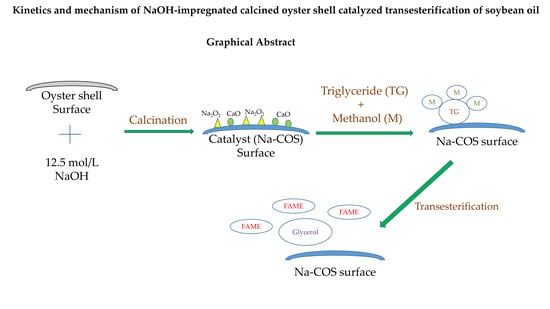
Kinetics and Mechanism of NaOH-Impregnated Calcined Oyster Shell-Catalyzed Transesterification of Soybean Oil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Catalyst Preparation
2.2. Catalyst Characterization
2.3. Batch Experiments
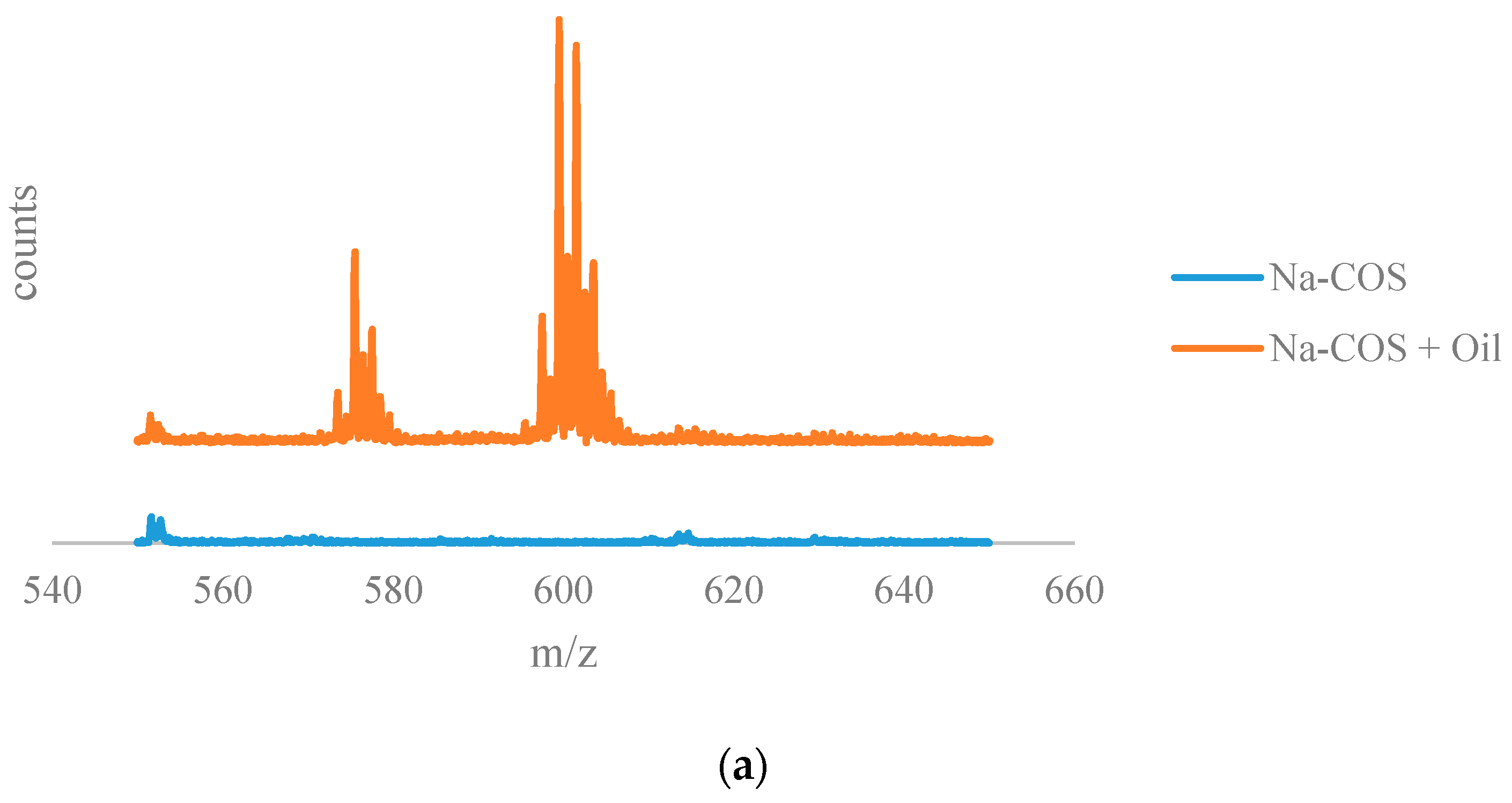
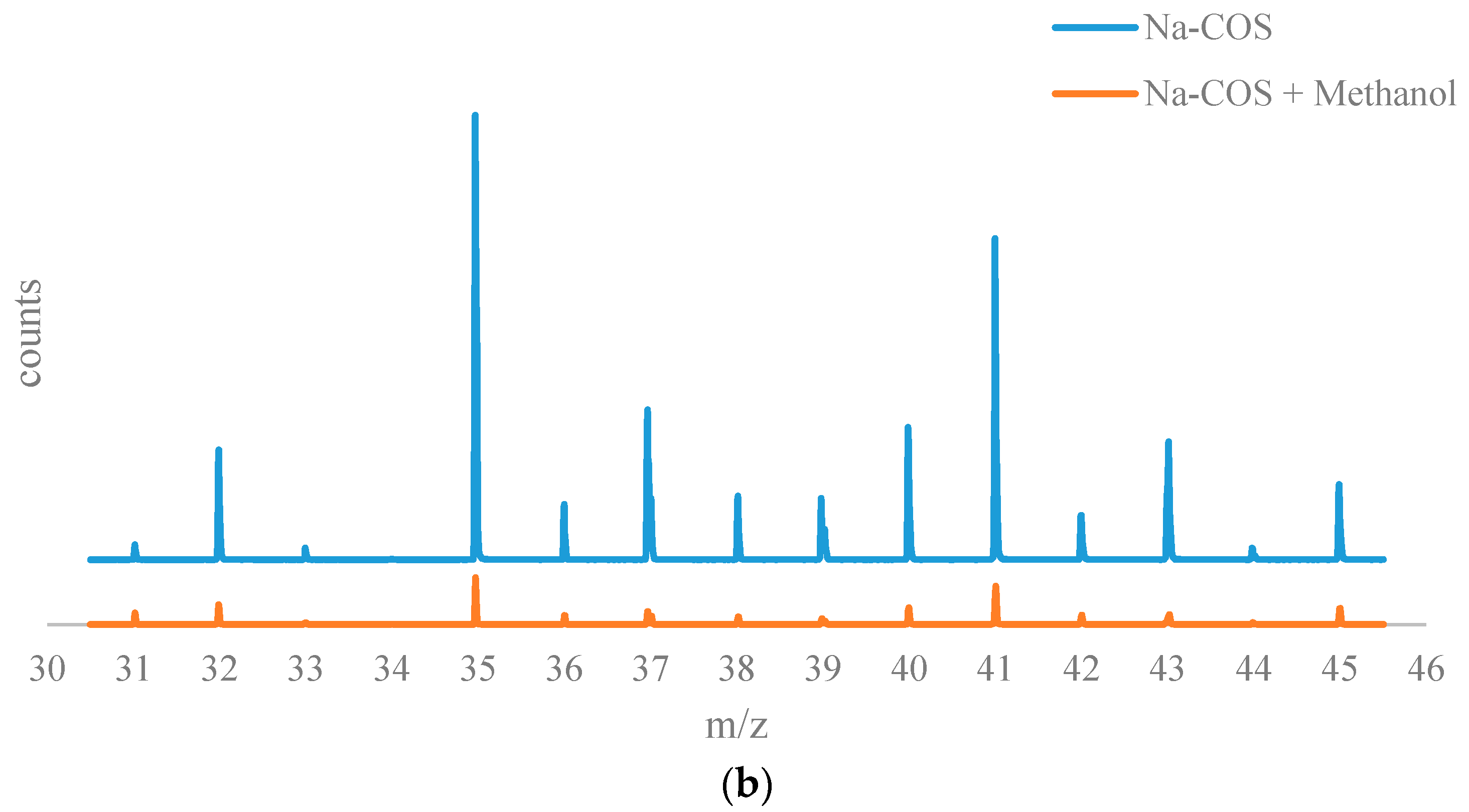
2.4. Gas Chromatograph–Mass Spectrometer (GC-MS) Analysis
2.5. Experimental Design
3. Results and Discussion
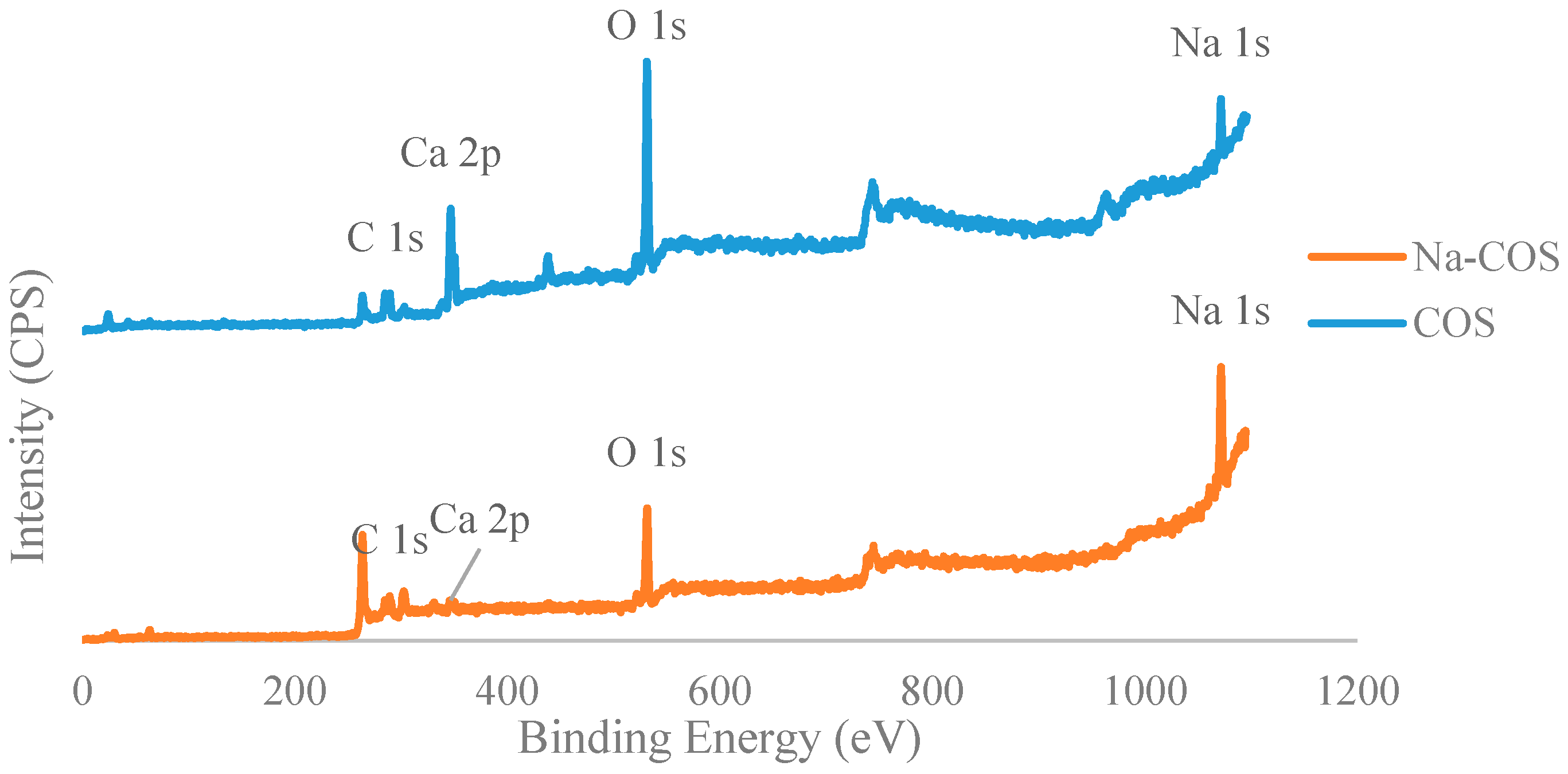
3.1. Catalyst Characterization
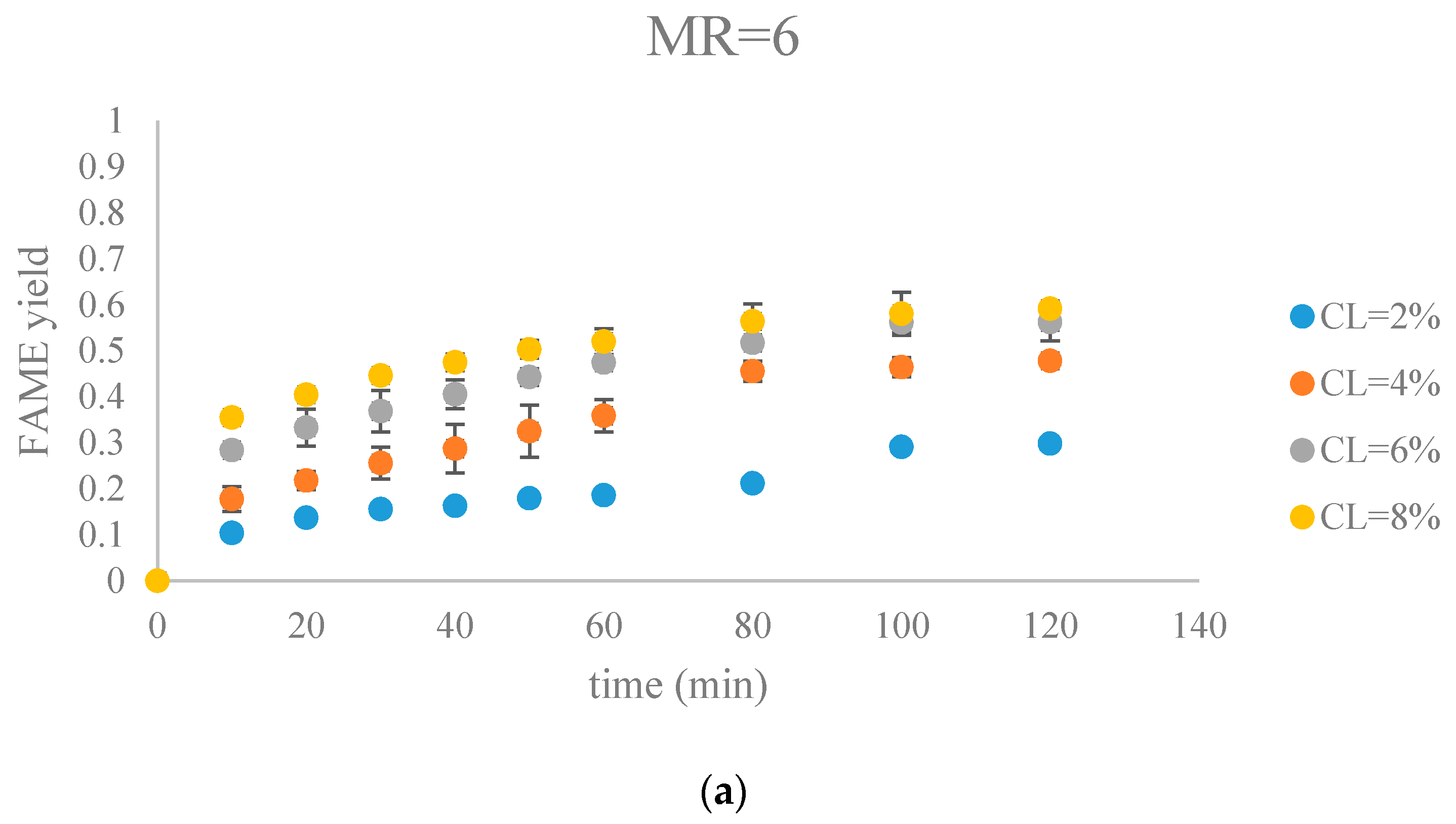
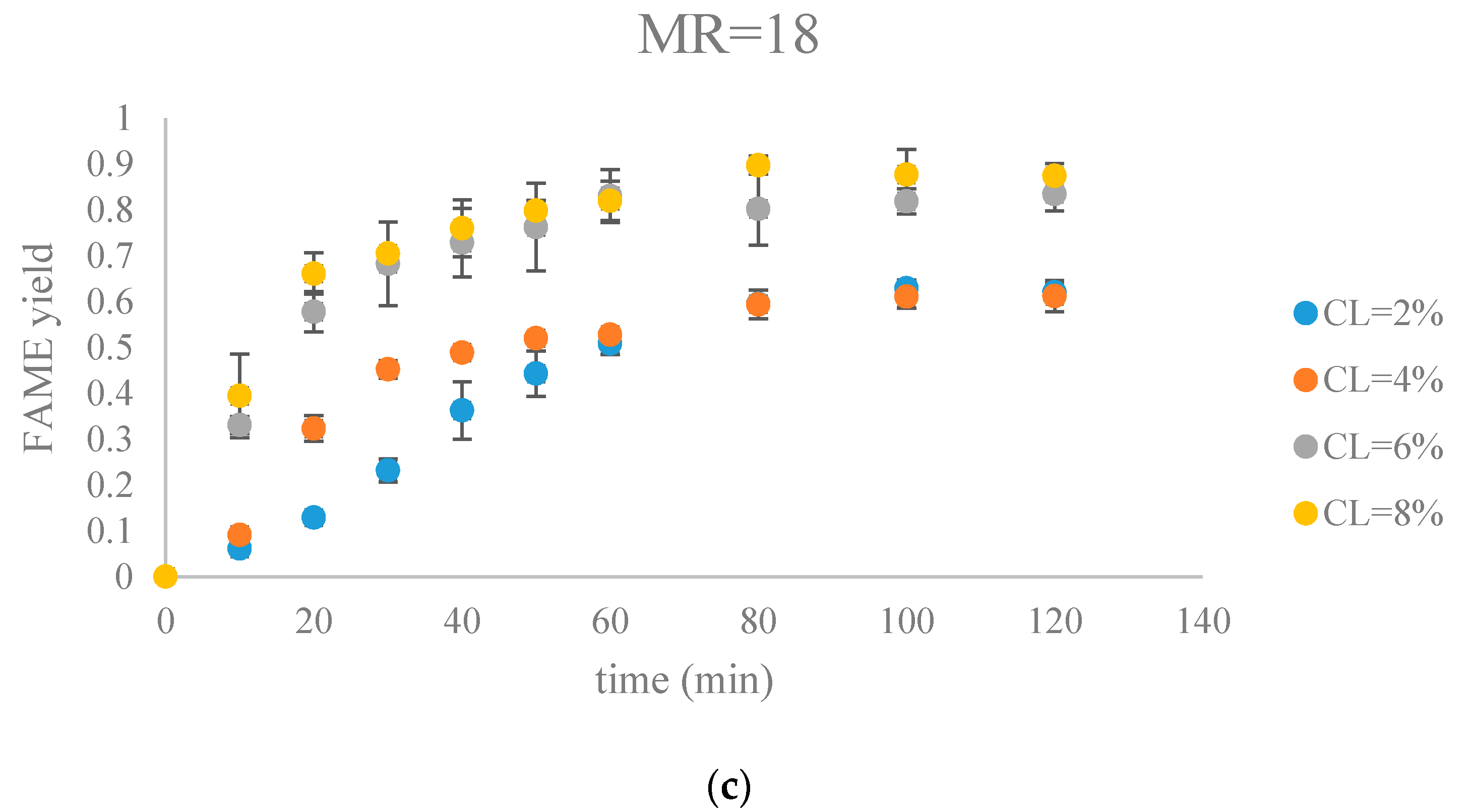
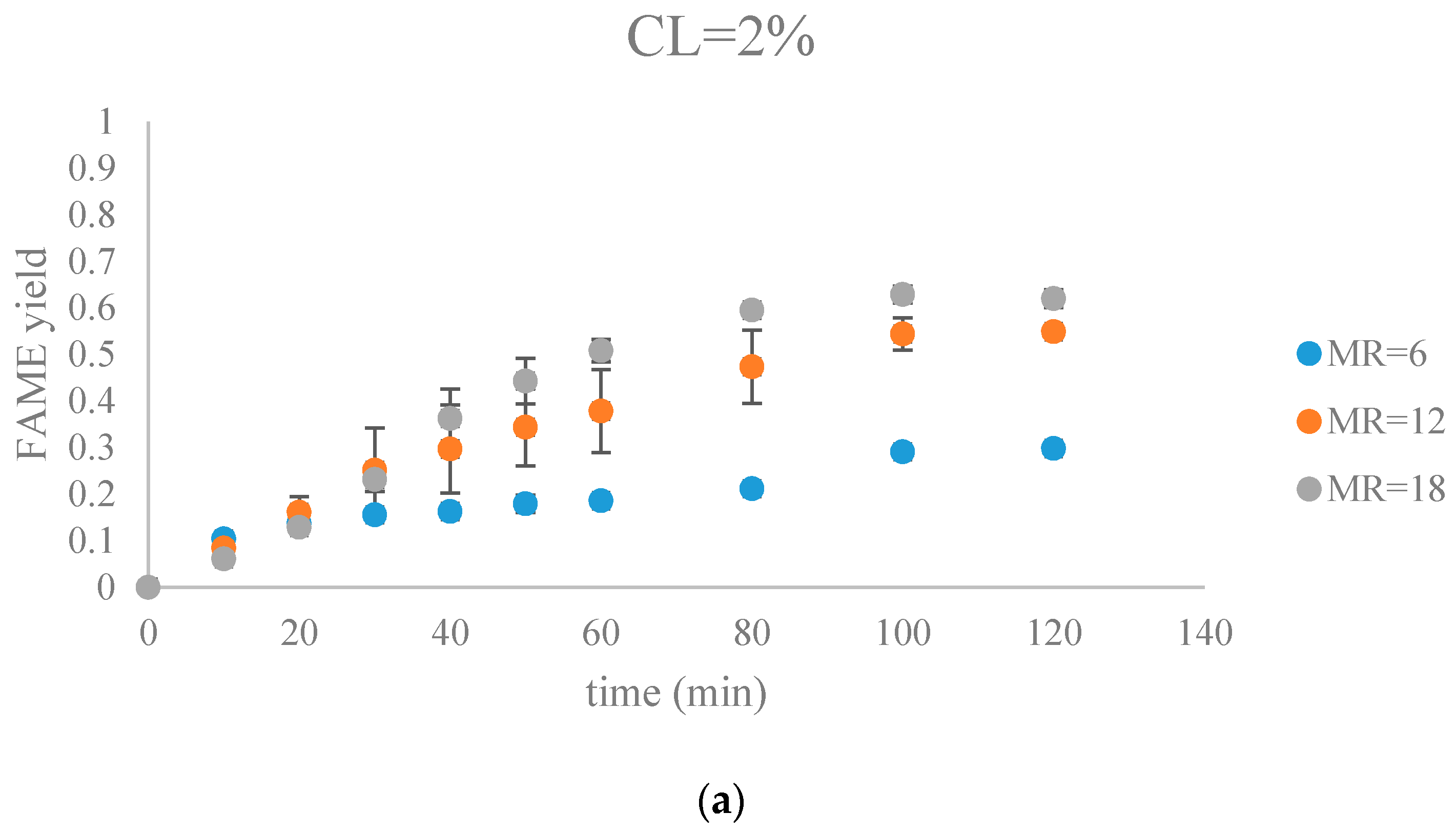
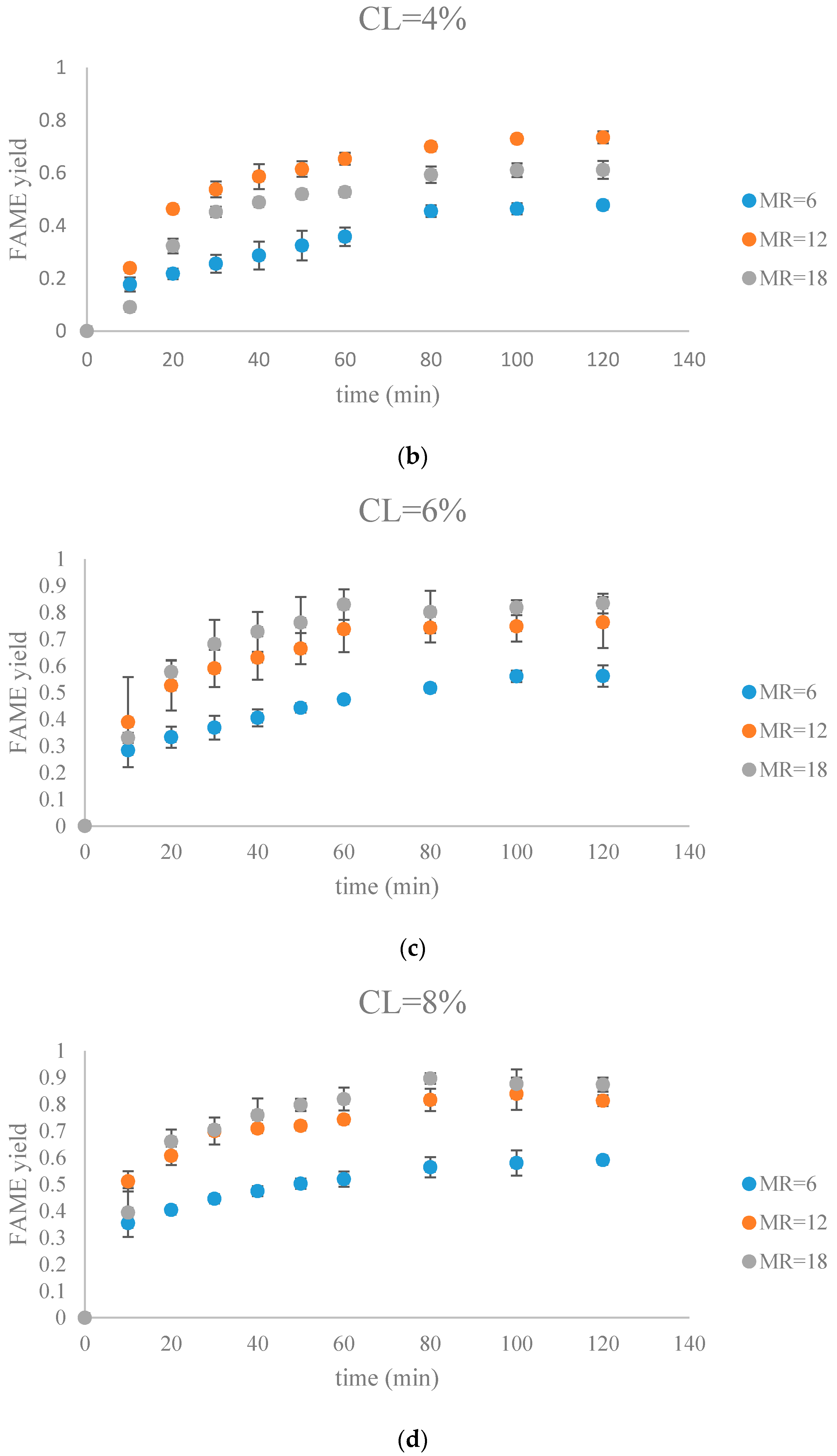
3.2. Batch Reactor Experiments
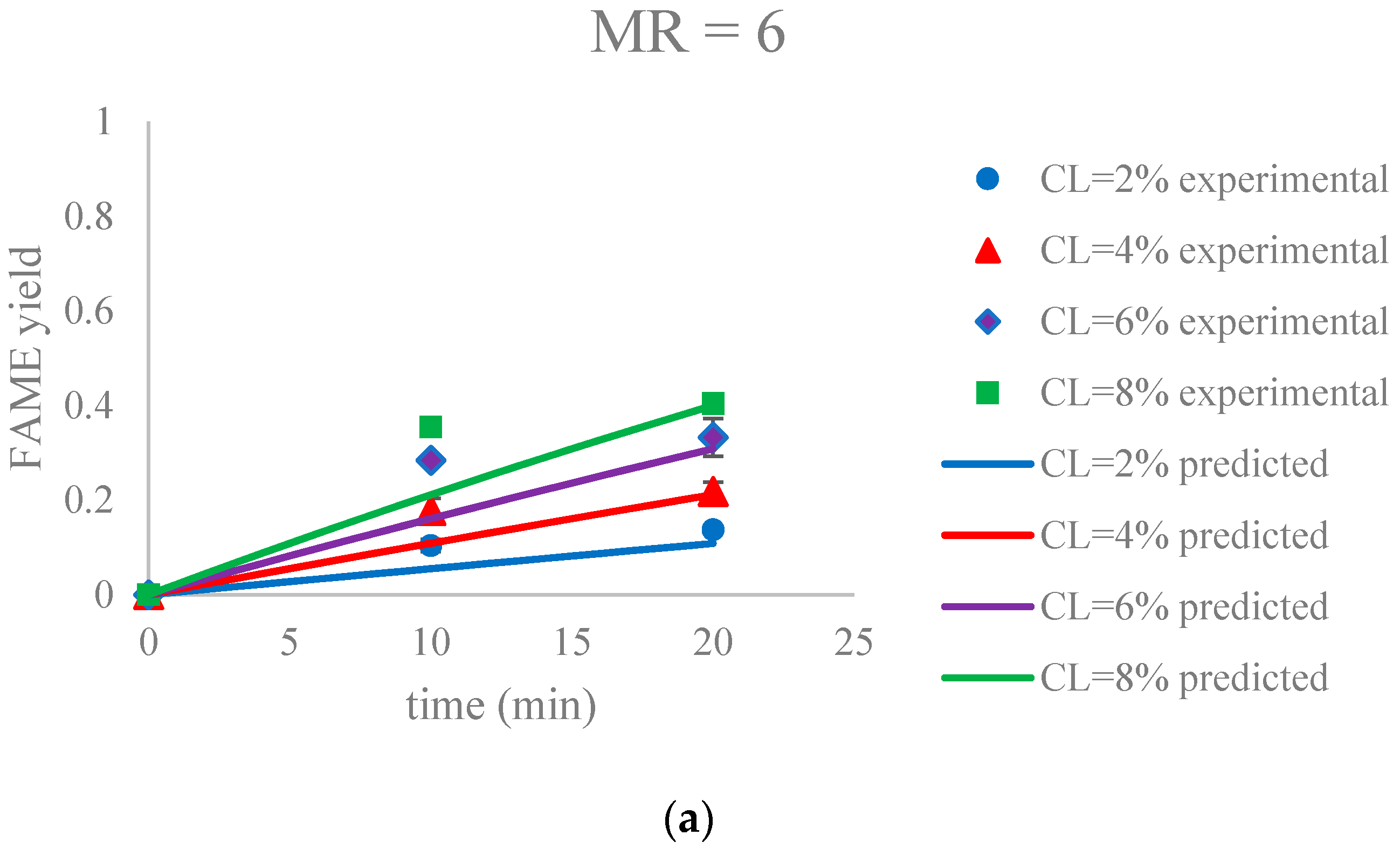
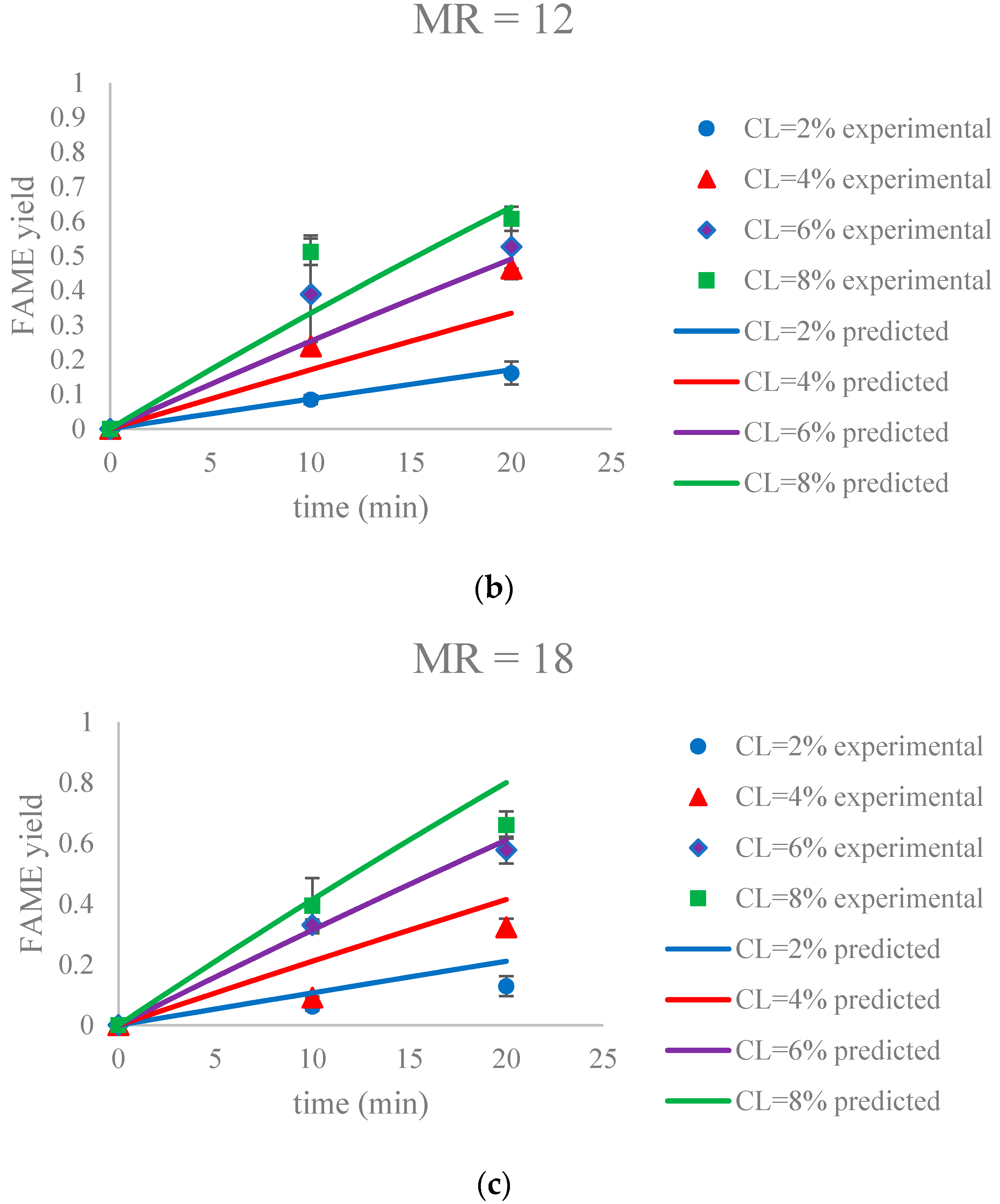
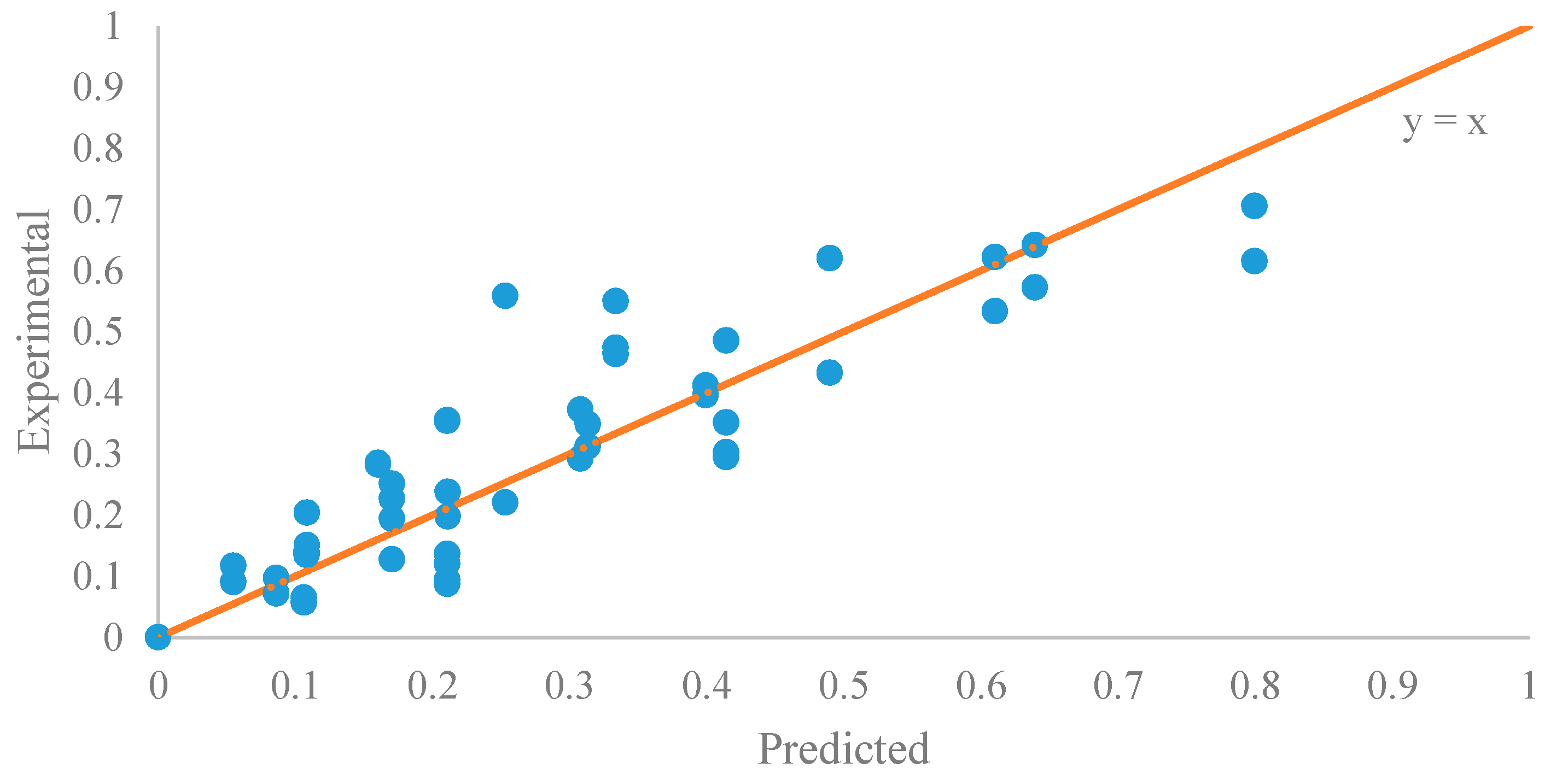
3.3. Kinetics of the Transesterification
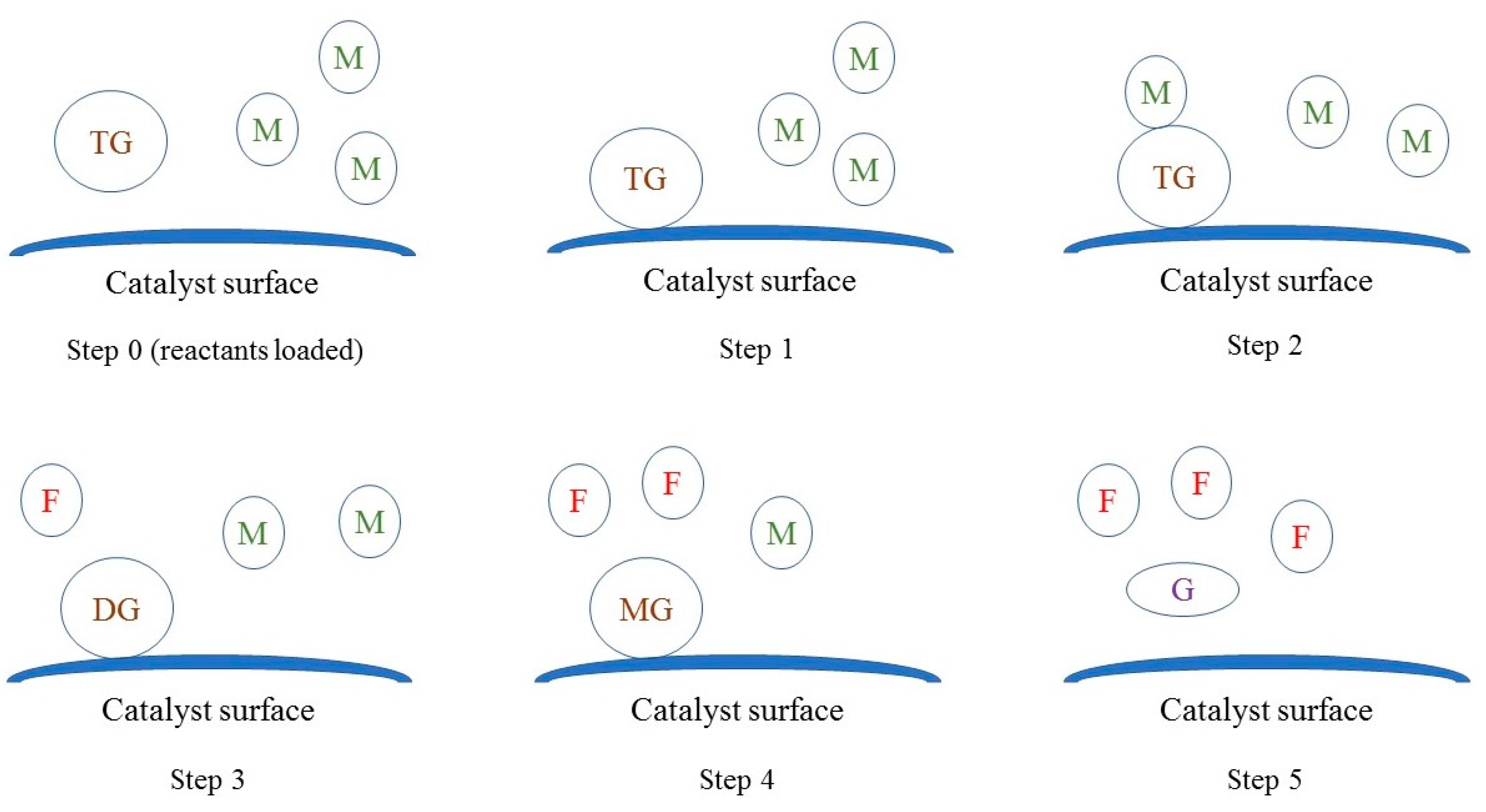
- The transesterification reaction occurred only on the Na-COS surface. The homogeneous transesterification reaction was negligible and thus was not considered.
- The produced FAMEs did not neutralize the basic site of the Na-COS. Therefore, the total amount of the basic site for the Na-COS did not change throughout the reaction.
- Because of continuous stirring of reactants (800 rpm), there was no mass transfer limitation between reactant and the Na-COS surface. Therefore, the production rate of FAMEs depended only on the transesterification reaction rate.
- The catalyst was assumed to be covered by triglyceride (TG*) only.
- TG was assumed to have high adsorption on the catalyst surface.
- The TG adsorption/desorption process (shown below) was fast and at equilibrium.
- The transesterification occurred in a stepwise manner as below:
- The stepwise reaction of transesterification was assumed to be irreversible in the initial period (0–20 min).
- The reaction from TG* to DG* was assumed to be the rate-determining step.
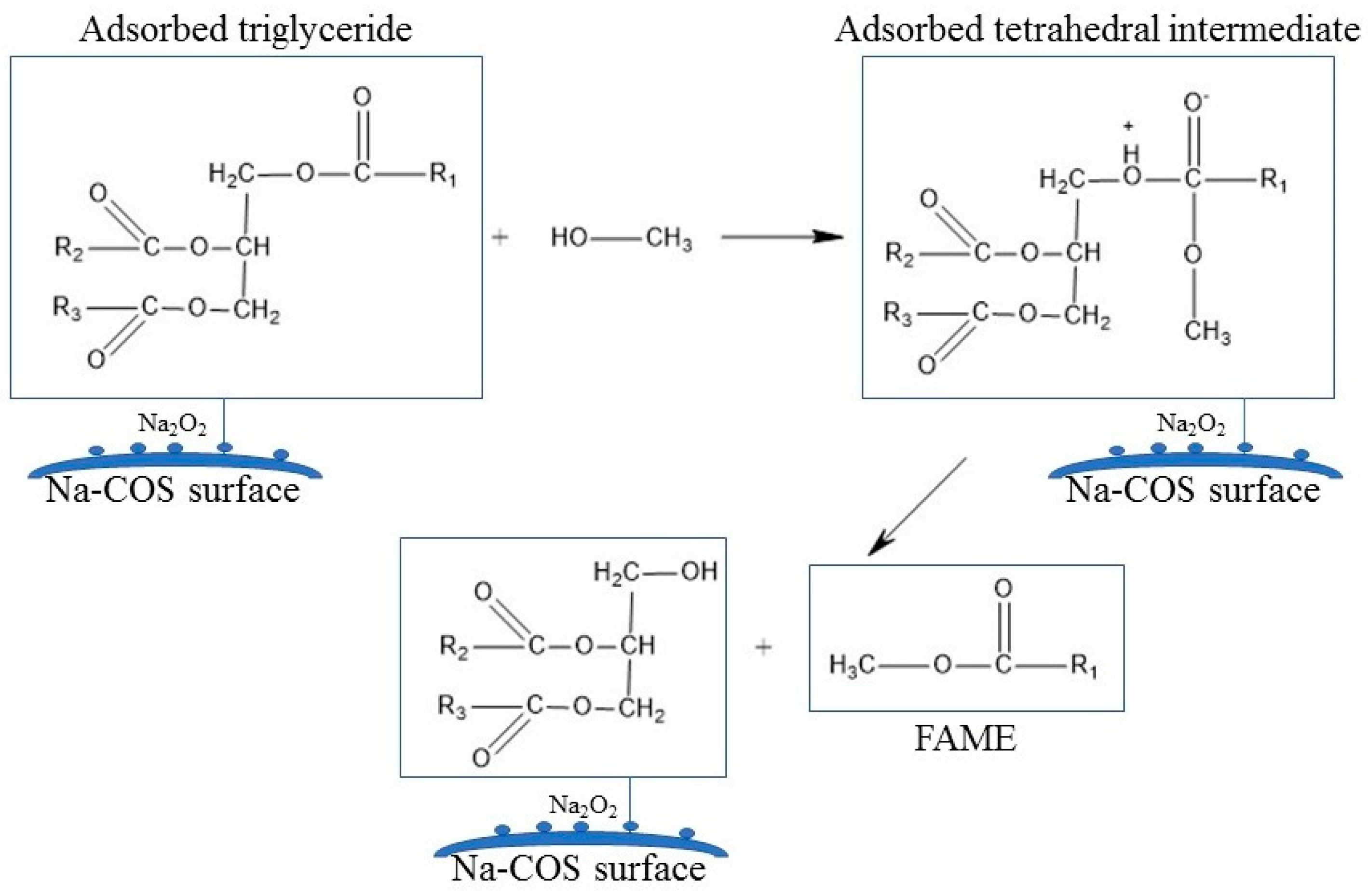
3.4. Proposed Reaction Mechanism
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Nomenclature
| a | Molar ratio of Methanol to oil (MR) |
| ΣA | Summation of peak areas of all methyl esters |
| ΣAIS | The peak area of internal standard |
| CIS | The concentration of internal standard in the final diluted solution |
| CF | Concentration of FAMEs (mol/L) |
| CM | Concentration of methanol (mol/L) |
| Coil | The concentration of soybean oil (g/mL) in the final diluted solution |
| Ctot | Concentration of total basic site of the catalyst (mol/L) |
| CTG | Concentration of triglyceride (mol/L) |
| C°TG | Starting concentration of triglyceride in the reaction (mol/L) |
| CTG* | Concentration of adsorbed triglyceride (mol/L) |
| C* | Concentration of vacant basic site of the catalyst (mol/L) |
| kad | Triglyceride adsorption rate constant (L mol−1 min−1) |
| k-ad | Triglyceride desorption rate constant (min−1) |
| k1 | Reaction rate constant of transesterifying triglyceride to diglyceride (L mol−1 min−1) |
| k2 | Reaction rate constant of transesterifying diglyceride to monoglyceride (L mol−1 min−1) |
| k3 | Reaction rate constant of transesterifying monoglyceride to glycerol (L mol−1 min−1) |
| mF | Mass of FAMEs (g) |
| m°TG | Starting mass of triglyceride (g) |
| MF | Averaged molecular weight of FAMEs (g/mol) |
| MTG | Molecular weight of triglyceride (g/mol) |
| r | Fatty acid methyl esters (FAMEs) production rate (mol L−1 min−1) |
| t | Reaction time (min) |
| V0 | Volume of the reaction mixture (L) |
| y | Biodiesel yield |
| z | Dilution ratio |
| Greek Symbols | |
| Density of soybean oil (g/mL) | |
| Abbreviation | |
| CL | Catalyst loadings (mass of catalyst/mass of oil) (%) |
| F | Fatty acid methyl esters |
| G | Glycerol |
| M | Methanol |
| MR | Molar ratio of methanol to oil |
| TG | Triglyceride |
References
- Leung, D.Y.C.; Wu, X.; Leung, M.K.H. A review on biodiesel production using catalyzed transesterification. Appl. Energy 2010, 87, 1083–1095. [Google Scholar] [CrossRef]
- Ma, F.; Hanna, M.A. Biodiesel production: A review. Bioresour. Technol. 1999, 70, 1–15. [Google Scholar] [CrossRef]
- Vicente, G.; Martínez, M.; Aracil, J. Optimisation of integrated biodiesel production. Part I. A study of the biodiesel purity and yield. Bioresour. Technol. 2007, 98, 1724–1733. [Google Scholar] [CrossRef] [PubMed]
- Zabeti, M.; Wan Daud, W.M.A.; Aroua, M.K. Activity of solid catalysts for biodiesel production: A review. Fuel Process. Technol. 2009, 90, 770–777. [Google Scholar] [CrossRef]
- Kawashima, A.; Matsubara, K.; Honda, K. Development of heterogeneous base catalysts for biodiesel production. Bioresour. Technol. 2008, 99, 3439–3443. [Google Scholar] [CrossRef] [PubMed]
- Yacob, A.R.; Khairul, M.; Amat, A.; Samadi, N.S. Calcination Temperature of Nano MgO Effect on Base Transesterification of Palm Oil. Eng. Technol. 2009, 3, 408–412. [Google Scholar]
- Liu, X.; He, H.; Wang, Y.; Zhu, S.; Piao, X. Transesterification of soybean oil to biodiesel using CaO as a solid base catalyst. Fuel 2008, 87, 216–221. [Google Scholar] [CrossRef]
- Liu, X.; He, H.; Wang, Y.; Zhu, S. Transesterification of soybean oil to biodiesel using SrO as a solid base catalyst. Catal. Commun. 2007, 8, 1107–1111. [Google Scholar] [CrossRef]
- Noiroj, K.; Intarapong, P.; Luengnaruemitchai, A.; Jai-In, S. A comparative study of KOH/Al2O3 and KOH/NaY catalysts for biodiesel production via transesterification from palm oil. Renew. Energy 2009, 34, 1145–1150. [Google Scholar] [CrossRef]
- Boz, N.; Degirmenbasi, N.; Kalyon, D.M. Conversion of biomass to fuel: Transesterification of vegetable oil to biodiesel using KF loaded nano-γ-Al2O3 as catalyst. Appl. Catal. B Environ. 2009, 89, 590–596. [Google Scholar] [CrossRef]
- Lukic, I.; Kesic, Ž.; Maksimovic, S.; Zdujic, M.; Liu, H.; Krstic, J.; Skala, D. Kinetics of sunflower and used vegetable oil methanolysis catalyzed by CaO·ZnO. Fuel 2013, 113, 367–378. [Google Scholar] [CrossRef]
- Jairam, S.; Kolar, P.; Sharma-Shivappa Ratna, R.; Osborne, J.A.; Davis, J.P. KI-impregnated oyster shell as a solid catalyst for soybean oil transesterification. Bioresour. Technol. 2012, 104, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Sheng, B.; Xin, Z.; Liu, Q.; Sun, S. Kinetics of transesterification of palm oil and dimethyl carbonate for biodiesel production at the catalysis of heterogeneous base catalyst. Bioresour. Technol. 2010, 101, 8144–8150. [Google Scholar] [CrossRef] [PubMed]
- Ramezani, K.; Rowshanzamir, S.; Eikani, M.H. Castor oil transesterification reaction: A kinetic study and optimization of parameters. Energy 2010, 35, 4142–4148. [Google Scholar] [CrossRef]
- Marjanovic, A.V.; Stamenkovic, O.S.; Todorovic, Z.B.; Lazic, M.L.; Veljkovic, V.B. Kinetics of the base-catalyzed sunflower oil ethanolysis. Fuel 2010, 89, 665–671. [Google Scholar] [CrossRef]
- Issariyakul, T.; Dalai, A.K. Comparative kinetics of transesterification for biodiesel production from palm oil and mustard oil. Can. J. Chem. Eng. 2012, 90, 342–350. [Google Scholar] [CrossRef]
- Xiao, Y.; Gao, L.; Xiao, G.; Lv, J. Kinetics of the transesterification reaction catalyzed by solid base in a fixed-bed reactor. Energy Fuels 2010, 24, 5829–5833. [Google Scholar] [CrossRef]
- Veljkovic, V.B.; Stamenkovic, O.S.; Todorovic, Z.B.; Lazic, M.L.; Skala, D.U. Kinetics of sunflower oil methanolysis catalyzed by calcium oxide. Fuel 2009, 88, 1554–1562. [Google Scholar] [CrossRef]
- Miladinovic, M.R.; Krstic, J.B.; Tasic, M.B.; Stamenkovic, O.S.; Veljkovic, V.B. A kinetic study of quicklime-catalyzed sunflower oil methanolysis. Chem. Eng. Res. Des. 2014, 92, 1740–1752. [Google Scholar] [CrossRef]
- Kouzu, M.; Hidaka, J.S. Transesterification of vegetable oil into biodiesel catalyzed by CaO: A review. Fuel 2012, 93, 1–12. [Google Scholar] [CrossRef]
- Wei, Z.; Xu, C.; Li, B. Application of waste eggshell as low-cost solid catalyst for biodiesel production. Bioresour. Technol. 2009, 100, 2883–2885. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, R.; Mohadesi, M.; Moradi, G.R. Optimization of biodiesel production using waste mussel shell catalyst. Fuel 2013, 109, 534–541. [Google Scholar] [CrossRef]
- Suryaputra, W.; Winata, I.; Indraswati, N.; Ismadji, S. Waste capiz (Amusium cristatum) shell as a new heterogeneous catalyst for biodiesel production. Renew. Energy 2013, 50, 795–799. [Google Scholar] [CrossRef]
- Xie, J.; Zheng, X.; Dong, A.; Xiao, Z.; Zhang, J. Biont shell catalyst for biodiesel production. Green Chem. 2009, 11, 355–364. [Google Scholar] [CrossRef]
- Jin, H.; Kolar, P.; Peretti, S.W.; Osborne, J.A.; Cheng, J.J. NaOH-impregnated oyster shell as a solid base catalyst for transesterification of soybean oil. Int. J. Agric. Biol. Eng. 2017. accepted. [Google Scholar]
- Chung, K.H.; Kim, J.; Lee, K.Y. Biodiesel production by transesterification of duck tallow with methanol on alkali catalysts. Biomass Bioenergy 2009, 33, 155–158. [Google Scholar] [CrossRef]
- Xie, W.; Peng, H.; Chen, L. Transesterification of soybean oil catalyzed by potassium loaded on alumina as a solid-base catalyst. Appl. Catal. A Gen. 2006, 300, 67–74. [Google Scholar] [CrossRef]
- Yoon, G.L.; Kim, B.T.; Kim, B.O.; Han, S.H. Chemical-mechanical characteristics of crushed oyster-shell. Waste Manag. 2003, 23, 825–834. [Google Scholar] [CrossRef]
- Albuquerque, M.C.G.; Jiménez-Urbistondo, I.; Santamaría-González, J.; Mérida-Robles, J.M.; Moreno-Tost, R.; Rodríguez-Castellón, E.; Jiménez-López, A.; Azevedo, D.C.S.; Cavalcante, C.L.; Maireles-Torres, P. CaO supported on mesoporous silicas as basic catalysts for transesterification reactions. Appl. Catal. A Gen. 2008, 334, 35–43. [Google Scholar] [CrossRef]
- Demri, B.; Muster, D. XPS study of some calcium compounds. J. Mater. Process. Technol. 1995, 55, 311–314. [Google Scholar] [CrossRef]
- Sosulnikov, M.I.; Teterin, Y.A. X-Ray Photoelectron Study of Calcium, Strontium, Barium and their Oxides. Dokl. Akad. Nauk SSSR 1991, 317, 418–421. [Google Scholar]
- Hammond, J.S.; Holubka, J.W.; de Vries, J.E.; Dickie, R.A. The application of X-ray photo-electron spectroscopy to a study of interfacial composition in corrosion-induced paint de-adhesion. Corros. Sci. 1981, 21, 239–253. [Google Scholar] [CrossRef]
- Siriwardane, R.V.; Cook, J.M. Interactions of NO and SO2 with iron deposited on silica. J. Colloid Interface Sci. 1985, 104, 250–257. [Google Scholar] [CrossRef]
- Wu, Q.H.; Thißen, A.; Jaegermann, W. XPS and UPS study of Na deposition on thin film V2O5. Appl. Surf. Sci. 2005, 252, 1801–1805. [Google Scholar] [CrossRef]
- Babu, N.S.; Sree, R.; Prasad, P.S.S.; Lingaiah, N. Room-temperature transesterification of edible and nonedible oils using a heterogeneous strong basic Mg/La catalyst. Energy Fuels 2008, 22, 1965–1971. [Google Scholar] [CrossRef]
- Zabeti, M.; Daud, W.M.A.W.; Aroua, M.K. Biodiesel production using alumina-supported calcium oxide: An optimization study. Fuel Process. Technol. 2010, 91, 243–248. [Google Scholar] [CrossRef]
- Vyas, A.P.; Subrahmanyam, N.; Patel, P.A. Production of biodiesel through transesterification of Jatropha oil using KNO3/Al2O3 solid catalyst. Fuel 2009, 88, 625–628. [Google Scholar] [CrossRef]
- Aransiola, E.F.; Daramola, M.O.; Ojumu, T.V.; Solomon, B.O.; Layokun, S.K. Homogeneously Catalyzed Transesterification of Nigerian Jatropha curcas Oil into Biodiesel: A Kinetic Study. Mod. Res. Catal. 2013, 2, 83–89. [Google Scholar] [CrossRef]





| Catalyst | MR | CL | Reaction Temperature (°C) | Reaction Time (h) | Yield | Reference |
|---|---|---|---|---|---|---|
| CaO | 12 | 8% | 65 | 1.5 h | ≥95% | [7] |
| CaO/Al2O3 | 12.14 | 5.97% | 64.29 | 5 h | 98.64% | [36] |
| KNO3/Al2O3 | 12 | 6% | 70 | 6 h | 84% | [37] |
| KOH/Al2O3 | 15 | 3% | 60 | 2 h | 91.07% | [9] |
| Eggshell | 9 | 3% | 65 | 3 h | 95% | [21] |
| Mussel shell | 24 | 12% | 60 | 8 h | 94.1% | [22] |
| Capiz shell | 8 | 3% | 60 | 6 h | 93% | [23] |
| Turtle shell | 9 | 3% | 70 | 3 h | 97.5% | [24] |
| Na-COS | 18 | 8% | 62 | 1.33 h | 89.7% | This research |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, H.; Kolar, P.; Peretti, S.W.; Osborne, J.A.; Cheng, J.J. Kinetics and Mechanism of NaOH-Impregnated Calcined Oyster Shell-Catalyzed Transesterification of Soybean Oil. Energies 2017, 10, 1920. https://doi.org/10.3390/en10111920
Jin H, Kolar P, Peretti SW, Osborne JA, Cheng JJ. Kinetics and Mechanism of NaOH-Impregnated Calcined Oyster Shell-Catalyzed Transesterification of Soybean Oil. Energies. 2017; 10(11):1920. https://doi.org/10.3390/en10111920
Chicago/Turabian StyleJin, Han, Praveen Kolar, Steven W. Peretti, Jason A. Osborne, and Jay J. Cheng. 2017. "Kinetics and Mechanism of NaOH-Impregnated Calcined Oyster Shell-Catalyzed Transesterification of Soybean Oil" Energies 10, no. 11: 1920. https://doi.org/10.3390/en10111920