1. Introduction
Mediterranean countries are responsible for a large part of the world olive oil production. The environmentally friend disposal of the olive pomace requires the implementation of waste-to-energy strategies through pomace gasification. A proper solution is to process the crude olive pomace (COP) aiming at obtaining dry olive pomace (DOP) as a fuel. In [
1,
2], an overview of the most common olive oil extraction processes is provided. A three-phases centrifugation system is generally adopted in Italy. The main products are: (i) olive oil—20% in weight of olives; (ii) COP—50% in weight of olives; (iii) a large amount of olive mill wastewater (OMW)—120% in weight of olives, which includes the water added during the process [
3]. The extraction of pomace oil from COP allows obtaining the DOP as a by-product (about 10% wb), generally used for energy conversion in CHP or thermal plants. The reduced demand for pomace oil (due to excess olive oil availability from emerging markets) and consequently the increased production of COP with high moisture content (>60%) has increased the problem of olive waste disposal [
4]. The option of discharging COP to the soil as fertilizer has the drawback of the high phenols, fatty acid and tannin content [
2,
5]. However, COP can be directly used for energy conversion, after drying [
6]. The main technical issues are in this case the energy consumption for the drying process, the air emissions from combustion (unburned carbon, particulate matter), the logistics of supply and storage [
7]. In Italy, the existing energy policies support the development of biomass small scale CHP plants, as described in [
8].
Thermo-economic approaches can be usefully employed to compare operation strategies and profitability of small scale CHP plants fed by syngas [
9]. In [
10] an analysis of a more sophisticated configuration (micro GT and bottoming ORC) is proposed to investigate the best operating conditions.
Among biomass energy conversion systems, gasification is one of the most promising. A wide number of gasification technologies are available, and for small-medium applications downdraft and updraft systems are the most common. When considering syngas as a fuel, internal combustion engines and micro GT are generally employed for power and heat generation.
The ChemCad software (Chemstations, Inc, Houston, TX, USA) has been demonstrated to be an appropriate tool for computing solid fuel gasification. In [
11] a ChemCad model of a biomass gasification plant coupled to a micro GT is validated through experimental data. In light of this, in this paper we adopted ChemCad to model the COP to syngas gasification process.
We assume an updraft gasifiers and its use to feed a micro GT because of its lower emissions and reduced O&M costs. In the next section, a review of gasification technologies and modelling approaches is proposed, and successively a description of the CHP plant under investigation is presented. The energy balance of the system as resulting from the simulation, informed by specific experimental tests, and the economic profitability of the investment in the Italian legislative scenario are then presented, and results are discussed in the last section.
2. Gasification Technologies and Modelling Approaches
The gasification process is the conversion of a solid carbonaceous material, such as biomass, in a gaseous energy carrier (syngas) through a partial high temperature oxidation [
12,
13,
14,
15]. The syngas is composed by CO, CO
2, H
2, CH
4, H
2O, N
2, other hydrocarbons such as C
2H
4 and C
2H
6 and further substances such as ash, coal particles, tar and oils. The gasifying agent can be air, steam, oxygen or a mixture of these.
The syngas can be used as a gaseous fuel, thus easy to convey and transport, or used in other industrial processes. The gasification process takes place within a reactor and can be divided into two main stages. In the first stage of pyrolysis, as a consequence of the thermochemical decomposition of biomass at temperatures above 350 °C, the volatile components of the fuel are released [
16]. These volatile vapors contain gaseous hydrocarbons, hydrogen, carbon monoxide, carbon dioxide, water vapor and tar. The solid fraction from the pyrolysis process (bio-char) is an agglomerate of complex nature consisting of carbon, ash, sulfur compounds and volatile hydrocarbons. In the second stage, the gasification of pyrolysis products is achieved, and the reaction with the gasifying agent leads to an increase of fuels concentration, and to the conversion of bio-char. This last stage is the most important; it is the slower phase, hence it affects the kinetics of the whole process and, consequently, both the sizing and the performance of the reactor.
A first classification of gasifiers proposes direct and indirect systems [
17,
18]. In the direct type, the burning of a part of the pyrolytic products provides heat to both pyrolysis and gasification, and the process is developed inside the reactor; in the indirect type, the combustion takes place in a separate combustion chamber and the heat is carried to the pyrolysis zone by means of a flow glowing sand or other material (heat transport is the critical point of this technology, slowing down the process [
19]). Further classification criteria are based on the operating pressure, the gasifying agent, the type of the reactor construction [
20,
21]. Indirect gasifiers normally operate at atmospheric pressure, whereas in the direct type considerably higher pressure can be reached, with the advantage of a produced syngas that does not need to be compressed. The choice of the gasifying agent is very important, because it highly affects the characteristics of the syngas. Both the composition and calorific value vary greatly with the gasifying agent: air gasification presents a low heating value (LHV) of the syngas in the order of 4–6 MJ/N·m
3, and large volumes of N
2, while pure oxygen gasification allows LHV ranging between 12 and 18 MJ/N·m
3 [
22]. Moreover, the gasifiers can be classified in fixed bed [
23,
24], fluidized bed [
25,
26] or bed dragged gasifiers. The fixed bed gasifiers are distinguished in co-current gasifiers (downdraft) and counter current ones (updraft) [
27,
28]. The configuration choice is dependent on the biomass characteristics, typology and quality required for the syngas, processing plant size and cost issues. In particular, the downdraft gasifier is usually used for the production of syngas with a high content of volatile matter and low tar content. This technology is the most popular in the small scale range [
29,
30].
The state-of-the-art of the fluidization technology for the gasification of biomass is reviewed in [
31], where the different gasifier types, fluidized bed technologies, gas cleaning systems and influence of process parameters on the syngas and biochar composition are discussed. In [
32], a review of thermochemical biomass gasification for producing biofuels and chemicals is presented, with a specific focus on the effects of operating conditions on gasification reactions, on the reliable prediction of the product compositions, and on conversion efficiency optimization. The promising conversion route of bio-hydrogen production via biomass air gasification is investigated in [
33,
34] focusing on agricultural wastes and olive kernels via bubbling fluidized bed gasifier. Mass and energy balances on a pre-pilot scale bubbling fluidized bed reactor fed with mixtures of plastic waste, wood, and coal were also discussed in [
35] in order to explore the influence of input fuel on gas composition and gas cleaning.
In the specific olive waste-to-energy sector, several experimental researches have been proposed. In particular, in [
36] the atmospheric gasification of untreated olive stones at 700–820 °C is proposed. The gasification process at higher temperatures reported higher carbon conversion and gas yield, despite decreasing the LHV of the gas when increasing the air flow rates. In addition, the high potassium content and low ash fusion temperature of the olive stone ashes were found to be responsible for the bed agglomeration problems, so requiring the use of two inert materials (sand and ofite), which however affected the fluidized bed operation and reliability.
The biochar yield, biomass burning rate, syngas composition and tar content of a updraft gasifier feed by pine wood chips at different particle size, moisture content and compactness was studied in [
37], reporting, among the others, an increase of biochar yield when increasing the particle size.
In [
38], the behavior of different biomasses (wood, torrefied biomass, agricultural and industrial wastes) after gasification in a bubbling fluidized bed gasifier at mild temperatures (600 °C) was investigated, using an air–steam mixture at different stoichiometric ratios. The low-temperature gasification of biomass in a purpose-built atmospheric bubbling fluidized bed reactor was optimized on the basis of the experimental results; it was found that the same gasification conditions (steam quantity) do not affect every biomass in the same way, and gasification conditions must be carefully tested for each biomass. The research also investigated the possibility of reusing the carbon-rich partially oxidized biochar in agricultural applications or as a catalyst-sorbent precursor. The properties of biochar depend heavily on biomass feedstock, gasifier design and operating conditions, and in light of this, ref. [
38] investigated the influence of biomass type and equivalence air ratio on the physiochemical properties of biochar from gasification. The production of biochar from hazelnut and olive pruning residues via carbonization was also investigated in [
39], performing a physicochemical characterisation of the produced biochar in light of the European standards.
A simulation of the gasification process requires the assessment of the different phases of pyrolysis, partial oxidation and gasification. The modelling of the chemical reactions is very complex, and the kinetics are far from the equilibrium conditions. For these reasons, simplified models are usually assumed, such as mono-dimensional ones [
40,
41,
42,
43]. To predict the real behaviour of the plant, numerical simulation codes can be successfully adopted. In [
16] a mathematical CFD model of biomass pyrolysis is presented. The model predicts the tar and light particulate matter in the syngas as well as char fraction released in a biomass packed porous bed system. In [
44], the gasification of lignocellulosic biomass coupled with a SOFC/ORC plant is modelled and validated. The influence of air enrichment and of biomass moisture content is also addressed.
A good and reliable theoretical model of a gasifier is very important, to provide useful information for the design, optimal operation and energy performance assessment Many theoretical models can be found in the literature simulating the performance of gasifiers [
44,
45,
46,
47,
48,
49], under different operating conditions (e.g., in terms of moisture content, type of biomass, sizes of the molecules, and ash contents). In particular, some approaches use kinetic models to represent the biomass gasification process [
44,
45,
46], which are suitable and accurate at moderately high temperatures (T < 800 °C), but very complex, while others apply equilibrium models and are particularly accurate at high temperature [
47,
48,
49]. Equilibrium models are based either on the use of the equilibrium constants or on the minimization of the Gibbs free energy. When equilibrium constants are considered, the equilibrium models are based on the use of fundamental chemical reaction mechanisms and of the chemical composition of the biomass. In this case, their complexity depends primarily on the number of reactions that are considered. In many cases these methods, using a limited number of reactions, greatly simplify the analysis, while compromising the reliability and accuracy of the results. On the opposite, when the minimization of the Gibbs free energy is considered, the exact knowledge of the chemical reactions mechanism is not required for the syngas components prediction.
Several review papers are available in literature in the field of modeling biomass gasification. In [
50], the state of art modeling works based on specific criteria such as type of gasifier and feedstock have been categorized in a comprehensive review, reporting comparative assessments of the modeling techniques and output for each category. In [
51], a similar review is proposed but including artificial neural networks and Aspen Plus gasification models, while [
52] focuses on mathematical and computational approaches for design of biomass gasification for hydrogen production. In [
53], biomass gasification in bubbling and circulating fluidized bed gasifiers is reviewed, including both black-box models and computational fluid-dynamic ones or comprehensive fluidization models, with semi-empirical correlations.
Several free commercial codes [
54] implement the Gibbs free energy minimization and propose a relatively simple and easy option to model the gasification, with accuracy that could be acceptable on the basis of the specific application. Most of the thermodynamic modeling and optimization tools are based on a modular structure, which considers the plant as a set of interconnected components; each of them can be modelled through specific equations and mass/energy flows. The research has recently moved from the simple model of gasification, based on the Gibbs free energy minimization, to more complex models, which attempt to simulate most of the physical phenomena taking place in a gasifier. For instance, in [
55] different models with increasingly high level of complexity have been proposed, and the results show that a simplistic use of the default gasifier module determines an under-estimation of the methane molar percentage into the syngas. The researches proposed in [
55,
56,
57,
58] suggests the following improvements to the downdraft biomass gasification model: (i) modeling three cascade reactors, simulating respectively the pyrolysis, oxidation and reduction zones; (ii) separation of a fraction (5% by mass) of the inlet carbon content, in order to take into account the unavoidable losses occurring in the gasifier due to char formation; (iii) bypass of a fraction of the methane formed during the pyrolysis directly to the reduction outlet, in order to reflect the unavoidable losses and the impossibility to achieve a complete equilibrium composition during the gasification. In light of this state of art on gasification modelling, we here propose an original approach based on interconnection of thermochemical components and informed by experimental data at lab scale, as described in the following section.
5. Profitability Analysis
This section proposes a profitability assessment of the small scale biomass CHP system under different thermal energy demand intensities, and assuming the Italian subsidy mechanism for biomass CHP plants. The key techno-economic factors influencing the feasibility of such biomass to energy systems are explored for the case studies of only electricity sale (case EL) and the combined sales of heat and electricity for residential heat demand (case RES, corresponding to 1500 equivalent hours/year of thermal energy demand at 60 °C) and industrial heat demand (case IND, corresponding to 4000 equivalent hours/year of thermal energy demand at 90 °C). In all the cases, a baseload operation mode is considered (6600 equivalent operating hours/year), which is assumed on the basis of data from manufacturers and small size gasification plants in operation [
8,
9,
66]. Different operational strategies have been investigated in previous researches [
10,
67], such as electric of heat driven modes. However, previous results show that, in absence of a specifically designed energy market structure that rewards the flexible operation of the on-site CHP system (load following operating mode, or operation only during high electricity price periods, or when both heat and power can be delivered to the end users), the high value of the bio-electricity feed in tariff (in comparison to the biomass purchase cost) and the high investment cost (CAPEX) of the investment make significantly more profitable a baseload operation mode, which maximizes the revenues from electricity sales, rather than other operational modes that maximize the global conversion efficiency but not the profitability.
On the basis of the assumed baseload operational mode, and in light of the energy conversion efficiency simulation results of the previous section, the wet olive pomace and dry olive cake consumption are respectively 2344 t/year and 1172 t/year and the thermal energy demand for biomass drying is 1199 MWh/year.
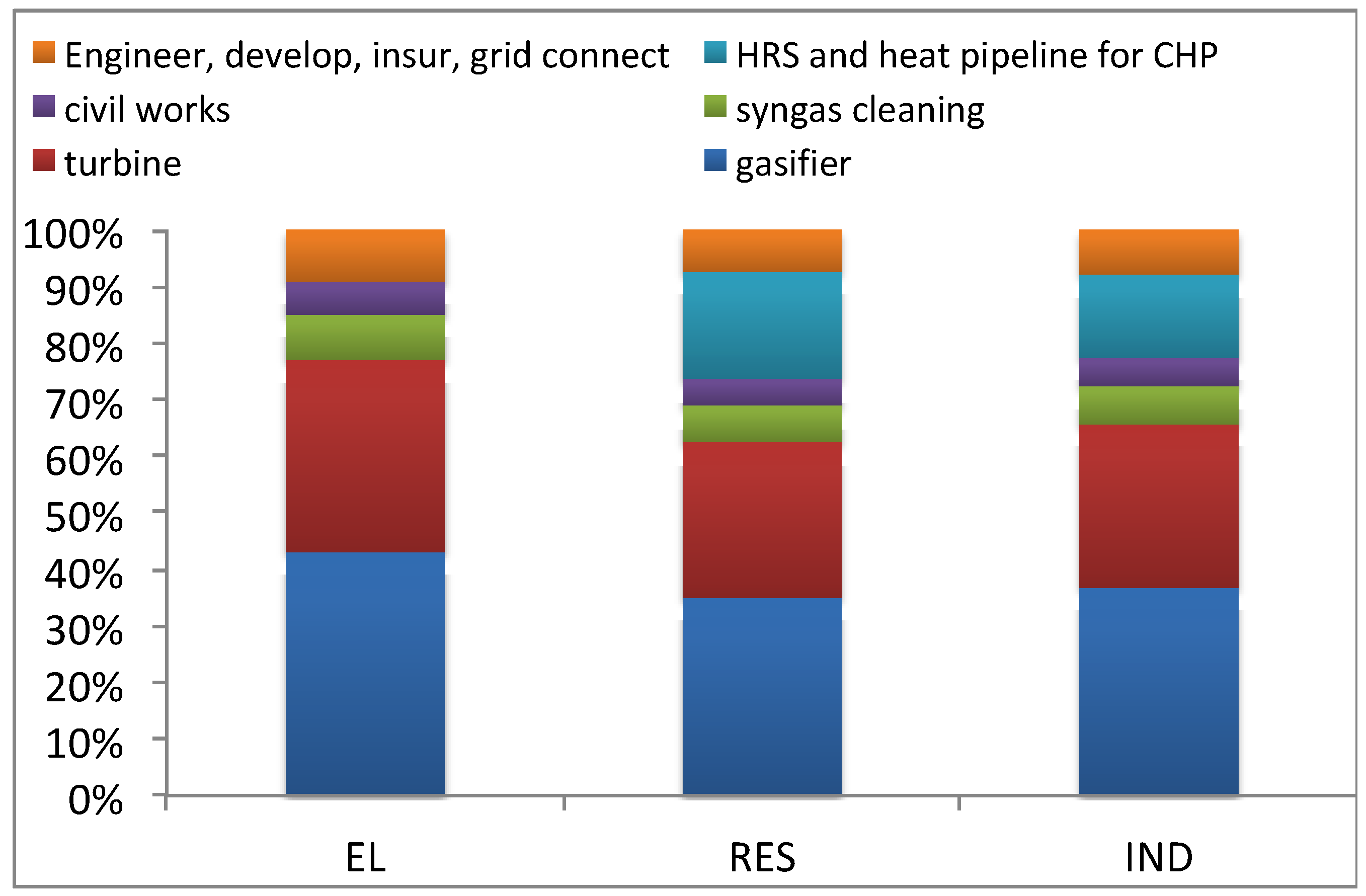
Figure 3 summarizes the CAPEX breakdown for the proposed case studies. The cost figures are author’s elaboration of data from manufacturers (for gasifiers [
68,
69,
70,
71,
72,
73], for MGT [
74,
75,
76]). These data consider average values for installation, engineering, grid connection costs for small scale plants in Italy [
66,
67]. The operational costs are calculated assuming a biomass purchase price of 30 €/t for wet olive pomace included transport (55% moisture content and LHV of 1.97 kWh/kg), according to [
1,
75] and on the basis of interviews to olive oil operators in the Puglia region in Southern Italy (the Italian region with the highest production of olive oil), while unitary ash discharge costs are assumed of 70 €/t ash (ash percentage in dry olive cake of 8% [
1]), and other annual O&M costs (labour, spare parts, insurances, global maintenance service) are equal to 4% of CAPEX, in line with literature data [
67,
77,
78].
The Levelized Cost of Energy, which is calculated assuming electric autoconsumption of CHP plant of 12%, results respectively of 208, 223 and 227 €/MWh for the cases EL, IND and RES. The energy revenues are calculated in light of the Italian support mechanism for RES [
79] and in particular biomass electricity feed-in tariff equal to 246 €/MWh. Moreover, thermal energy selling price is assumed on the basis of avoided natural gas cost with purchase price respectively of 80 and 35 €/Nm
3 for residential and industrial demand (estimates from Italian Energy Authority data for 2017). The financial appraisal of the investment is carried out assuming 20 years of operating life and the same duration of the electricity feed in tariff, no ‘re-powering’ throughout the lifetime nor decommissioning costs, discount rate of 5%, maintenance costs, fuel supply costs, electricity and heat selling prices held constant (in real 2017 values). Moreover, corporation tax is here neglected, while capital investments and income do not benefit from any further support except the feed-in tariff.
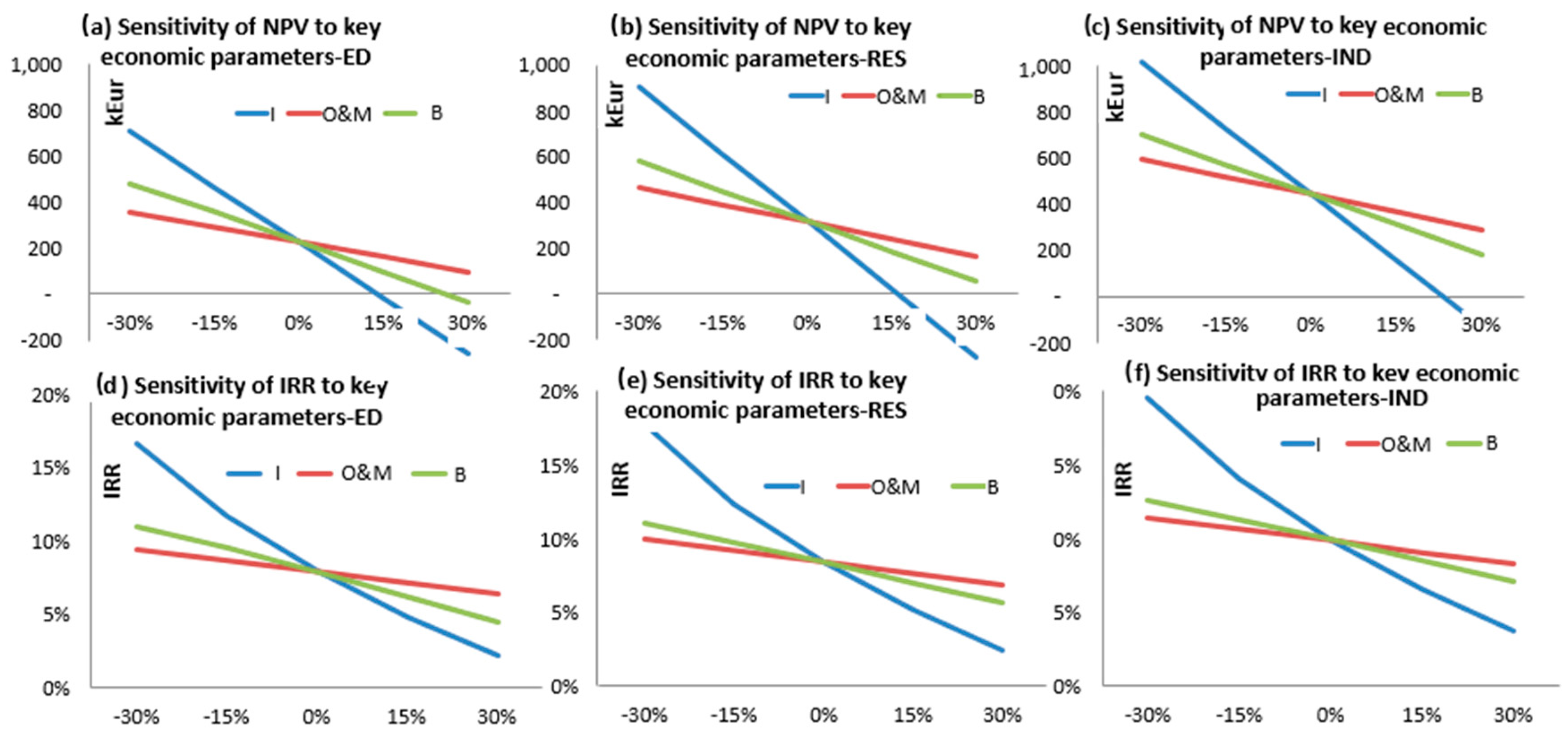
Figure 4 reports the results of the profitability analysis and the sensitivity to the three most influencing factors (the capex I, the biomass supply cost B and the operation and maintenance cost factor O&M, expressed as % of I. In the baseline scenario, Net Present Value (NPV) is positive and Internal rate of return (IRR) above 8% for all the considered energy demand and system operation typologies (ED, RES and IND).
As expected, the best results are obtained in Case IND where the highest revenues from thermal energy sales (because of the high thermal energy demand intensity) compensate the lower electric conversion efficiency and the higher investment cost in comparison to the other scenarios.
6. Conclusions
The coupling of an 800 kW updraft pomace gasifier to a MGT for CHP was modeled using ChemCad and the proposed model was validated with experimental data. The thermodynamic analysis demonstrated that, starting from a gasifier with thermal input size of 800 kW, an electric output power of 200 kW can be obtained, with a gross electric conversion efficiency of 25%, while the cogeneration section is able to provide 250 kW of thermal energy for low grade heat demand (90 °C) with 30% thermal efficiency. These results are aligned with literature data.
In order to assess the global conversion efficiency and the investment profitability, a thermo-economic analysis was also proposed, with different categories of energy end users for cogenerated heat, in order to evaluate the investment profitability and the sensitivity to the main cost items. The case studies of only electricity generation (ED), cogeneration for residential energy demand (RES) and cogeneration for industrial energy demand (IND) were analyzed, to assess the influence of thermal energy demand and cogeneration option on the investment profitability. When considering the feed-in tariff currently implemented in Italy, all the cases resulted profitable, with IRR around 8% for the baseline scenarios, and best economic indices were obtained when the CHP plant serves an industrial energy demand. However, a 15% increase of turnkey cost reduces significantly the investment profitability, with negative NPV, when only electricity is sold (case ED). In the case of cogeneration (both for industrial and residential heat demand), the investment is still profitable with an increase of investment cost up to 15% or increase of O&M or biomass supply cost up to 30%, proving that, in the scenario of industrial cogeneration with high thermal energy demand intensity, the quite low conversion efficiency of the biomass gasification plant is compensated by the high revenues from electricity sales with the feed in tariff and cogenerated heat sales to the end user.