Experimental Study on the Mechanical Properties of CH4 and CO2 Hydrate Remodeling Cores in Qilian Mountain
Abstract
:1. Introduction
2. Experimental Methods
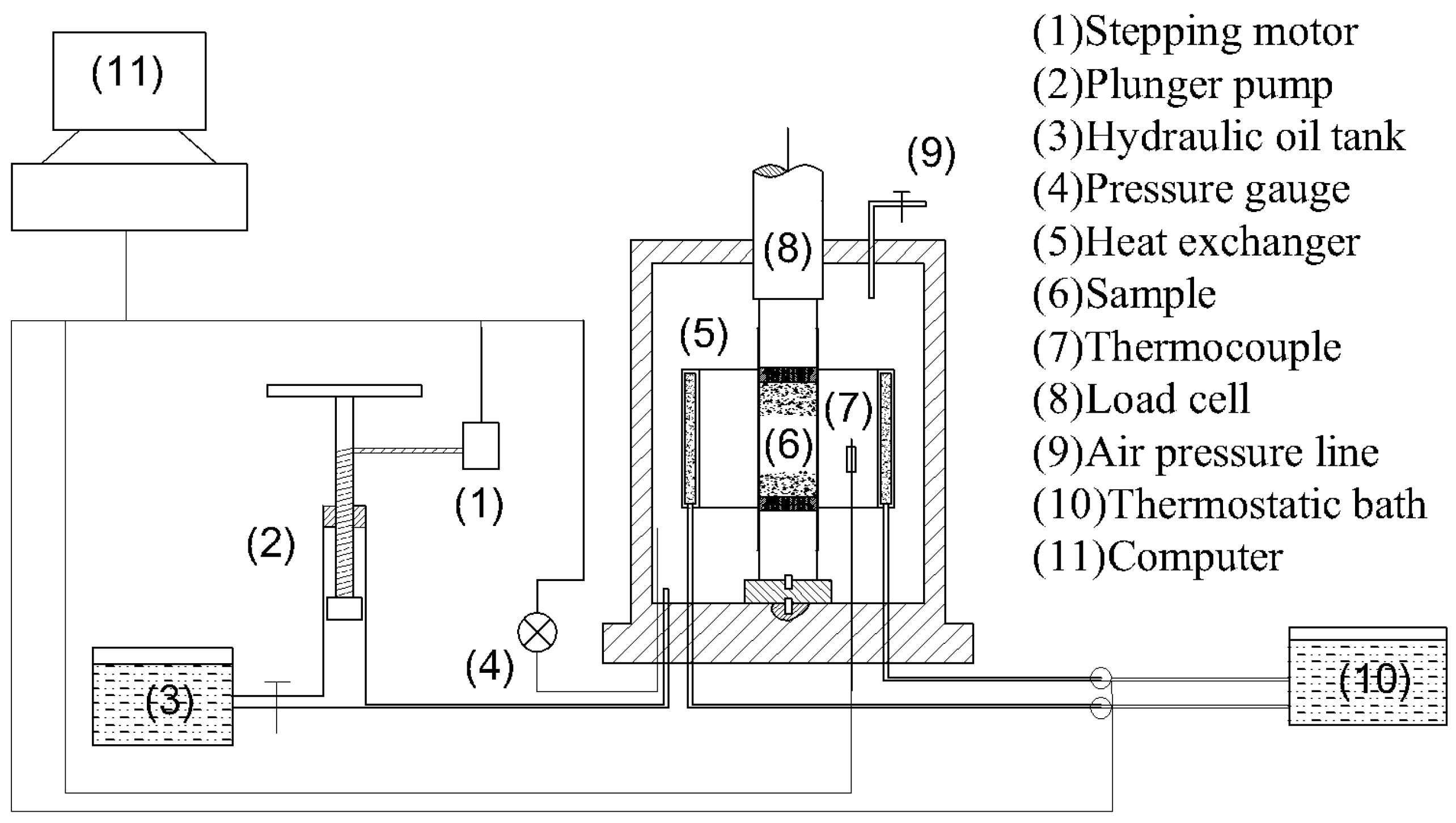
2.1. Experimental Apparatus
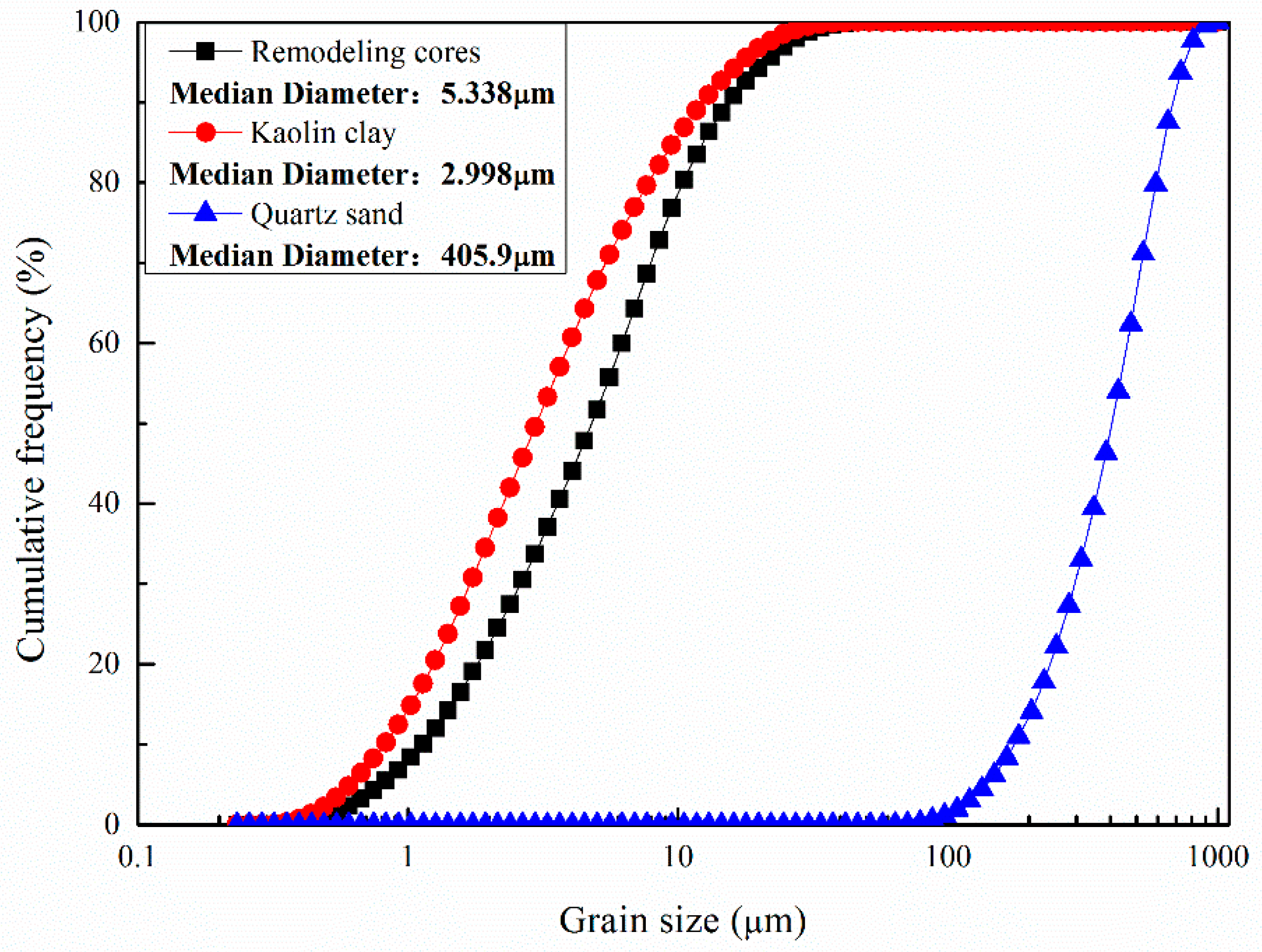
2.2. Sample Preparation
2.3. Experimental Procedures
3. Results and Discussions
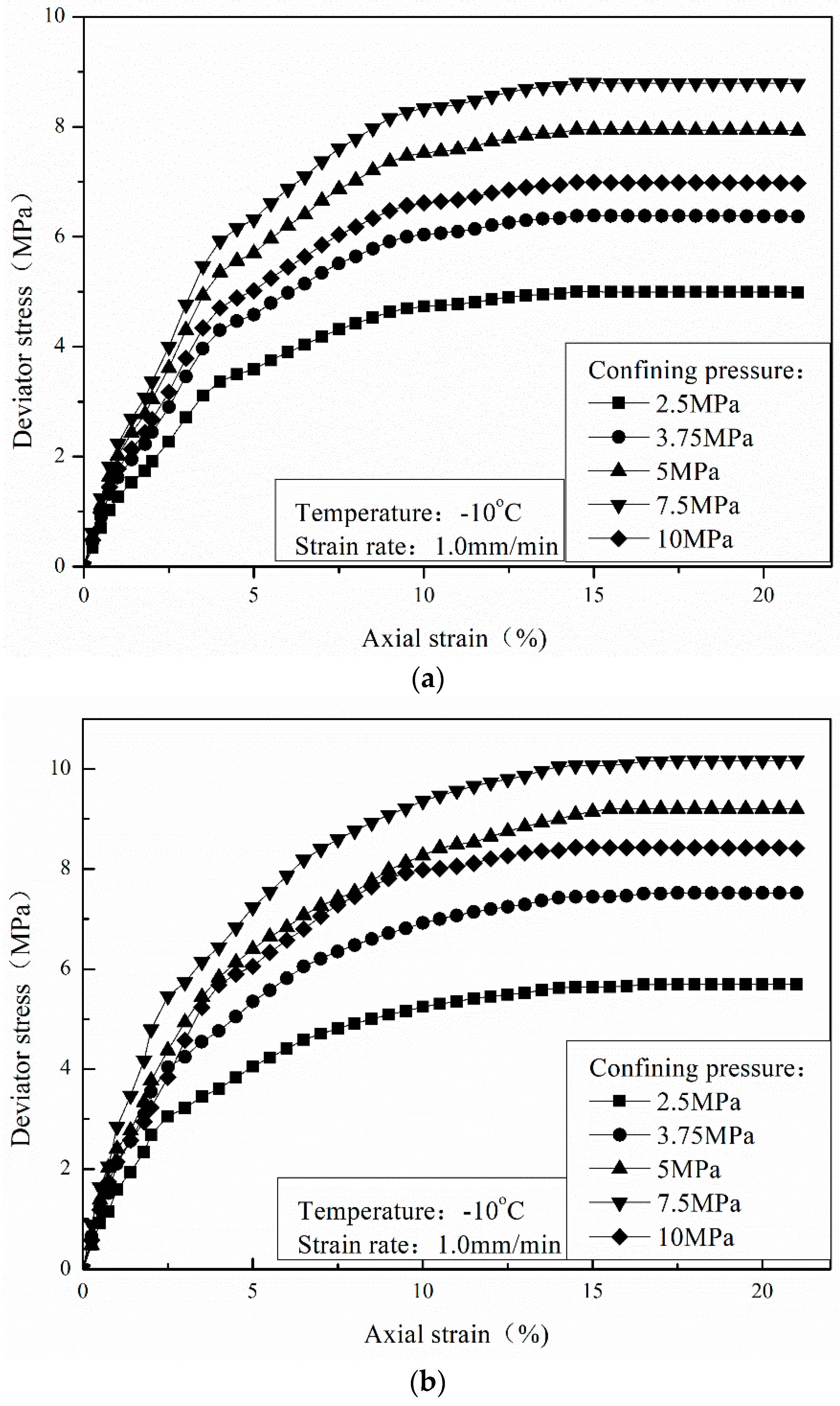
3.1. The Effect of Confining Pressure
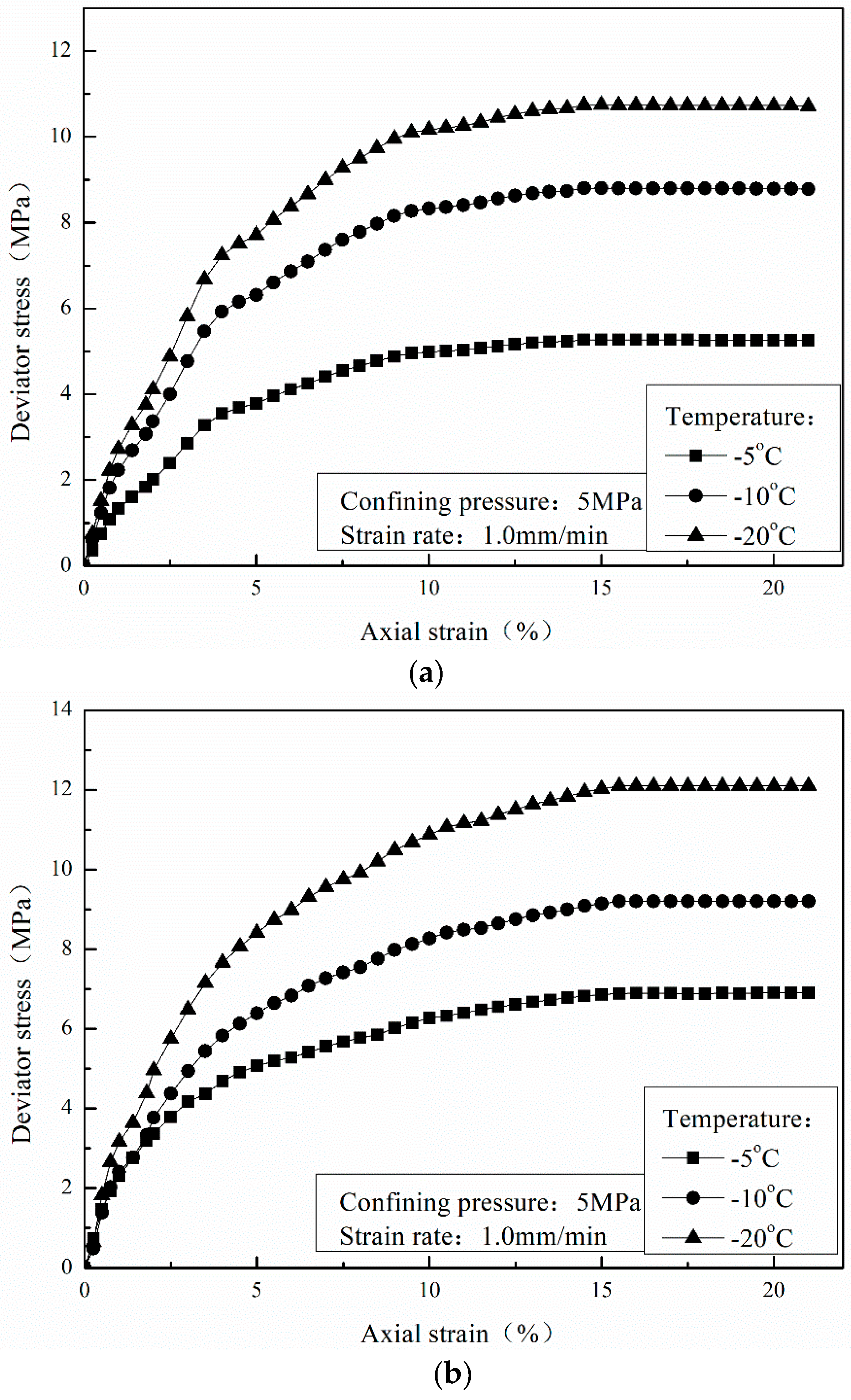
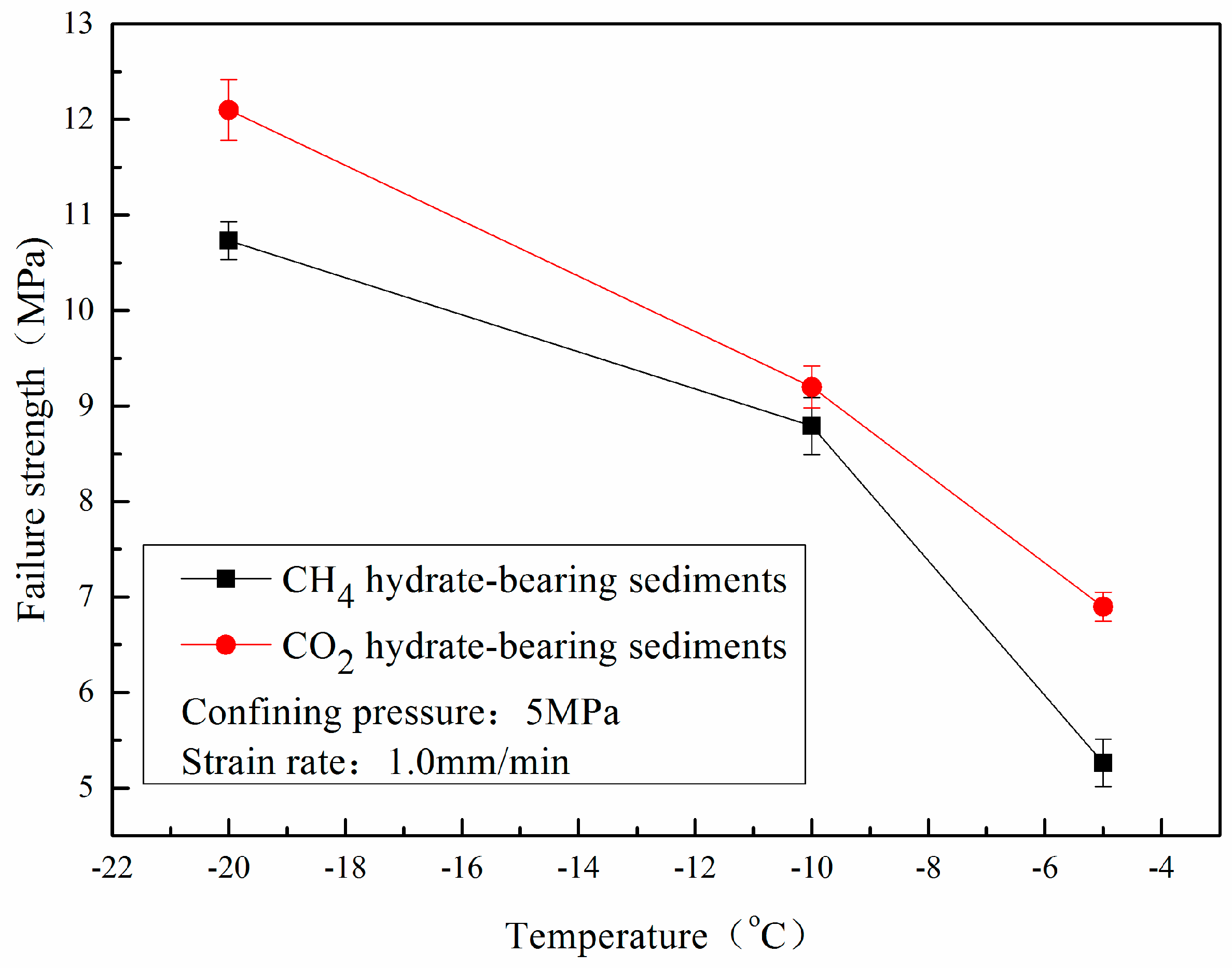
3.2. Effect of Temperature
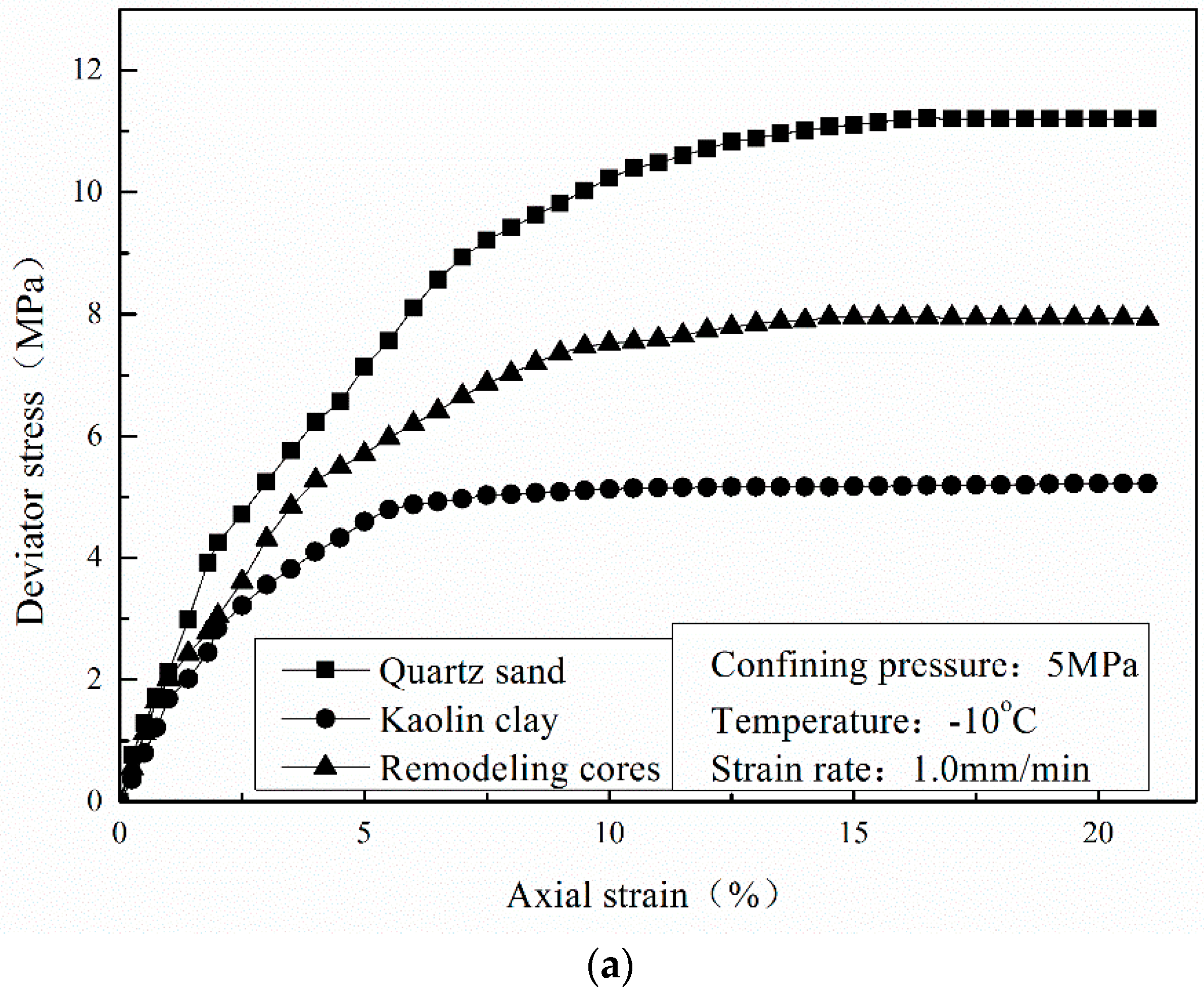
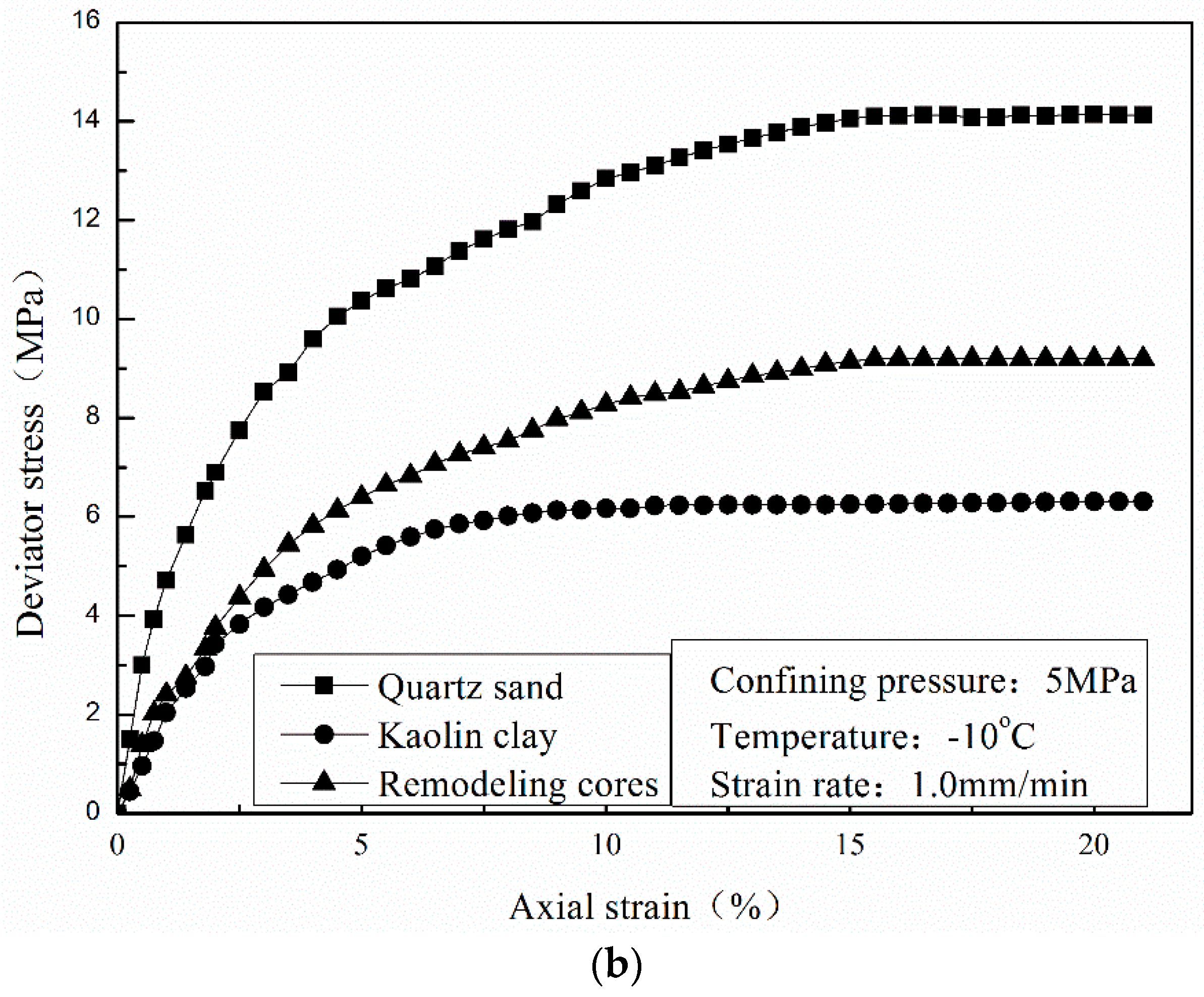
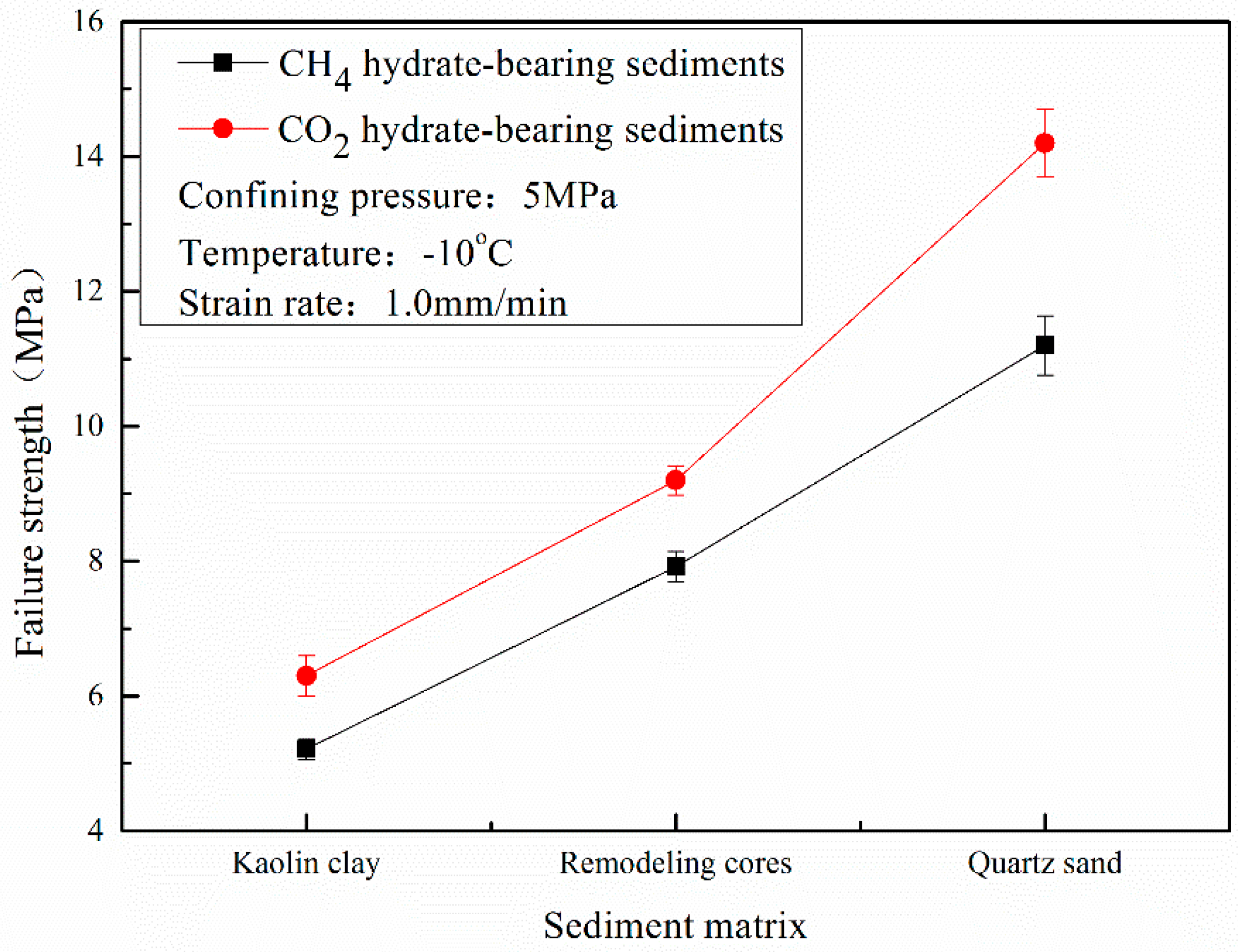
3.3. Effect of Sediment Matrix
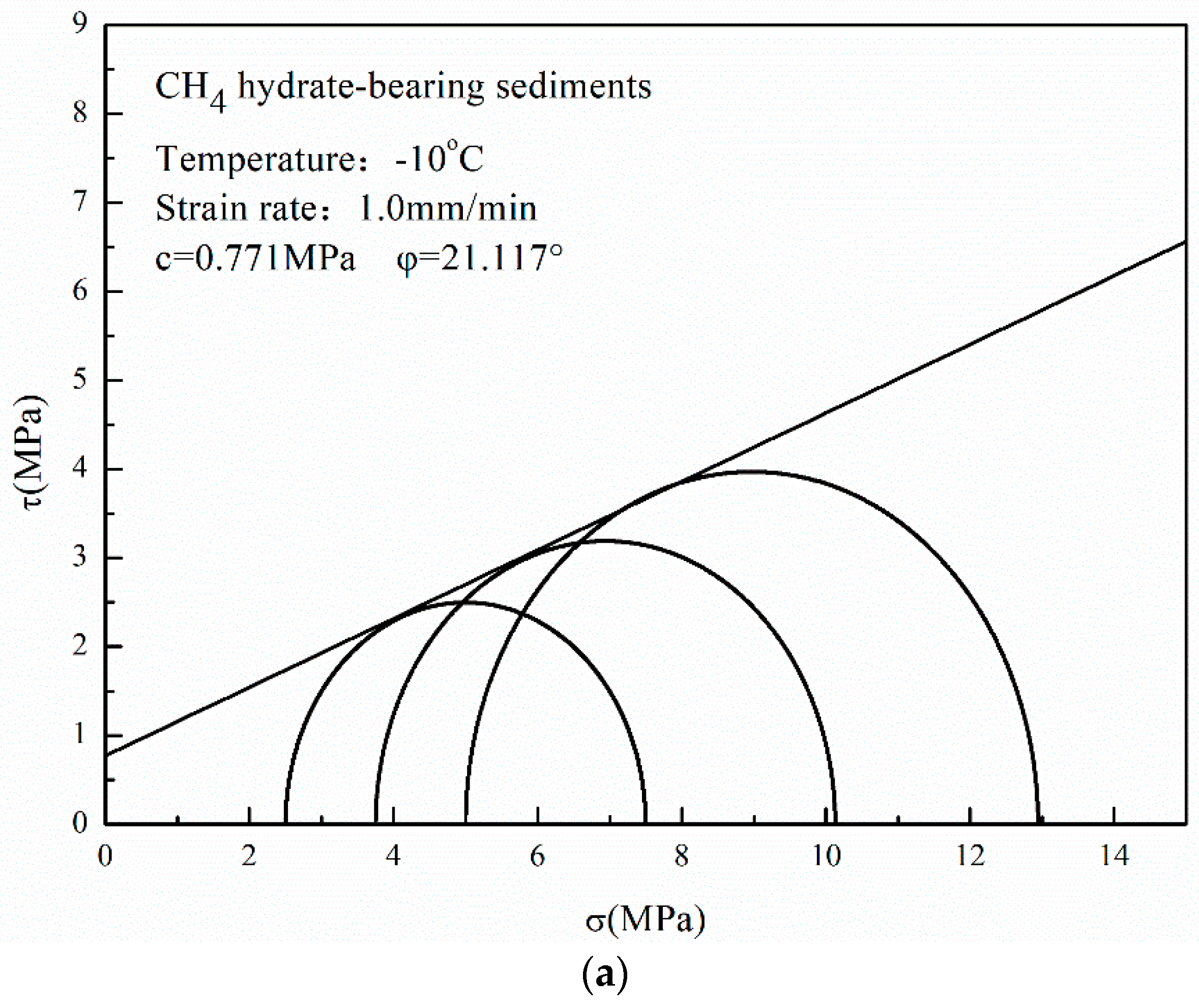
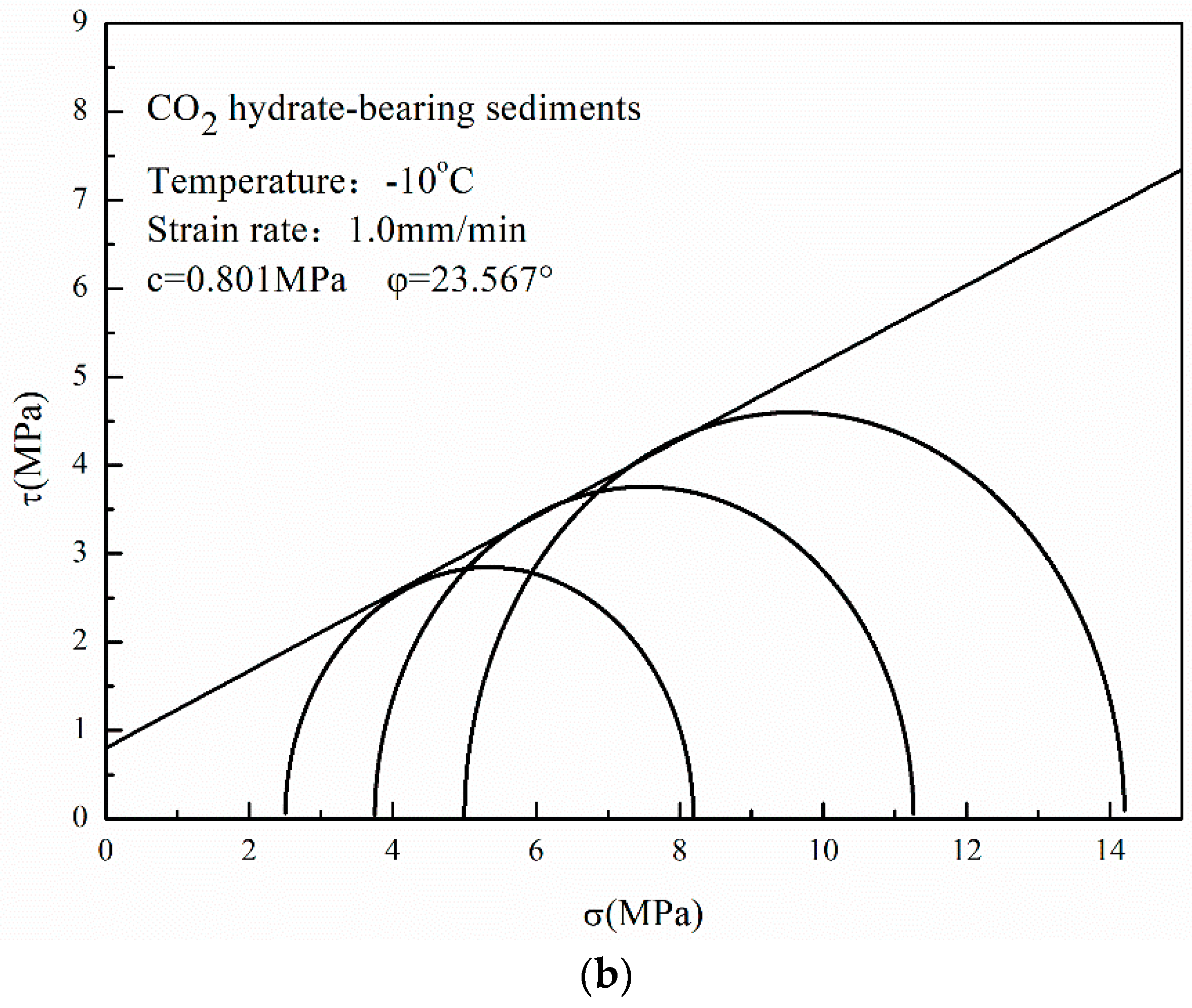
3.4. Mohr–Coulomb Strength Theory
4. Conclusions
- (1)
- The stress-strain curves of CO2 hydrate-bearing sediments are consistent with that of CH4 hydrate-bearing sediments. The effects of temperature, confining pressure, and sediment matrices on the stress-strain relationships of CH4 hydrate-bearing sediments are consistent with the effects of those on CO2 hydrate-bearing sediments.
- (2)
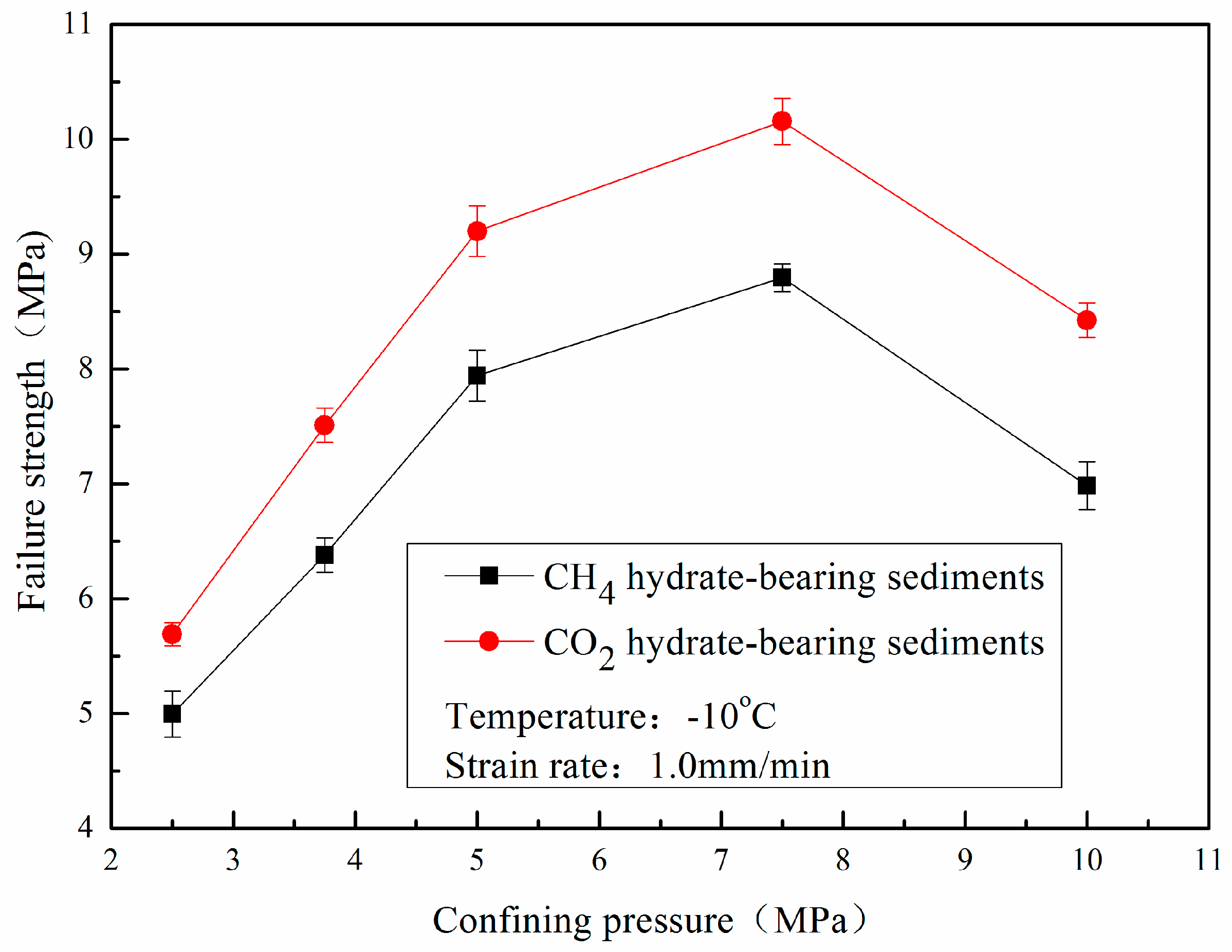
- The failure strength of CO2 hydrate-bearing sediments is higher than that of CH4 hydrate sediments under different confining pressures, temperatures, and sediment matrices. The failure strength of the CH4 and CO2 hydrate-bearing sediments first increased and then decreased with an increase in confining pressure, and achieved a maximum at 7.5 MPa.
- (3)
- The cohesion and the internal friction angle of CO2 hydrate-bearing sediments are both higher than those of CH4 hydrate-bearing sediments. The internal friction angle plays a dominant role in the failure strength of CH4 and CO2 hydrate-bearing sediments. In this study, all of the experiments and the theoretical analysis proved the stability of the reservoir in Qilian Mountain under CH4-CO2 replacement.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Daigle, H.; Cook, A.; Malinverno, A. Permeability and porosity of hydrate-bearing sediments in the northern gulf of Mexico. Mar. Pet. Geol. 2015, 68, 551–564. [Google Scholar] [CrossRef]
- Kim, Y.G.; Lee, S.M.; Jin, Y.K.; Baranov, B.; Obzhirov, A.; Salomatin, A.; Shoji, H. The stability of gas hydrate field in the northeastern continental slope of Sakhalin island, sea of Okhotsk, as inferred from analysis of heat flow data and its implications for slope failures. Mar. Pet. Geol. 2013, 45, 198–207. [Google Scholar] [CrossRef]
- Kayen, R.E.; Lee, H.J. Pleistocene slope instability of gas hydrate-laden sediment on the beaufort sea margin. Mar. Georesour. Geotechnol. 1991, 10, 125–141. [Google Scholar] [CrossRef]
- Miyazaki, K.; Masui, A.; Aoki, K.; Sakamoto, Y.; Yamaguchi, T.; Okubo, S. Strain-rate dependence of triaxial compressive strength of artificial methane-hydrate-bearing sediment. Int. J. Offshore Polar Eng. 2010, 20, 256–264. [Google Scholar]
- Priest, J.A.; Clayton, C.R.I.; Rees, E.V.L. Potential impact of gas hydrate and its dissociation on the strength of host sediment in the Krishna-Godavari Basin. Mar. Pet. Geol. 2014, 58, 187–198. [Google Scholar] [CrossRef]
- Priest, J.A.; Druce, M.; Roberts, J.; Schultheiss, P.; Nakatsuka, Y.; Suzuki, K. Pcats triaxial: A new geotechnical apparatus for characterizing pressure cores from the Nankai Trough, Japan. Mar. Pet. Geol. 2015, 66, 460–470. [Google Scholar] [CrossRef]
- Yoneda, J.; Hyodo, M.; Yoshimoto, N.; Nakata, Y.; Kato, A. Development of high-pressure low-temperature plane strain testing apparatus for methane hydrate-bearing sand. Soils Found. 2013, 53, 774–783. [Google Scholar] [CrossRef]
- Yun, T.S.; Santamarina, J.C.; Ruppel, C. Mechanical properties of sand, silt, and clay containing tetrahydrofuran hydrate. J. Geophys. Res. Solid Earth 2007, 112. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, D.; Yang, M.; Song, Y. Analysis of heat transfer effects on gas production from methane hydrate by depressurization. Int. J. Heat Mass Transf. 2014, 77, 529–541. [Google Scholar] [CrossRef]
- Zhao, J.; Yao, L.; Song, Y.; Xue, K.; Cheng, C.; Liu, Y.; Zhang, Y. In situ observations by magnetic resonance imaging for formation and dissociation of tetrahydrofuran hydrate in porous media. Magn. Reson. Imaging 2011, 29, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Yu, T.; Song, Y.; Liu, D.; Liu, W.; Liu, Y.; Yang, M.; Ruan, X.; Li, Y. Numerical simulation of gas production from hydrate deposits using a single vertical well by depressurization in the QiLian Mountain permafrost, QingHai-Tibet Plateau, China. Energy 2013, 52, 308–319. [Google Scholar] [CrossRef]
- Zhao, J.; Zhu, Z.; Song, Y.; Liu, W.; Zhang, Y.; Wang, D. Analyzing the process of gas production for natural gas hydrate using depressurization. Appl. Energy 2015, 142, 125–134. [Google Scholar] [CrossRef]
- Lu, Z.; Zhu, Y.; Zhang, Y.; Wen, H.; Li, Y. Basic geological characteristics of gas hydrates in QiLian Mountain permafrost area, QingHai Province. Miner. Depos. China 2010, 29, 182–193. [Google Scholar]
- Pang, S.; Su, X.; He, H.; Zhao, Q. Geological controlling factors of gas hydrate occurrence in QiLian Mountain permafrost. Earth Sci. Front. China 2013, 20, 223–229. [Google Scholar]
- Jung, J.W.; Espinoza, D.N.; Santamarina, J.C. Properties and phenomena relevant to CH4-CO2 replacement in hydrate-bearing sediments. J. Geophys. Res. 2010, 115. [Google Scholar] [CrossRef]
- Jung, J.W.; Santamarina, J.C. CH4-CO2 replacement in hydrate-bearing sediments: A pore-scale study. Geochem. Geophys. Geosyst. 2010, 11. [Google Scholar] [CrossRef]
- Ota, M.; Abe, Y.; Watanabe, M.; Smith, R.L.; Inomata, H. Methane recovery from methane hydrate using pressurized CO2. Fluid Phase Equilib. 2005, 228–229, 553–559. [Google Scholar] [CrossRef]
- Ota, M.; Saito, T.; Aida, T.; Watanabe, M.; Sato, Y.; Smith, R.L.; Inomata, H. Macro and microscopic CH4-CO2 replacement in CH4 hydrate under pressurized CO2. AIChE J. 2007, 53, 2715–2721. [Google Scholar] [CrossRef]
- Ohgaki, K.T.; Sangawa, H.; Matsubara, T.; Nakano, S. Methane exploitation by carbon dioxide from gas hydrates-phase equilibria for CH4-CO2 mixed hydrate system. J. Chem. Eng. Jpn. 1996, 29, 478–483. [Google Scholar] [CrossRef]
- Hirohama, Y.S.; Wakabayashi, A.; Tatsuta, S.; Nishida, N. Conversion of CH4-hydrate to CO2-hydrate in liquid CO2. J. Chem. Eng. Jpn. 1996, 29, 1014–1020. [Google Scholar] [CrossRef]
- Yoon, J.H.; Kawamura, T.; Yamamoto, Y.; Komai, T. Transformation of methane hydrate to CO2 hydrate: In situ raman spectroscopic observations. Phys. Chem. A 2004, 108, 5057–5059. [Google Scholar] [CrossRef]
- Wang, X.H.; Li, F.G.; Xu, Y.X.; Sun, C.Y.; Pan, H.; Liu, B.; Yang, L.Y.; Chen, G.J.; Li, Q.P. Elastic properties of hydrate-bearing sandy sediment during CH4-CO2 replacement. Energy Convers. Manag. 2015, 99, 274–281. [Google Scholar] [CrossRef]
- Liu, B.; Pan, H.; Wang, X.; Li, F.; Sun, C.; Chen, G. Evaluation of different CH4-CO2 replacement processes in hydrate-bearing sediments by measuring P-wave velocity. Energies 2013, 6, 6242–6254. [Google Scholar] [CrossRef]
- Hyodo, M.; Li, Y.; Yoneda, J.; Nakata, Y.; Yoshimoto, N.; Kajiyama, S.; Nishimura, A.; Song, Y. A comparative analysis of the mechanical behavior of carbon dioxide and methane hydrate-bearing sediments. Am. Miner. 2014, 99, 178–183. [Google Scholar] [CrossRef]
- Sloan, E.D.; Koh, C. Clathrate Hydrates of Natural Gases; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Andersen, G.R.; Swan, C.W.; Ladd, C.C.; Germaine, J.T. Small-strain behavior of frozen sand in triaxial compression. Can. Geotech. J. 1995, 32, 428–451. [Google Scholar] [CrossRef]
- Cameron, I.; Handa, Y.P.; Baker, T.H.W. Compressive strength and creep behavior of hydrate-consolidated sand. Can. Geotech. J. 1990, 27, 255–258. [Google Scholar] [CrossRef]
- Ting, J.M.; Torrence Martin, R.; Ladd, C.C. Mechanisms of strength for frozen sand. J. Geotech. Eng. 1983, 109, 1286–1302. [Google Scholar] [CrossRef]
- Durham, W.B.; Kirby, S.H.; Stern, L.A.; Zhang, W. The strength and rheology of methane clathrate hydrate. J. Geophys. Res. Solid Earth 2003, 108. [Google Scholar] [CrossRef]
- Udachin, K.A.; Ratcliffe, C.I.; Ripmeester, J.A. Structure, composition, and thermal expansion of CO2 hydrate from single crystal X-ray diffraction measurements. J. Phys. Chem. B 2001, 105, 4200–4204. [Google Scholar] [CrossRef]
- Circone, S.; Stern, L.A.; Kirby, S.H.; Durham, W.B.; Chakoumakos, B.C.; Rawn, C.J.; Rondinone, A.J.; Ishii, Y. CO2 hydrate: Synthesis, composition, structure, dissociation behavior, and a comparison to structure i CH4 hydrate. J. Phys. Chem. B 2003, 107, 5529–5539. [Google Scholar] [CrossRef]
- Miyazaki, K.; Masui, A.; Sakamoto, Y.; Aoki, K.; Tenma, N.; Yamaguchi, T. Triaxial compressive properties of artificial methane-hydrate-bearing sediment. Geophys. Res. Solid Earth 2011, 116. [Google Scholar] [CrossRef]
- Miyazaki, K.; Tenma, N.; Aoki, K.; Sakamoto, Y.; Yamaguchi, T. Effects of confining pressure on mechanical properties of artificial methane-hydrate-bearing sediment in triaxial compression test. Int. J. Offshore Polar Eng. 2011, 21, 148–154. [Google Scholar]
- Li, Y.; Song, Y.; Yu, F.; Liu, W.; Zhao, J. Experimental study on mechanical properties of gas hydrate-bearing sediments using KaoLin Clay. China Ocean Eng. 2011, 25, 113–122. [Google Scholar] [CrossRef]
- Sun, Z.; Zhang, J.; Liu, C.; Zhao, S.; Ye, Y. Experimental study on the in situ mechanical properties of methane hydrate-bearing sediments. In Applied Mechanics and Materials; Trans Tech Publications: Zürich, Switzerland, 2013; pp. 326–331. [Google Scholar]
- Yu, F.; Song, Y.; Liu, W.; Li, Y.; Lam, W. Analyses of stress strain behavior and constitutive model of artificial methane hydrate. J. Pet. Sci. Eng. 2011, 77, 183–188. [Google Scholar] [CrossRef]
- Masui, A.; Haneda, H.; Ogata, Y.; Aoki, K. Effects of methane hydrate formation on shear strength of synthetic methane hydrate sediments. In Proceedings of the 50th International Offshore and Polar Engineering Conference, Seoul, Korea, 19–24 June 2005. [Google Scholar]
- Masui, A.; Haneda, H.; Ogata, Y.; Aoki, K. Mechanical properties of sandy sediment containing marine gas hydrates in deep sea offshore japan. In Proceedings of the 7th ISOPE Ocean Mining Symposium, Lisbon, Portugal, 1–6 July 2007. [Google Scholar]
- Chamberlain, E.; Groves, C.; Perham, R. The mechanical behaviour of frozen earth materials under high pressure triaxial test conditions. Geotechnique 1972, 22, 469–483. [Google Scholar] [CrossRef]
- Hyodo, M.; Nishimura, A.; Kajiyama, S. Effect of fines on shear strength of methane hydrate bearing sand. In Proceedings of the 11th Ocean Mining and Gas Hydrates Symposium, Kona, HI, USA, 21–27 June 2015. [Google Scholar]
- Miyazaki, K.T.N.; Aoki, K.; Sakamoto, Y.; Yamaguchi, T. Loading-rate dependence of triaxial compressive strength of artificial methane-hydrate-bearing sediment containing fine fraction. In Proceedings of the 22nd International Offshore and Polar Engineering Conference, Rhodes, Greece, 17–22 June 2012. [Google Scholar]






© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, T.; Li, Y.; Liu, W.; Sun, X.; Shen, S. Experimental Study on the Mechanical Properties of CH4 and CO2 Hydrate Remodeling Cores in Qilian Mountain. Energies 2017, 10, 2078. https://doi.org/10.3390/en10122078
Luo T, Li Y, Liu W, Sun X, Shen S. Experimental Study on the Mechanical Properties of CH4 and CO2 Hydrate Remodeling Cores in Qilian Mountain. Energies. 2017; 10(12):2078. https://doi.org/10.3390/en10122078
Chicago/Turabian StyleLuo, Tingting, Yanghui Li, Weiguo Liu, Xiang Sun, and Shi Shen. 2017. "Experimental Study on the Mechanical Properties of CH4 and CO2 Hydrate Remodeling Cores in Qilian Mountain" Energies 10, no. 12: 2078. https://doi.org/10.3390/en10122078




