Experimental and One-Dimensional Mathematical Modeling of Different Operating Parameters in Direct Formic Acid Fuel Cells
Abstract
:1. Introduction
2. Model Development
2.1. Conventional Model
2.2. Anode Overpotential
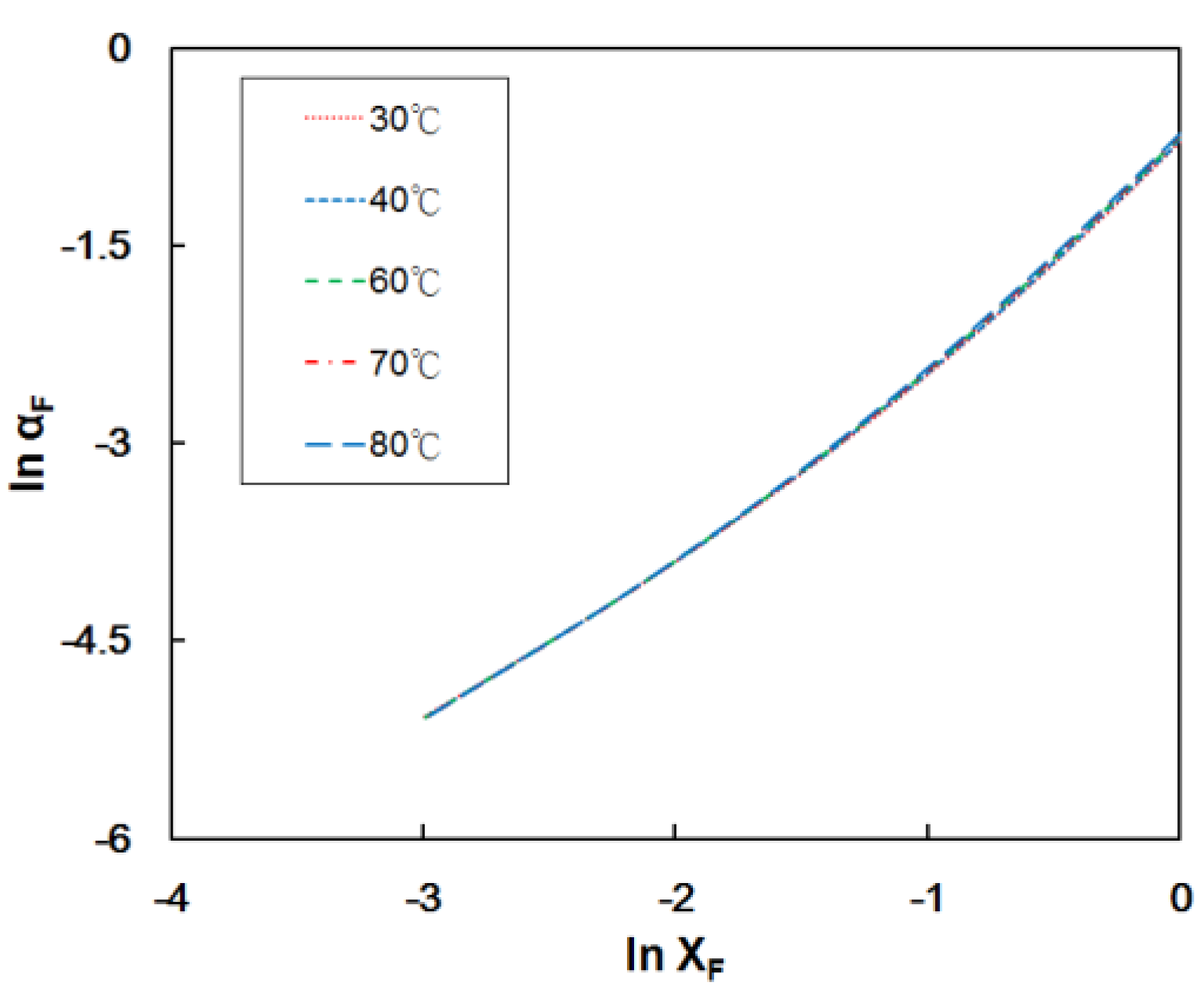
2.3. Formic Acid Diffusion Coefficient at the Anode Electrode (Dba)
2.4. Cathode Overpotential
3. Experimental Section
3.1. Electrolyte Preparation
3.2. Conductivity and Formic Acid Permeability Measurements
3.3. Formic Acid Fuel Cell Test
4. Results and Discussion
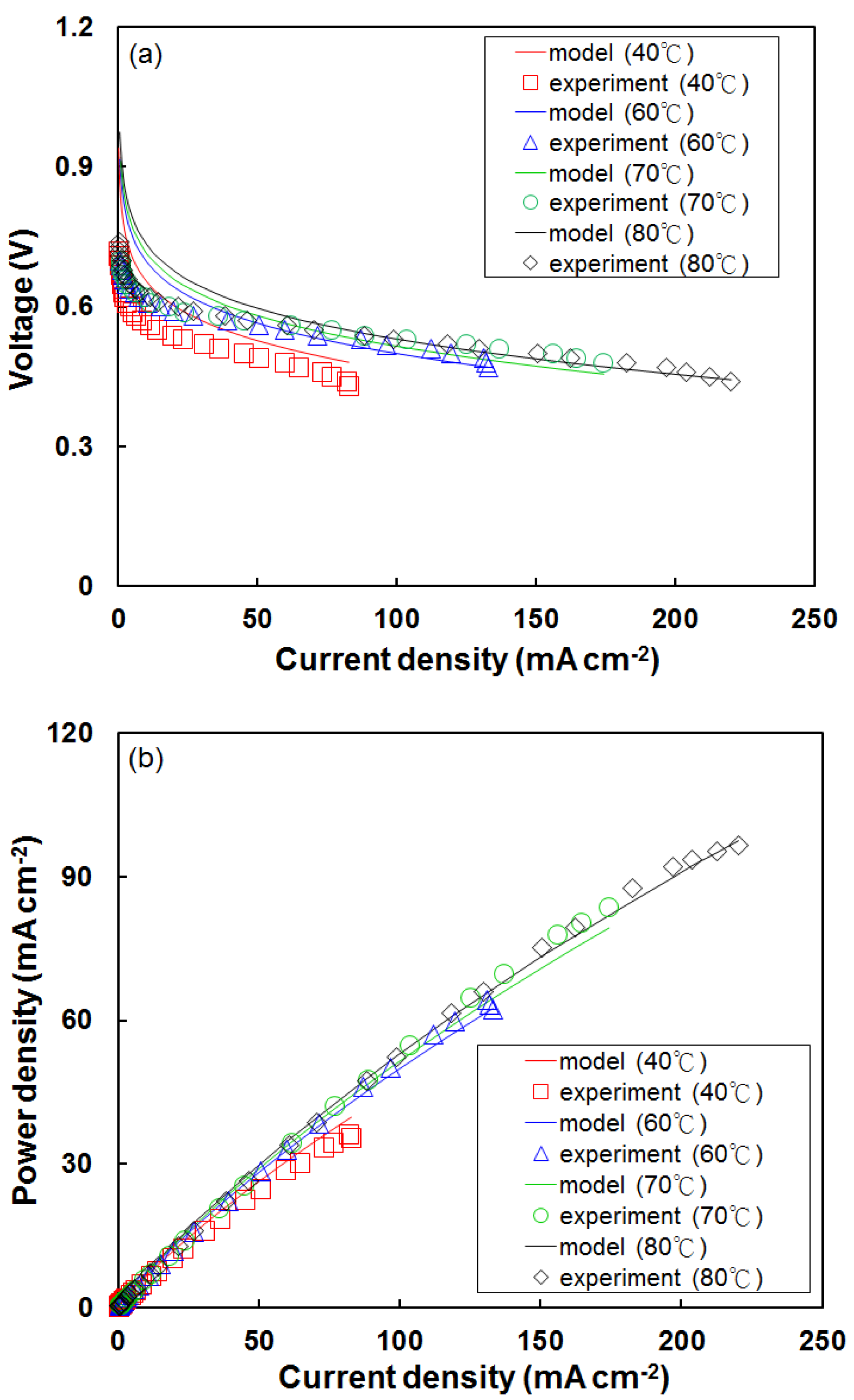
4.1. Effect of Temperature on Fuel Cell Performance Using the Modified Model
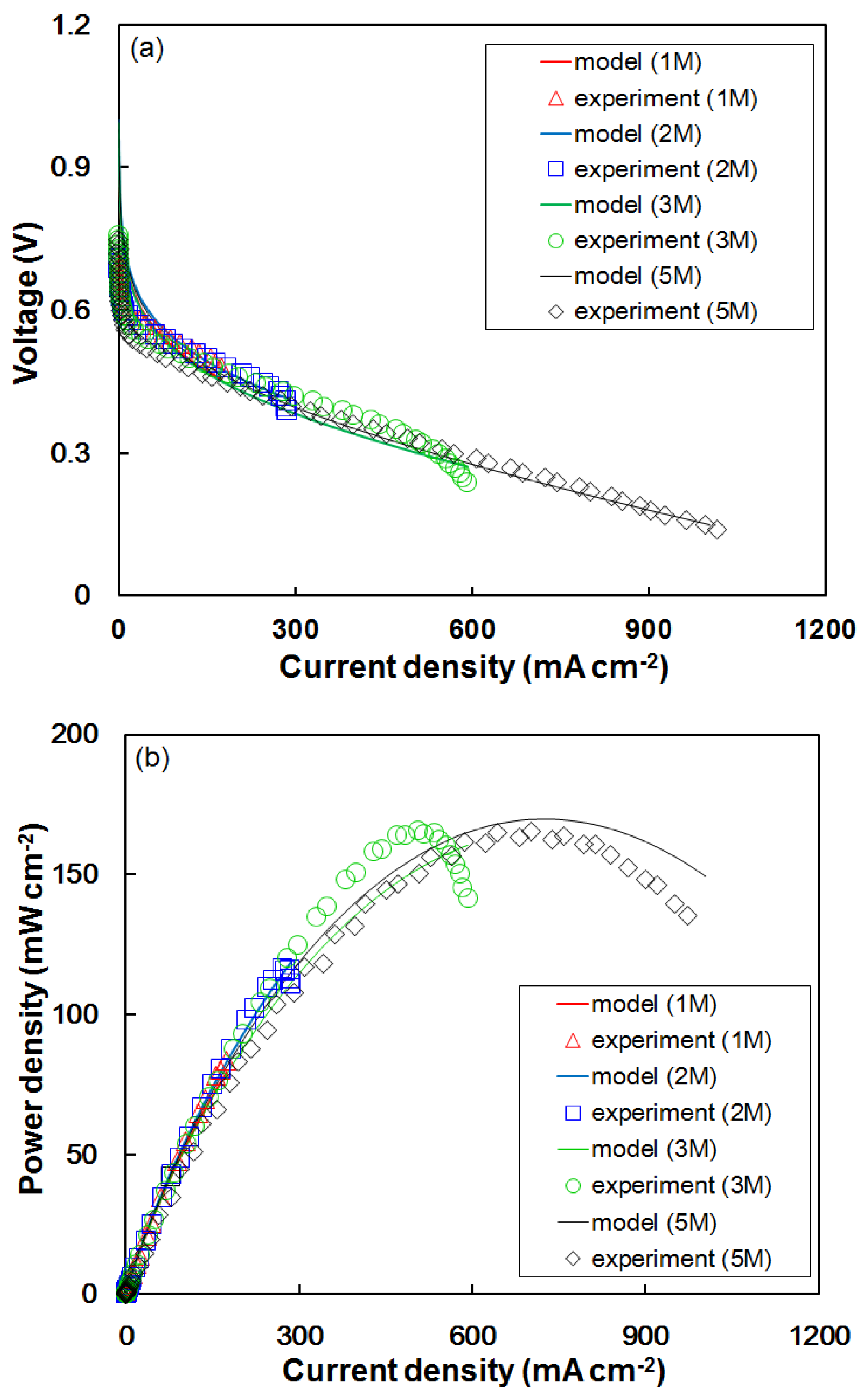
4.2. Effect of Formic Acid Concentration on Cell Performance Using the Modified Model
4.3. Modeling for sSEBS/PTFE Membrane Electrolytes
5. Conclusions
Supplementary Materials
Acknowledgment
Author Contributions
Conflicts of Interest
Nomenclature
| a | activity of reactant or product (−) |
| ba | Tafel slope at the anode (V) |
| bc | Tafel slope at the cathode (V) |
| CO | oxygen concentration (mol·cm−3) |
| CF | formic acid concentration (mol·cm−3) |
| Dba | formic acid diffusion coefficient in the anode diffusion layer (cm2·s−1) |
| Dbc | oxygen diffusion coefficient in the cathode diffusion layer (cm2·s−1) |
| D° | solute diffusion coefficient at infinite dilution (cm2·s−1) |
| Deff | effective diffusion coefficient of reactant in the porous diffusion layer (cm2·s−1) |
| DIJ | diffusion coefficient of component I in mixture with J (cm2·s−1) |
| E0 | thermodynamic cell potential at standard state (V) |
| E | thermodynamic cell potential at any condition (V) |
| F | Faraday’s constant (96,485 C·mol−1) |
| ∆G0 | Gibbs free energy change in overall reaction for a DFAFC at standard state (J·mol−1) |
| iec | exchange current density for the cathode (A·cm−2) |
| iea | exchange current density for the anode (A·cm−2) |
| i | current density (A·cm−2) |
| ilc | limiting current density at the cathode (A·cm−2) |
| ila | limiting current density at the anode (A·cm−2) |
| L | thickness of the electrolyte (cm) |
| Lbc | thickness of backing layer at the cathode (cm) |
| Lba | thickness of backing layer at the anode (cm) |
| M | molar mass (g·mol−1) |
| n | number of electrons in half-reaction |
| p | gas pressure (bar) |
| P | power density (mW·cm−2) |
| Rc | dimensionless parameter accounting for potential drop due to formic acid crossover (−) |
| R | gas constant (8.314 J·mol−1·K−1) |
| T* | reduced temperature (−) |
| T | temperature (K) |
| v | molar volume (cm3) |
| V | cell voltage (V) |
| x | mole fraction (−) |
| Greek | |
| α | thermodynamic correction term (−) |
| β | formic acid permeability (cm2·s−1) |
| ε | characteristic Lennard-Jones energy (J) |
| ζ | solvent viscosity (cP) |
| ηΩ | ohmic potential drop (V) |
| ηa | overpotential at the anode (V) |
| ηc | overpotential at the cathode (V) |
| θ | porosity of diffusion layer (−) |
| Φ | solvent association constant (−) |
| κ | Boltzmann’s constant (J·K−1) |
| μ | dimensionless parameter (−) |
| σ | proton conductivity of the solid electrolyte membrane (S·m−1) |
| σOA | characteristic Lennard-Jones length (Å) |
| ΩD | diffusion collision integral (−) |
References
- Zhu, Y.; Ha, S.Y.; Masel, R.I. High power density direct formic acid fuel cells. J. Power Sources 2004, 130, 8–14. [Google Scholar] [CrossRef]
- Sebastian, D.; Serov, A.; Matanovic, I.; Artyushkova, K.; Atanassov, P.; Arico, A.; Baglio, V. Insights on the extraordinary tolerance to alcohols of Fe-NC cathode catalysts in highly performing direct alcohol fuel cells. Nano Energy 2017, 34, 195–204. [Google Scholar] [CrossRef]
- Mohammad, A.M.; El-Nagar, G.A.; Al-Akraa, I.M.; El-Deab, M.S.; El-Anadouli, B.E. Towards improving the catalytic activity and stability of platinum-based anodes in direct formic acid fuel cells. Int. J. Hydrog. Energy 2015, 40, 7808–7816. [Google Scholar] [CrossRef]
- Baik, S.; Kim, J.; Han, J.; Kwon, Y. Performance improvement in direct formic acid fuel cells (DFAFCs) using metal catalyst prepared by dual mode spraying. Int. J. Hydrog. Energy 2011, 36, 12583–12590. [Google Scholar] [CrossRef]
- Yu, X.; Pickup, P.G. Recent advances in direct formic acid fuel cells (DFAFC). J. Power Sources 2008, 182, 124–132. [Google Scholar] [CrossRef]
- Wang, B.-Y.; Lin, H.-K.; Liu, N.-Y.; Mahesh, K.; Lue, S.J. Cell performance modeling of direct methanol fuel cells using proton-exchange solid electrolytes: Effective reactant diffusion coefficients in porous diffusion layers. J. Power Sources 2013, 227, 275–283. [Google Scholar] [CrossRef]
- Bauskar, A.S.; Rice, C.A. Impact of anode catalyst layer porosity on the performance of a direct formic acid fuel cell. Electrochim. Acta 2012, 62, 36–41. [Google Scholar] [CrossRef]
- Jiang, J.; Kucernak, A. Probing anodic reaction kinetics and interfacial mass transport of a direct formic acid fuel cell using a nanostructured palladium–gold alloy microelectrode. Electrochim. Acta 2009, 54, 4545–4551. [Google Scholar] [CrossRef]
- Rice, C.; Ha, S.; Masel, R.; Waszczuk, P.; Wieckowski, A.; Barnard, T. Direct formic acid fuel cells. J. Power Sources 2002, 111, 83–89. [Google Scholar] [CrossRef]
- Krishnamurthy, D.; Johansson, E.O.; Lee, J.W.; Kjeang, E. Computational modeling of microfluidic fuel cells with flow-through porous electrodes. J. Power Sources 2011, 196, 10019–10031. [Google Scholar] [CrossRef]
- Perales-Rondón, J.V.; Herrero, E.; Feliu, J.M. On the activation energy of the formic acid oxidation reaction on platinum electrodes. J. Electroanal. Chem. 2015, 742, 90–96. [Google Scholar] [CrossRef] [Green Version]
- Reis, A.; Mert, S.O. Performance assessment of a direct formic acid fuel cell system through exergy analysis. Int. J. Hydrog. Energy 2015, 40, 12776–12783. [Google Scholar] [CrossRef]
- Ha, S.; Dunbar, Z.; Masel, R. Characterization of a high performing passive direct formic acid fuel cell. J. Power Sources 2006, 158, 129–136. [Google Scholar] [CrossRef]
- Gao, W.; Keith, J.A.; Anton, J.; Jacob, T. Theoretical elucidation of the competitive electro-oxidation mechanisms of formic acid on Pt (111). J. Am. Chem. Soc. 2010, 132, 18377–18385. [Google Scholar] [CrossRef] [PubMed]
- Shaegh, S.A.M.; Nguyen, N.-T.; Chan, S.H. An air-breathing microfluidic formic acid fuel cell with a porous planar anode: Experimental and numerical investigations. J. Micromech. Microeng. 2010, 20, 105008. [Google Scholar] [CrossRef]
- Wang, X.; Hu, J.-M.; Hsing, I.-M. Electrochemical investigation of formic acid electro-oxidation and its crossover through a Nafion® membrane. J. Electroanal. Chem. 2004, 562, 73–80. [Google Scholar] [CrossRef]
- Li, X.; Roberts, E.; Holmes, S. Evaluation of composite membranes for direct methanol fuel cells. J. Power Sources 2006, 154, 115–123. [Google Scholar] [CrossRef]
- Kulikovsky, A. A method for analysis of DMFC performance curves. Electrochem. Commun. 2003, 5, 1030–1036. [Google Scholar] [CrossRef]
- Hirschfelder, J.; Bird, R.B.; Curtiss, C.F. Molecular Theory of Gases and Liquids; Wiley: New York, NY, USA, 1964. [Google Scholar]
- Wilke, C.; Chang, P. Correlation of diffusion coefficients in dilute solutions. AIChE J. 1955, 1, 264–270. [Google Scholar] [CrossRef]
- Smith, J.M.; Van Ness, H.C.; Abbott, V.N. Introduction to Chemical Engineering Thermodynamics; McGraw-Hill Book: Boston, MA, USA, 2005. [Google Scholar]
- Das, P.K.; Li, X.; Liu, Z.-S. Effective transport coefficients in PEM fuel cell catalyst and gas diffusion layers: Beyond Bruggeman approximation. Appl. Energy 2010, 87, 2785–2796. [Google Scholar] [CrossRef]
- Nguyen, T.V.; White, R.E. A water and heat management model for Proton-Exchange-Membrane fuel cells. J. Electrochem. Soc. 1993, 140, 2178–2186. [Google Scholar] [CrossRef]
- Alberti, G.; Casciola, M. Solid state protonic conductors, present main applications and future prospects. Solid State Ion. 2001, 145, 3–16. [Google Scholar] [CrossRef]
- Weiss, R.; Sen, A.; Willis, C.; Pottick, L. Block copolymer ionomers: 1. Synthesis and physical properties of sulphonated poly (styrene-ethylene/butylene-styrene). Polymer 1991, 32, 1867–1874. [Google Scholar] [CrossRef]
- Won, J.; Choi, S.W.; Kang, Y.S.; Ha, H.Y.; Oh, I.-H.; Kim, H.S.; Kim, K.T.; Jo, W.H. Structural characterization and surface modification of sulfonated polystyrene-(ethylene-butylene)-styrene triblock proton exchange membranes. J. Membr. Sci. 2003, 214, 245–257. [Google Scholar] [CrossRef]
- Kim, B.; Kim, J.; Jung, B. Morphology and transport properties of protons and methanol through partially sulfonated block copolymers. J. Membr. Sci. 2005, 250, 175–182. [Google Scholar] [CrossRef]
- Divisek, J.; Fuhrmann, J.; Gärtner, K.; Jung, R. Performance modeling of a direct methanol fuel cell. J. Electrochem. Soc. 2003, 150, A811–A825. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Deng, C.; Wu, B.; Gao, Y. Kinetic study of formic acid oxidation on carbon supported platinum electrocatalyst. Electrochemistry 2010, 78, 662–665. [Google Scholar] [CrossRef]
- Velázquez-Palenzuela, A.; Centellas, F.; Garrido, J.A.; Arias, C.; Rodríguez, R.M.; Brillas, E.; Cabot, P.-L. Kinetic analysis of carbon monoxide and methanol oxidation on high performance carbon-supported Pt–Ru electrocatalyst for direct methanol fuel cells. J. Power Sources 2011, 196, 3503–3512. [Google Scholar] [CrossRef]
- Darken, L.S. Diffusion, mobility and their interrelation through free energy in binary metallic system. Trans. Am. Inst. Min. Metall. Eng. 1948, 175, 184. [Google Scholar]
- Ghai, R.; Ertl, H.; Dullien, F. Liquid diffusion of nonelectrolytes: Part I. AIChE J. 1973, 19, 881–900. [Google Scholar] [CrossRef]
- Reid, R.C.; Prausnitz, J.; Poling, B. The Properties of Gases and Liquids; McGraw-Hill: New York, NY, USA, 1987; Volume 4. [Google Scholar]
- Yang, C.; Wei, G.; Li, Y. Densities and Viscosities of N,N-Dimethylformamide+ Formic Acid, and+ Acetic Acid in the Temperature Range from (303.15 to 353.15) K. J. Chem. Eng. Data 2008, 53, 1211–1215. [Google Scholar] [CrossRef]
- Zamel, N.; Astrath, N.G.; Li, X.; Shen, J.; Zhou, J.; Astrath, F.B.; Wang, H.; Liu, Z.-S. Experimental measurements of effective diffusion coefficient of oxygen–nitrogen mixture in PEM fuel cell diffusion media. Chem. Eng. Sci. 2010, 65, 931–937. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, C. Mathematical modeling of liquid-feed direct methanol fuel cells. J. Electrochem. Soc. 2003, 150, A508–A519. [Google Scholar] [CrossRef]
- Parthasarathy, A.; Srinivasan, S.; Appleby, A.J.; Martin, C.R. Temperature dependence of the electrode kinetics of oxygen reduction at the platinum/Nafion® interface—A microelectrode investigation. J. Electrochem. Soc. 1992, 139, 2530–2537. [Google Scholar] [CrossRef]
- Lue, S.J.; Wang, W.-T.; Mahesh, K.; Yang, C.-C. Enhanced performance of a direct methanol alkaline fuel cell (DMAFC) using a polyvinyl alcohol/fumed silica/KOH electrolyte. J. Power Sources 2010, 195, 7991–7999. [Google Scholar] [CrossRef]
- Rajesh Kumar, S.; Juan, C.-H.; Liao, G.-M.; Lin, J.-S.; Yang, C.-C.; Ma, W.-T.; You, J.-H.; Jessie Lue, S. Fumed silica nanoparticles incorporated in quaternized poly (vinyl alcohol) nanocomposite membrane for enhanced power densities in direct alcohol alkaline fuel cells. Energies 2016, 9, 15. [Google Scholar] [CrossRef]
- Lin, J.-S.; Ma, W.-T.; Shih, C.-M.; Yu, B.-C.; Teng, L.-W.; Wang, Y.-C.; Cheng, K.-W.; Chiu, F.-C.; Lue, S.J. Reorientation of magnetic graphene oxide nanosheets in crosslinked quaternized polyvinyl alcohol as effective solid electrolyte. Energies 2016, 9, 1003. [Google Scholar] [CrossRef]
- Rajesh Kumar, S.; Ma, W.-T.; Lu, H.-C.; Teng, L.-W.; Hsu, H.-C.; Shih, C.-M.; Yang, C.-C.; Lue, S.J. Surfactant-Assisted Perovskite Nanofillers Incorporated in Quaternized Poly (Vinyl Alcohol) Composite Membrane as an Effective Hydroxide-Conducting Electrolyte. Energies 2017, 10, 615. [Google Scholar] [CrossRef]
- Lue, S.J.; Juang, H.J.; Hou, S.Y. Permeation of xylene isomers through supported liquid membranes containing cyclodextrins. Sep. Sci. Technol. 2002, 37, 463–480. [Google Scholar] [CrossRef]
- Lue, S.J.; Shih, T.-S.; Wei, T.-C. Plasma modification on a Nafion membrane for direct methanol fuel cell applications. Korean J. Chem. Eng. 2006, 23, 441–446. [Google Scholar] [CrossRef]
- Lue, S.J.; Mahesh, K.; Wang, W.-T.; Chen, J.-Y.; Yang, C.-C. Permeant transport properties and cell performance of potassium hydroxide doped poly (vinyl alcohol)/fumed silica nanocomposites. J. Membr. Sci. 2011, 367, 256–264. [Google Scholar] [CrossRef]
- Lin, J.-S.; Kumar, S.R.; Ma, W.-T.; Shih, C.-M.; Teng, L.-W.; Yang, C.-C.; Lue, S.J. Gradiently distributed iron oxide@ graphene oxide nanofillers in quaternized polyvinyl alcohol composite to enhance alkaline fuel cell power density. J. Membr. Sci. 2017, 543, 28–39. [Google Scholar] [CrossRef]
- Gold, S.; Chu, K.-L.; Lu, C.; Shannon, M.A.; Masel, R.I. Acid loaded porous silicon as a proton exchange membrane for micro-fuel cells. J. Power Sources 2004, 135, 198–203. [Google Scholar] [CrossRef]
- Jiang, J.; Kucernak, A. Nanostructured platinum as an electrocatalyst for the electrooxidation of formic acid. J. Electroanal. Chem. 2002, 520, 64–70. [Google Scholar] [CrossRef]
- Lović, J.; Tripković, A.; Gojković, S.L.; Popović, K.D.; Tripković, D.; Olszewski, P.; Kowal, A. Kinetic study of formic acid oxidation on carbon-supported platinum electrocatalyst. J. Electroanal. Chem. 2005, 581, 294–302. [Google Scholar] [CrossRef]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lue, S.J.; Liu, N.-Y.; Rajesh Kumar, S.; Tseng, K.C.-Y.; Wang, B.-Y.; Leung, C.-H. Experimental and One-Dimensional Mathematical Modeling of Different Operating Parameters in Direct Formic Acid Fuel Cells. Energies 2017, 10, 1972. https://doi.org/10.3390/en10121972
Lue SJ, Liu N-Y, Rajesh Kumar S, Tseng KC-Y, Wang B-Y, Leung C-H. Experimental and One-Dimensional Mathematical Modeling of Different Operating Parameters in Direct Formic Acid Fuel Cells. Energies. 2017; 10(12):1972. https://doi.org/10.3390/en10121972
Chicago/Turabian StyleLue, Shingjiang Jessie, Nai-Yuan Liu, Selvaraj Rajesh Kumar, Kevin Chi-Yang Tseng, Bo-Yan Wang, and Chieh-Hsin Leung. 2017. "Experimental and One-Dimensional Mathematical Modeling of Different Operating Parameters in Direct Formic Acid Fuel Cells" Energies 10, no. 12: 1972. https://doi.org/10.3390/en10121972





