Status and prospects. Biomass Bioenergy 2005, 29, 269–292. [Google Scholar] [CrossRef]
- Dinjus, E.; Kruse, A. Hot compressed water—A suitable and sustainable solvent and reaction medium? J. Phys. Condens. Matter 2004, 16, S1161–S1169. [Google Scholar] [CrossRef]
- Castello, D.; Fiori, L. Supercritical water gasification of biomass: Thermodynamic constraints. Bioresour. Technol. 2011, 102, 7574–7582. [Google Scholar] [CrossRef] [PubMed]
- Kruse, A. Hydrothermal biomass gasification. J. Supercrit. Fluids 2009, 47, 391–399. [Google Scholar] [CrossRef]
- Molino, A.; Migliori, M.; Blasi, A.; Davoli, M.; Marino, T.; Chianese, S.; Catizzone, E.; Giordano, G. Municipal waste leachate conversion via catalytic supercritical water gasification process. Fuel 2017, 206, 155–161. [Google Scholar] [CrossRef]
- Yakaboylu, O.; Harinck, J.; Smit, K.G.; de Jong, W. Supercritical water gasification of biomass: A literature and technology overview. Energies 2015, 8, 859–894. [Google Scholar] [CrossRef]
- Boukis, N.; Habicht, W.; Franz, G.; Dinjus, E. Behavior of Ni-base alloy 625 in methanol-supercritical water systems. Mater. Corros. 2003, 54, 326–330. [Google Scholar] [CrossRef]
- Boukis, N.; Claussen, N.; Ebert, K.; Janssen, R.; Schacht, M. Corrosion Screening Tests of High-Performance Ceramics in Supercritical Water Containing Oxygen and Hydrochloric Acid. J. Eur. Ceram. Soc. 1997, 17, 71–76. [Google Scholar] [CrossRef]
- Schacht, M.; Schacht, M.; Boukis, N.; Dinjus, E. Corrosion of alumina ceramics in acidic aqueous solutions at high temperatures and pressures. J. Mater. Sci. 2000, 35, 6251–6258. [Google Scholar] [CrossRef]
- Richard, T.; Poirier, J.; Reverte, C.; Aymonier, C.; Loppinet-Serani, A.; Iskender, G.; Pablo, E.-B.; Marias, F. Corrosion of ceramics for vinasse gasification in supercritical water. J. Eur. Ceram. Soc. 2012, 32, 2219–2233. [Google Scholar] [CrossRef]
- Vogel, F.; DiNaro Blanchard, J.L.; Marrone, P.A.; Rice, S.F.; Webley, P.A.; Peters, W.A.; Smith, K.A.; Tester, J.W. Critical review of kinetic data for the oxidation of methanol in supercritical water. J. Supercrit. Fluids 2005, 34, 249–286. [Google Scholar] [CrossRef]
- Boukis, N.; Diem, V.; Galla, U.; Dinjus, E. Methanol reforming in supercritical water for hydrogen production. Combust. Sci. Technol. 2006, 178, 467–485. [Google Scholar] [CrossRef]
- Antal, M.J., Jr.; Allen, S.G.; Schulman, D.; Xu, X.; Divilio, R.J. Biomass gasification in supercritical water. Ind. Eng. Chem. Res. 2000, 39, 4040–4053. [Google Scholar] [CrossRef]
- Castello, D.; Kruse, A.; Fiori, L. Biomass gasification in supercritical and subcritical water: The effect of the reactor material. Chem. Eng. J. 2013, 228. [Google Scholar] [CrossRef]
- Tanksale, A.; Beltramini, J.N.; Lu, G.M. A review of catalytic hydrogen production processes from biomass. Renew. Sustain. Energy Rev. 2010, 14, 166–182. [Google Scholar] [CrossRef]
- Flego, C.; Carati, A.; Perego, C. Methanol interaction with mesoporous silica-aluminas. Microporous Mesoporous Mater. 2001, 44–45, 733–744. [Google Scholar] [CrossRef]
- Castello, D.; Kruse, A.; Fiori, L. Supercritical water gasification of hydrochar. Chem. Eng. Res. Des. 2014, 92, 1864–1875. [Google Scholar] [CrossRef]
- DiLeo, G.J.; Neff, M.E.; Kim, S.; Savage, P.E. Supercritical Water Gasification of Phenol and Glycine as Models for Plant and Protein Biomass. Energy Fuels 2008, 22, 871–877. [Google Scholar] [CrossRef]
- Kruse, A.; Krupka, A.; Schwarzkopf, V.; Gamard, C.; Henningsen, T. Influence of Proteins on the Hydrothermal Gasification and Liquefaction of Biomass. 1. Comparison of Different Feedstocks. Ind. Eng. Chem. Res. 2005, 44, 3013–3020. [Google Scholar] [CrossRef]
- Kruse, A.; Maniam, P.; Spieler, F. Influence of proteins on the hydrothermal gasification and liquefaction of biomass. 2. Model compounds. Ind. Eng. Chem. Res. 2007, 46, 87–96. [Google Scholar] [CrossRef]
- Byrd, A.J.; Pant, K.K.; Gupta, R.B. Hydrogen production from glucose using Ru/Al2O3 catalyst in supercritical water. Ind. Eng. Chem. Res. 2007, 46, 3574–3579. [Google Scholar] [CrossRef]
- Hao, X.H.; Guo, L.J.; Mao, X.; Zhang, X.M.; Chen, X.J. Hydrogen production from glucose used as a model compound of biomass gasified in supercritical water. Int. J. Hydrogen Energy 2003, 28, 55–64. [Google Scholar] [CrossRef]
- Muangrat, R.; Onwudili, J.A.; Williams, P.T. Influence of alkali catalysts on the production of hydrogen-rich gas from the hydrothermal gasification of food processing waste. Appl. Catal. B Environ. 2010, 100, 440–449. [Google Scholar] [CrossRef]
- Williams, P.; Onwudili, J. Composition of products from the supercritical water gasification of glucose: A model biomass compound. Ind. Eng. Chem. Res. 2005, 44, 8739–8749. [Google Scholar] [CrossRef]
- Knezevic, D.; van Swaaij, W.P.M.; Kersten, S.R.A. Hydrothermal Conversion of Biomass: I, Glucose Conversion in Hot Compressed Water. Ind. Eng. Chem. Res. 2009, 48, 4731–4743. [Google Scholar] [CrossRef]
- Huelsman, C.M.; Savage, P.E. Reaction pathways and kinetic modeling for phenol gasification in supercritical water. J. Supercrit. Fluids 2013, 81, 200–209. [Google Scholar] [CrossRef]
- Yong, T.L.-K.; Matsumura, Y. Kinetics analysis of phenol and benzene decomposition in supercritical water. J. Supercrit. Fluids 2014, 87, 73–82. [Google Scholar] [CrossRef]
- DiLeo, G.J.; Neff, M.E.; Savage, P.E. Gasification of Guaiacol and Phenol in Supercritical Water. Energy Fuels 2007, 21, 2136–2340. [Google Scholar] [CrossRef]
- Castello, D.; Kruse, A.; Fiori, L. Low temperature supercritical water gasification of biomass constituents: Glucose/phenol mixtures. Biomass Bioenergy 2015, 73, 84–94. [Google Scholar] [CrossRef]
- Weiss-Hortala, E.; Kruse, A.; Ceccarelli, C.; Barna, R. Influence of phenol on glucose degradation during supercritical water gasification. J. Supercrit. Fluids 2010, 53, 42–47. [Google Scholar] [CrossRef]
- Fujita, S.; Terunuma, H.; Kobayashi, H.; Takezawa, N. Methanation of carbon monoxide and carbon dioxide over nickel catalyst under the transient state. React. Kinet. Catal. Lett. 1987, 33, 179–184. [Google Scholar] [CrossRef]
- Busca, G. Acid catalysts in industrial hydrocarbon chemistry. Chem. Rev. 2007, 107, 5366–5410. [Google Scholar] [CrossRef] [PubMed]
- Karayıldırım, T.; Sınağ, A.; Kruse, A. Char and Coke Formation as Unwanted Side Reaction of the Hydrothermal Biomass Gasification. Chem. Eng. Technol. 2008, 31, 1561–1568. [Google Scholar] [CrossRef]
- Chianese, S.; Fail, S.; Binder, M.; Rauch, R.; Hofbauer, H.; Molino, A.; Blasi, A.; Musmarra, D. Experimental investigations of hydrogen production from CO catalytic conversion of tar rich syngas by biomass gasification. Catal. Today 2016, 277, 182–191. [Google Scholar] [CrossRef]
- Chakinala, A.G.; Brilman, D.W.F.; van Swaaij, W.P.M.; Kersten, S. Catalytic and non-catalytic supercritical water gasification of microalgae and glycerol. Ind. Eng. Chem. Res. 2010, 49, 1113–1122. [Google Scholar] [CrossRef]
- Matsumura, Y.; Harada, M.; Nagata, K.; Kikuchi, Y. Effect of heating rate of biomass feedstock on carbon gasification efficiency in supercritical water gasification. Chem. Eng. Commun. 2006, 193, 649–659. [Google Scholar] [CrossRef]
- Sinag, A.; Kruse, A.; Rathert, J. Influence of the Heating Rate and the Type of Catalyst on the Formation of Key Intermediates and on the Generation of Gases During Hydropyrolysis of Glucose in Supercritical Water in a Batch Reactor. Ind. Eng. Chem. Res. 2004, 43, 502–508. [Google Scholar] [CrossRef]
- Louw, J.; Schwarz, C.E.; Burger, A.J. Catalytic supercritical water gasification of primary paper sludge using a homogeneous and heterogeneous catalyst: Experimental vs. thermodynamic equilibrium results. Bioresour. Technol. 2016, 201, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Kruse, A.; Faquir, M. Hydrothermal Biomass Gasification—Effects of Salts, Backmixing and Their Interaction. Chem. Eng. Technol. 2007, 30, 749–754. [Google Scholar] [CrossRef]
- Chuntanapum, A.; Matsumura, Y. Char formation mechanism in supercritical water gasification process: A study of model compounds. Ind. Eng. Chem. Res. 2010, 49, 4055–4062. [Google Scholar] [CrossRef]
- Lucian, M.; Fiori, L. Hydrothermal Carbonization of Waste Biomass: Process Design, Modeling, Energy Efficiency and Cost Analysis. Energies 2017, 10, 211. [Google Scholar] [CrossRef]
- Wagner, W.; Pruß, A. The IAPWS formulation 1995 for the thermodynamic properties of ordinary water substance for general and scientific use. J. Phys. Chem. Ref. Data 2002, 31, 387–535. [Google Scholar] [CrossRef]


| Feedstock | Elemental Composition (wt %) | HHV (MJ/kg) | ||||
|---|---|---|---|---|---|---|
| C | H | N | S | O | ||
| Beech sawdust | 48.93 | 6.17 | 1.03 | 0.10 | 40.61 | 18.2 |
| Municipal waste | 45.21 | 6.61 | 2.06 | 0.22 | 41.83 | 17.3 |
| Hydrothermal char | 69.68 | 6.82 | 1.06 | 0.10 | 19.71 | 29.9 |
| Malt spent grains | 49.72 | 6.94 | 4.33 | 0.30 | 34.25 | 20.7 |
| Glucose | 40.00 | 6.67 | − | − | 53.33 | 13.5 |
| Glucose + phenol | 45.43 | 6.62 | − | − | 47.95 | 16.3 |
| Feedstock | Reactor | Gas | Solid | Liquid |
|---|---|---|---|---|
| Beech sawdust | Inconel | 32.1 | 26.1 | 41.8 |
| Stainless steel | 33.3 | 22.0 | 44.7 | |
| Ceramics | 34.2 | 21.3 | 44.5 | |
| Municipal waste | Inconel | 33.1 | 25.4 | 41.5 |
| Stainless steel | 35.0 | 23.5 | 41.5 | |
| Ceramics | 34.8 | 20.5 | 44.7 | |
| Hydrothermal char | Inconel | 28.7 | 49.3 | 22.0 |
| Stainless steel | 27.6 | 48.1 | 24.2 | |
| Ceramics | 29.5 | 47.8 | 22.7 | |
| Malt spent grains | Inconel | 31.8 | 18.9 | 49.3 |
| Stainless steel | 27.7 | 18.7 | 53.7 | |
| Ceramics | 30.9 | 23.1 | 46.1 | |
| Glucose | Inconel | 29.8 | 28.8 | 41.3 |
| Stainless steel | 30.2 | 38.5 | 31.3 | |
| Ceramics | 29.8 | 23.0 | 47.2 | |
| Glucose + phenol | Inconel | 20.7 | 19.5 | 59.8 |
| Stainless steel | 18.2 | 23.8 | 58.0 | |
| Ceramics | 19.0 | 19.9 | 61.0 |
| Feedstock | Equilibrium Prod. (g/kg) | Measured CGE (wt %) | ||
|---|---|---|---|---|
| H2 | CH4 | CO2 | ||
| Beech sawdust | 2.2 | 355.0 | 957.3 | 22.6–25.8 |
| Municipal waste | 2.2 | 338.9 | 872.0 | 26.4–28.5 |
| Hydrothermal char | 2.6 | 561.6 | 1180.0 | 14.9–16.5 |
| Malt spent grains | 2.4 | 398.5 | 892.0 | 20.9–25.2 |
| Glucose | 2.0 | 262.6 | 811.6 | 21.4–23.3 |
| Glucose + phenol | 2.2 | 311.0 | 883.4 | 12.3–14.3 |
| Surface of the Reactor | C | O | Al | Mg |
|---|---|---|---|---|
| Unreacted surface | - | 54.4 | 45.1 | 0.5 |
| Reacted surface (black) | 89.7 | 10.0 | 0.3 | - |
| Reacted surface (white) | 13.4 | 48.4 | 38.2 | - |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castello, D.; Rolli, B.; Kruse, A.; Fiori, L. Supercritical Water Gasification of Biomass in a Ceramic Reactor: Long-Time Batch Experiments. Energies 2017, 10, 1734. https://doi.org/10.3390/en10111734
Castello D, Rolli B, Kruse A, Fiori L. Supercritical Water Gasification of Biomass in a Ceramic Reactor: Long-Time Batch Experiments. Energies. 2017; 10(11):1734. https://doi.org/10.3390/en10111734
Chicago/Turabian StyleCastello, Daniele, Birgit Rolli, Andrea Kruse, and Luca Fiori. 2017. "Supercritical Water Gasification of Biomass in a Ceramic Reactor: Long-Time Batch Experiments" Energies 10, no. 11: 1734. https://doi.org/10.3390/en10111734






